Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis
Abstract
1. Introduction
2. Results
2.1. General and Clinical Characteristics of the Study Group
2.1.1. General and Clinical Characteristics of the Groups with VitD Deficiency and Normal VitD Levels
2.1.2. Assessment of the Selected Parameters of Brain Injury in the CSF in the Groups with VitD Deficiency and Normal VitD Levels
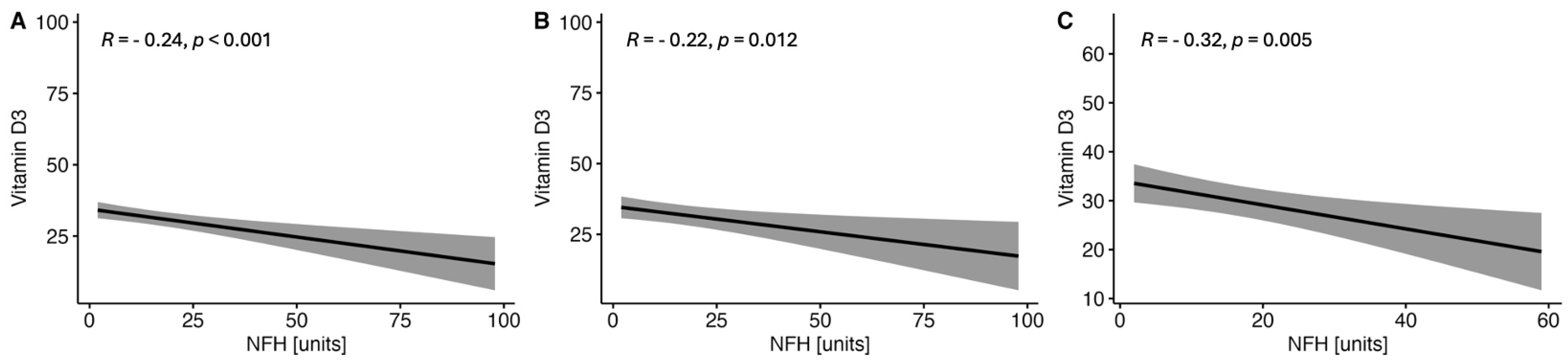
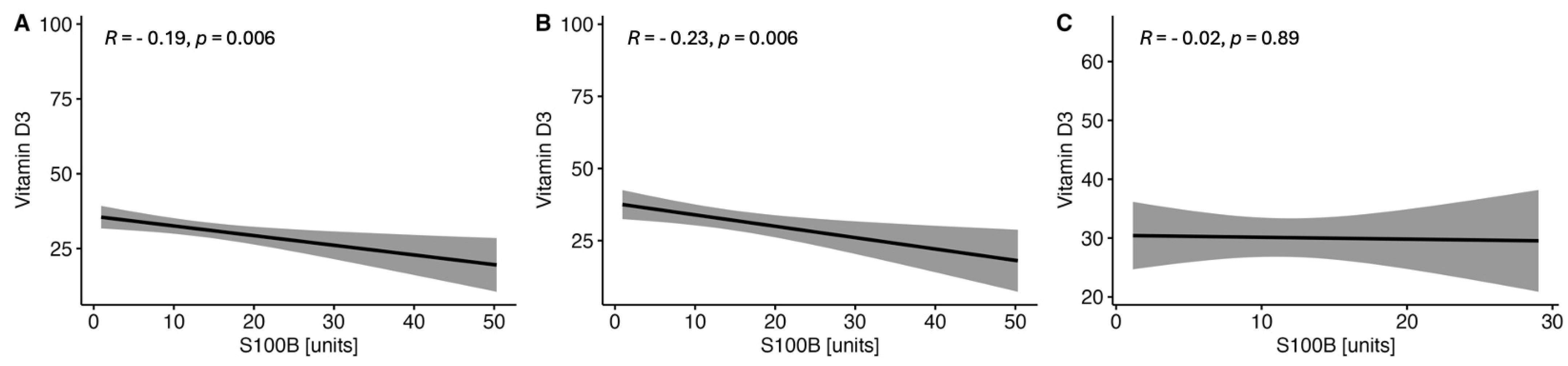
2.1.3. Correlations between the Serum VitD Concentration and the Selected Parameters of Brain Injury in CSF in the Study MS Group Depending on Sex
3. Discussion
Future Directions
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA—J. Am. Med. Assoc. 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, R.; Hosseini-Asl, S.S.; Arefhosseini, S.R.; Morshedi, M. The Impact of Vitamin D3 Intake on Inflammatory Markers in Multiple Sclerosis Patients and Their First-Degree Relatives. PLoS ONE 2020, 15, e0231145. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between Genetic, Lifestyle and Environmental Risk Factors for Multiple Sclerosis. Nat. Rev. Neurol. 2016, 13, 25–36. [Google Scholar] [CrossRef]
- Verdoia, M.; De Luca, G. Potential Role of Hypovitaminosis D and Vitamin D Supplementation during COVID-19 Pandemic. QJM An. Int. J. Med. 2021, 114, 3–10. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis: Lessons from Molecular Neuropathology. Exp. Neurol. 2014, 262 Pt A, 2–7. [Google Scholar] [CrossRef]
- Simons, M.; Misgeld, T.; Kerschensteiner, M. A Unified Cell Biological Perspective on Axon-Myelin Injury. J. Cell Biol. 2014, 206, 335–345. [Google Scholar] [CrossRef]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.S. Immunomodulatory and Anti-Inflammatory Effects of Probiotics in Multiple Sclerosis: A Systematic Review. J. Neuroinflammat. 2019, 16, 231. [Google Scholar] [CrossRef]
- Haase, S.; Linker, R.A. Inflammation in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211007687. [Google Scholar] [CrossRef]
- Tobore, T.O. Towards a Comprehensive Etiopathogenetic and Pathophysiological Theory of Multiple Sclerosis. Int. J. Neurosci. 2020, 130, 279–300. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple Sclerosis Progression: Time for a New Mechanism-Driven Framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S.; et al. Autoreactive Lymphocytes in Multiple Sclerosis: Pathogenesis and Treatment Target. Front. Immunol. 2022, 13, 996469. [Google Scholar] [CrossRef] [PubMed]
- Arrambide, G.; Rovira, A.; Sastre-Garriga, J.; Tur, C.; Castilló, J.; Río, J.; Vidal-Jordana, A.; Galán, I.; Rodríguez-Acevedo, B.; Midaglia, L.; et al. Spinal Cord Lesions: A Modest Contributor to Diagnosis in Clinically Isolated Syndromes but a Relevant Prognostic Factor. Mult. Scler. 2018, 24, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Attfield, K.E.; Jensen, L.T.; Kaufmann, M.; Friese, M.A.; Fugger, L. The Immunology of Multiple Sclerosis. Nat. Rev. Immunol. 2022, 22, 734–750. [Google Scholar] [CrossRef]
