Enhancement of Stress Granule Formation by a Chiral Compound Targeting G3BP1 via eIF2α Phosphorylation
Abstract
1. Introduction
2. Results
2.1. Identifying Promising Chiral Compounds for Binding to the NTF2-like Domain of G3BP1
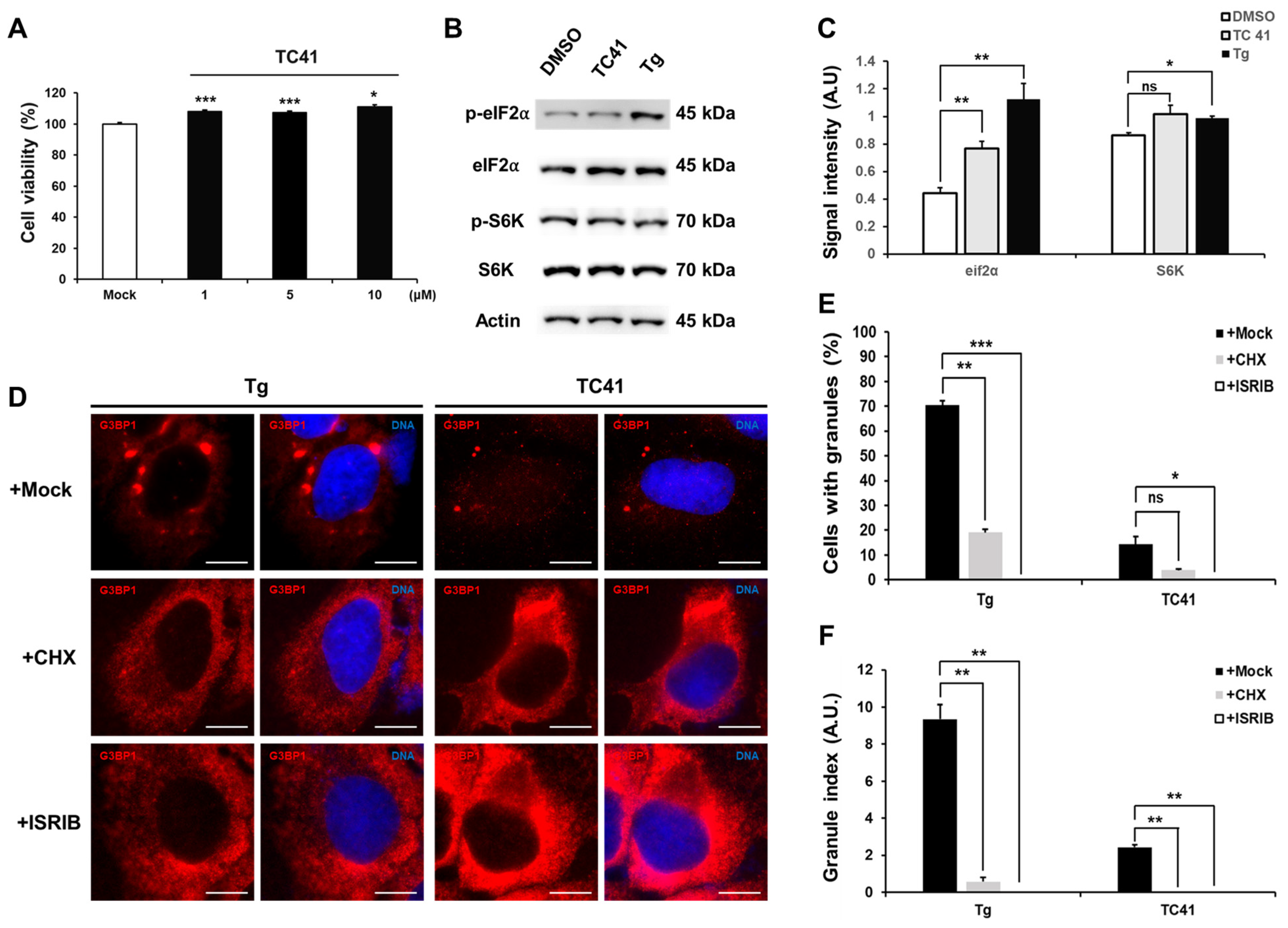
2.2. Dependence of Induced Formation of G3BP1-Positive Granules by TC41 on eIF2α Phosphorylation without Exhibiting Toxicity
2.3. Chirality Analysis of TC41
2.4. Molecular-Docking Simulations of Stereoisomers with G3BP1
2.5. Analysis of the Interaction of Stereoisomers with G3BP1 in Comparison to the FGDF Motif
3. Discussion
4. Material and Methods
4.1. In Silico Molecular-Docking Simulation and Chiral Structure Analysis
4.2. Formation of a Reliable Cell Line That Demonstrates Expression of mEmerald-LaminB1 and RFP-G3BP
4.3. Cell Culture
4.4. Compound Treatments
4.5. Immunofluorescence Imaging and Quantification of Granule Formation
4.6. Assessment of Cell Viability
4.7. Immunoblotting
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Guan, Y.; Wang, Y.; Fu, X.; Bai, G.; Li, X.; Mao, J.; Yan, Y.; Hu, L. Multiple functions of stress granules in viral infection at a glance. Front. Microbiol. 2023, 14, 1138864. [Google Scholar] [CrossRef] [PubMed]
- Freibaum, B.D.; Messing, J.; Nakamura, H.; Yurtsever, U.; Wu, J.; Kim, H.J.; Hixon, J.; Lemieux, R.; Duffner, J.; Huynh, W. Identification of small molecule inhibitors of G3BP-driven stress granule formation. J. Cell Biol. 2024, 223, e202308083. [Google Scholar] [CrossRef] [PubMed]