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobeus, E.; Jensen, M.B.; García-Domínguez, J.M.; Sousa, L.; De Rossi, N.; Hupperts, R.; et al. Smouldering Multiple Sclerosis: The ‘Real MS. ’ Ther. Adv. Neurol. Disord. 2022, 15, 17562864211066751. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in Multiple Sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- Slezáková, D.; Kadlic, P.; Jezberová, M.; Boleková, V.; Valkovič, P.; Minár, M. Brain Volume Loss in Multiple Sclerosis Is Independent of Disease Activity and Might Be Prevented by Early Disease-Modifying Therapy. Neurol. Neurochir. Pol. 2023, 57, 282–288. [Google Scholar] [CrossRef]
- Ruiz, F.; Vigne, S.; Pot, C. Resolution of Inflammation during Multiple Sclerosis. Semin. Immunopathol. 2019, 41, 711–726. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Shobeiri, P.; Saghazadeh, A.; Teunissen, C.E.; Burman, J.; Szalardy, L.; Klivenyi, P.; Bartos, A.; Fernandes, A.; Rezaei, N. Neuronal and Glial CSF Biomarkers in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2021, 32, 573–595. [Google Scholar] [CrossRef]
- Brenner, M. Role of GFAP in CNS Injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Górska, E.; Tylicka, M.; Hermanowicz, A.; Matuszczak, E.; Sankiewicz, A.; Gorodkiewicz, E.; Hermanowicz, J.; Karpińska, E.; Socha, K.; Kochanowicz, J.; et al. UCHL1, besides Leptin and Fibronectin, Also Could Be a Sensitive Marker of the Relapsing-Remitting Type of Multiple Sclerosis. Sci. Rep. 2023, 13, 3423. [Google Scholar] [CrossRef] [PubMed]
- Langeh, U.; Singh, S. Targeting S100B Protein as a Surrogate Biomarker and Its Role in Various Neurological Disorders. Curr. Neuropharmacol. 2021, 19, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sánchez, E.D. Immunology and Oxidative Stress in Multiple Sclerosis: Clinical and Basic Approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef] [PubMed]
- Motamed, S.; Nikooyeh, B.; Anari, R.; Motamed, S.; Mokhtari, Z.; Neyestani, T. The Effect of Vitamin D Supplementation on Oxidative Stress and Inflammatory Biomarkers in Pregnant Women: A Systematic Review and Meta-Analysis of Clinical Trials. BMC Pregnancy Childbirth 2022, 22, 141–152. [Google Scholar] [CrossRef]
- Koduah, P.; Paul, F.; Dörr, J.M. Vitamin D in the Prevention, Prediction and Treatment of Neurodegenerative and Neuroinflammatory Diseases. EPMA J. 2017, 8, 313–325. [Google Scholar] [CrossRef]
- Sangha, A.; Quon, M.; Pfeffer, G.; Orton, S.M. The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An Update. Nutrients 2023, 15, 2978. [Google Scholar] [CrossRef]
- Anwar, M.J.; Alenezi, S.K.; Alhowail, A.H. Molecular Insights into the Pathogenic Impact of Vitamin D Deficiency in Neurological Disorders. Biomed. Pharmacother. 2023, 162, 114718. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Bäärnhielm, M.; Olsson, T.; Alfredsson, L. Fatty Fish Intake Is Associated with Decreased Occurrence of Multiple Sclerosis. Mult. Scler. 2014, 20, 726–732. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.C. Vitamin D and Multiple Sclerosis: An Update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Van Schependom, J.; Guldolf, K.; D’Hooghe, M.B.; Nagels, G.; D’Haeseleer, M. Detecting Neurodegenerative Pathology in Multiple Sclerosis before Irreversible Brain Tissue Loss Sets In. Transl. Neurodegener. 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Mr, A.; Pga, B.; Scpl, D. Citation: Almeida MR, Brasiel PGA and Dutra SCPL. Effects and Mechanisms of Action of Vitamin D in Multiple Sclerosis Effects and Mechanisms of Action of Vitamin D in Multiple Sclerosis. Austin J. Nutr. Food Sci. 2018, 1109, 6. [Google Scholar]
- Jahromi, S.R.; Sahraian, M.A.; Togha, M.; Sedighi, B.; Shayegannejad, V.; Nickseresht, A.; Nafissi, S.; Mohebbi, N.; Majdinasab, N.; Foroughipour, M.; et al. Iranian Consensus on Use of Vitamin D in Patients with Multiple Sclerosis. BMC Neurol. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.P.; Li, S.M.; Lv, W.Y.; Jv, M.L.; He, H.Y. Vitamin D3 Repressed Astrocyte Activation Following Lipopolysaccharide Stimulation in Vitro and in Neonatal Rats. Neuroreport 2017, 28, 492–497. [Google Scholar] [CrossRef]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132–1140. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Storoni, M.; Verbeek, M.M.; Illes, Z.; Marignier, R.; Teunissen, C.E.; Grabowska, M.; Confavreux, C.; Plant, G.T.; Petzold, A. Serum GFAP Levels in Optic Neuropathies. J. Neurol. Sci. 2012, 317, 117–122. [Google Scholar] [CrossRef]
- Sun, M.J.; Liu, N.; Xie, Q.F.; Li, X.; Sun, J.; Wang, H.; Wang, M.X. A Candidate Biomarker of Glial Fibrillary Acidic Protein in CSF and Blood in Differentiating Multiple Sclerosis and Its Subtypes: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2021, 51, 102870. [Google Scholar] [CrossRef]
- Thouvenot, E.; Orsini, M.; Daures, J.P.; Camu, W. Vitamin D Is Associated with Degree of Disability in Patients with Fully Ambulatory Relapsing-Remitting Multiple Sclerosis. Eur. J. Neurol. 2015, 22, 564–569. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Simão, A.N.C.; Alfieri, D.F.; Flauzino, T.; Kallaur, A.P.; Mezzaroba, L.; Lozovoy, M.A.B.; Sabino, B.S.; Ferreira, K.P.Z.; Pereira, W.L.C.J.; et al. Vitamin D Deficiency Is Associated with Disability and Disease Progression in Multiple Sclerosis Patients Independently of Oxidative and Nitrosative Stress. J. Neurol. Sci. 2017, 381, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Muris, A.H.; Rolf, L.; Broen, K.; Hupperts, R.; Damoiseaux, J.; Smolders, J. A Low Vitamin D Status at Diagnosis Is Associated with an Early Conversion to Secondary Progressive Multiple Sclerosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, A.G.; Condlin, M.L.; Loban, A.; DeMar, J.C. Omega-3 Fatty Acids and Vitamin D Decrease Plasma T-Tau, GFAP, and UCH-L1 in Experimental Traumatic Brain Injury. Front. Nutr. 2021, 8, 685220. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; McGlasson, S.; Hunt, D.; Overell, J. Cerebrospinal Fluid Neurofilament Light Chain in Multiple Sclerosis and Its Subtypes: A Meta-Analysis of Case-Control Studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Munger, K.L.; Cortese, M.; Barro, C.; Healy, B.C.; Niebuhr, D.W.; Scher, A.I.; Kuhle, J.; Ascherio, A. Serum Neurofilament Light Chain Levels in Patients with Presymptomatic Multiple Sclerosis. JAMA Neurol. 2020, 77, 58–64. [Google Scholar] [CrossRef]
- Sandberg, L.; Biström, M.; Salzer, J.; Vågberg, M.; Svenningsson, A.; Sundström, P. Vitamin D and Axonal Injury in Multiple Sclerosis. Mult. Scler. 2016, 22, 1027–1031. [Google Scholar] [CrossRef]
- Holmøy, T.; Røsjø, E.; Zetterberg, H.; Blennow, K.; Lindstrøm, J.C.; Steffensen, L.H.; Kampman, M.T. Vitamin D Supplementation and Neurofilament Light Chain in Multiple Sclerosis. Acta Neurol. Scand. 2019, 139, 172–176. [Google Scholar] [CrossRef]
- Røsjø, E.; Lindstrøm, J.C.; Holmøy, T.; Myhr, K.M.; Varhaug, K.N.; Torkildsen, Ø. Natural Variation of Vitamin D and Neurofilament Light Chain in Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2020, 11, 329. [Google Scholar] [CrossRef]
- Smolders, J.; Mimpen, M.; Oechtering, J.; Damoiseaux, J.; van den Ouweland, J.; Hupperts, R.; Kuhle, J. Vitamin D 3 Supplementation and Neurofilament Light Chain in Multiple Sclerosis. Acta Neurol. Scand. 2020, 141, 77–80. [Google Scholar] [CrossRef]
- Smolders, J.; Torkildsen, Ø.; Camu, W.; Holmøy, T. An Update on Vitamin D and Disease Activity in Multiple Sclerosis. CNS Drugs 2019, 33, 1187–1199. [Google Scholar] [CrossRef]
- Haindl, M.T.; Üçal, M.; Wonisch, W.; Lang, M.; Nowakowska, M.; Adzemovic, M.Z.; Khalil, M.; Enzinger, C.; Hochmeister, S. Vitamin D-An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis. Nutrients 2023, 15, 3309. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.; Silva, C.; Gohill, J.; Yong, V.W.; Koch, M. Serum Neurofilament-Light and Glial Fibrillary Acidic Protein Levels in Hydroxychloroquine-Treated Primary Progressive Multiple Sclerosis. Eur. J. Neurol. 2023, 30, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Bianchi, R.; Riuzzi, F.; Tubaro, C.; Arcuri, C.; Giambanco, I. S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc. Psychiatry Neurol. 2010, 2010, 656481. [Google Scholar] [CrossRef]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef] [PubMed]
- Mañé-Martínez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo-Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and Neuronal Markers in Cerebrospinal Fluid in Different Types of Multiple Sclerosis. J. Neuroimmunol. 2016, 299, 112–117. [Google Scholar] [CrossRef]
- Mi, Z.; Graham, S.H. Role of UCHL1 in the Pathogenesis of Neurodegenerative Diseases and Brain Injury. Ageing Res. Rev. 2023, 86, 101856. [Google Scholar] [CrossRef]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J.; Oli, M.W.; Zheng, W.; Robinson, G.; Robicsek, S.A.; Gabrielli, A.; et al. Ubiquitin C-Terminal Hydrolase Is a Novel Biomarker in Humans for Severe Traumatic Brain Injury. Crit. Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef]
- Langlois, J.; Denimal, D. Clinical and Imaging Outcomes after Vitamin D Supplementation in Patients with Multiple Sclerosis: A Systematic Review. Nutrients 2023, 15, 1945. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Kochert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.H.; Montalban, X.; et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef]
- Cadavid, D.; Cohen, J.A.; Freedman, M.S.; Goldman, M.D.; Hartung, H.P.; Havrdova, E.; Jeffery, D.; Kapoor, R.; Miller, A.; Sellebjerg, F.; et al. The EDSS-Plus, an Improved Endpoint for Disability Progression in Secondary Progressive Multiple Sclerosis. Mult. Scler. 2017, 23, 94–105. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Oczkowicz, M. Sex Differences in Vitamin D Metabolism, Serum Levels and Action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, T.H.; da Rocha Lemos Costa, T.M.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Body Composition in Nonobese Individuals According to Vitamin D Level. PLoS ONE 2020, 15, e0241858. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, F.; Jhaveri, S.; Avanthika, C.; Singh, A.; Jain, N.; Gulraiz, A.; Shah, P.; Nasir, F. Impact of Vitamin D Supplementation on Multiple Sclerosis. Cureus 2021, 13, e18487. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. Defining Secondary Progressive Multiple Sclerosis. Brain 2016, 139, 2395–2405. [Google Scholar] [CrossRef]


| Parameter | RRMS (n = 123) | PMS (n = 88) | p-Value |
|---|---|---|---|
| Age (years) | 36 (29–41) | 52 (47–54) | <0.001 |
| Sex (females) | 87/123 (71%) | 48/88 (55%) | 0.016 |
| MS relapse | 30/123 (24%) | 0/88 (0%) | <0.001 |
| MS duration (years) | 0 (0–1) | 1 (0–12) | <0.001 |
| MS symptoms duration (years) | 1 (0–3) | 5 (1–13) | <0.001 |
| EDSS (score) | 2.50 (2.00–3.00) | 4.50 (4.00–5.00) | <0.001 |
| DMT | 18/123 (15%) | 8/88 (9.1%) | 0.227 |
| Gd+ lesions (brain MRI) | 48/123 (39%) | 8/88 (9.1%) | <0.001 |
| Gd+ lesions (cervical and thoracic MRI) | 12/123 (9.8%) | 8/88 (9.1%) | 0.871 |
| Elevated IgG in the CSF | 75/105 (71%) | 64/88 (73%) | 0.841 |
| OCBs | 78/117 (67%) | 48/88 (55%) | 0.078 |
| Vitamin D3 deficiency | 78/120 (65%) | 48/88 (55%) | 0.127 |
| Vitamin D3 concentration (ng/mL) | 27 (18–36) | 26 (21–51) | 0.131 |
| Dyslipidemia | 75/123 (61%) | 64/88 (73%) | 0.076 |
| Total cholesterol (mmoL/L) | 5.00 (4.29–5.55) | 6.21 (4.15–6.38) | <0.001 |
| LDL cholesterol (mmoL/L) | 2.75 (2.23–3.37) | 3.65 (2.43–4.19) | <0.001 |
| HDL cholesterol (mmoL/L) | 1.56 (1.30–2.00) | 1.41 (1.09–1.66) | 0.002 |
| Triglycerides (mmoL/L) | 1.11 (0.78–1.43) | 1.36 (1.18–1.98) | <0.001 |
| Overweight or obesity | 39/123 (32%) | 32/88 (36%) | 0.480 |
| Hypertension | 15/123 (12%) | 40/88 (45%) | <0.001 |
| Diabetes mellitus | 9/123 (7.3%) | 0/88 (0%) | 0.011 |
| Thyroid function abnormalities | 18/123 (15%) | 0/88 (0%) | <0.001 |
| Smoking | 12/123 (9.8%) | 16/88 (18%) | 0.075 |
| Parameter | Overall n = 208 | VitDn n = 82 | VitDd n = 126 | p-Value |
|---|---|---|---|---|
| SM group | 0.127 | |||
| RRMS | 120/208 (58%) | 42/82 (51%) | 78/126 (62%) | |
| PMS | 88/208 (42%) | 40/82 (49%) | 48/126 (38%) | |
| Age (years) | 41 (31, 52) | 48 (39, 52) | 37 (29, 48) | <0.001 |
| Sex (females) | 135/208 (65%) | 59/82 (72%) | 76/126 (60%) | 0.086 |
| SM relapse | 30/208 (14%) | 15/82 (18%) | 15/126 (12%) | 0.200 |
| SM duration [years] | 0 (0, 1) | 1 (0, 12) | 0 (0, 1) | 0.004 |
| Duration of SM symptoms [years] | 2 (0, 6) | 2 (0, 14) | 2 (1, 5) | 0.442 |
| EDSS (score) | 3.50 (2.50, 4.50) | 3.75 (3.00, 5.00) | 3.25 (2.00, 4.00) | <0.001 |
| DMT | 26/208 (13%) | 23/82 (28%) | 3/126 (2.4%) | <0.001 |
| Gd+ lesions (brain MRI) | 56/208 (27%) | 18/82 (22%) | 38/126 (30%) | 0.192 |
| Gd+ lesions (cervical and thoracic MRI) | 20/208 (9.6%) | 6/82 (7.3%) | 14/126 (11%) | 0.364 |
| Elevated IgG in the CSF | 136/190 (72%) | 57/79 (72%) | 79/111 (71%) | 0.883 |
| OCBs | 123/202 (61%) | 48/82 (59%) | 75/120 (63%) | 0.571 |
| Vitamin D3 (ng/mL) | 26 (20, 37) | 41 (34, 54) | 22 (17, 25) | <0.001 |
| Dyslipidemia | 136/208 (65%) | 62/82 (76%) | 74/126 (59%) | 0.012 |
| Total cholesterol (mmoL/L) | 5.29 (4.23, 6.16) | 5.77 (4.76, 6.33) | 4.76 (4.22, 5.66) | 0.011 |
| LDL cholesterol (mmoL/L) | 2.75 (2.36, 3.65) | 3.42 (2.47, 3.92) | 2.72 (2.30, 3.44) | 0.004 |
| HDL cholesterol (mmoL/L) | 1.51 (1.15, 1.79) | 1.51 (1.15, 1.89) | 1.56 (1.23, 1.76) | 0.799 |
| Triglycerides (mmoL/L) | 1.20 (0.86, 1.64) | 1.26 (0.89, 1.38) | 1.20 (0.89, 1.98) | 0.194 |
| Overweight or obesity | 71/208 (34%) | 28/82 (34%) | 43/126 (34%) | 0.998 |
| Hypertension | 55/208 (26%) | 35/82 (43%) | 20/126 (16%) | <0.001 |
| Diabetes mellitus | 9/208 (4.3%) | 0/82 (0%) | 9/126 (7.1%) | 0.013 |
| Thyroid function abnormalities | 18/208 (8.7%) | 12/82 (15%) | 6/126 (4.8%) | 0.013 |
| Smoking | 28/208 (13%) | 3/82 (3.7%) | 25/126 (20%) | <0.001 |
| Parameter | Overall n = 208 | VitDn n = 82 | VitDd n = 126 | p-Value |
|---|---|---|---|---|
| GFAP (pg/mL) | 2210 (1457, 4450) | 2143 (1450, 3626) | 3055 (1548, 5045) | 0.157 |
| NF-H (pg/mL) | 4 (3, 25) | 3 (3, 4) | 4 (3, 25) | <0.001 |
| S100B (pg/mL) | 12 (4, 16) | 12 (3, 14) | 12 (8, 17) | 0.113 |
| UCHL1 (pg/mL) | 55 (49, 60) | 52 (49, 59) | 56 (48, 61) | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedziela, N.; Nowak-Kiczmer, M.; Malciene, L.; Stasiołek, M.; Niedziela, J.T.; Czuba, Z.P.; Lis, M.; Sowa, A.; Adamczyk-Sowa, M. Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 10502. https://doi.org/10.3390/ijms251910502
Niedziela N, Nowak-Kiczmer M, Malciene L, Stasiołek M, Niedziela JT, Czuba ZP, Lis M, Sowa A, Adamczyk-Sowa M. Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis. International Journal of Molecular Sciences. 2024; 25(19):10502. https://doi.org/10.3390/ijms251910502
Chicago/Turabian StyleNiedziela, Natalia, Maria Nowak-Kiczmer, Lina Malciene, Mariusz Stasiołek, Jacek T. Niedziela, Zenon P. Czuba, Martyna Lis, Agata Sowa, and Monika Adamczyk-Sowa. 2024. "Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis" International Journal of Molecular Sciences 25, no. 19: 10502. https://doi.org/10.3390/ijms251910502
APA StyleNiedziela, N., Nowak-Kiczmer, M., Malciene, L., Stasiołek, M., Niedziela, J. T., Czuba, Z. P., Lis, M., Sowa, A., & Adamczyk-Sowa, M. (2024). Serum Vitamin D3 as a Potential Biomarker for Neuronal Damage in Smoldering Multiple Sclerosis. International Journal of Molecular Sciences, 25(19), 10502. https://doi.org/10.3390/ijms251910502