- Younas, N.; Zafar, S.; Shafiq, M.; Noor, A.; Siegert, A.; Arora, A.S.; Galkin, A.; Zafar, A.; Schmitz, M.; Stadelmann, C. SFPQ and Tau: Critical factors contributing to rapid progression of Alzheimer’s disease. Acta Neuropathol. 2020, 140, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Shin, O.S. Stress Granules Inhibit Coxsackievirus B3-Mediated Cell Death via Reduction of Mitochondrial Reactive Oxygen Species and Viral Extracellular Release. J. Microbiol. Biotechnol. 2023, 33, 582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mañán-Mejías, P.M.; Miles, H.N.; Putnam, A.A.; MacGillivray, L.R.; Ricke, W.A. DDX3X and Stress Granules: Emerging Players in Cancer and Drug Resistance. Cancers 2024, 16, 1131. [Google Scholar] [CrossRef]
- Bradshaw, W.J.; Rehman, S.; Pham, T.T.; Thiyagarajan, N.; Lee, R.L.; Subramanian, V.; Acharya, K.R. Structural insights into human angiogenin variants implicated in Parkinson’s disease and Amyotrophic Lateral Sclerosis. Sci. Rep. 2017, 7, 41996. [Google Scholar] [CrossRef]
- Lee, J.I.; Namkoong, S. Stress granules dynamics: Benefits in cancer. BMB Rep. 2022, 55, 577–586. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e328. [Google Scholar] [CrossRef]
- Schulte, T.; Liu, L.; Panas, M.D.; Thaa, B.; Dickson, N.; Götte, B.; Achour, A.; McInerney, G.M. Combined structural, biochemical and cellular evidence demonstrates that both FGDF motifs in alphavirus nsP3 are required for efficient replication. Open Biol. 2016, 6, 160078. [Google Scholar] [CrossRef]
- Song, D.; Kuang, L.; Yang, L.; Wang, L.; Li, H.; Li, X.; Zhu, Z.; Shi, C.; Zhu, H.; Gong, W. Yin and yang regulation of stress granules by Caprin-1. Proc. Natl. Acad. Sci. USA 2022, 119, e2207975119. [Google Scholar] [CrossRef]
- Panas, M.D.; Schulte, T.; Thaa, B.; Sandalova, T.; Kedersha, N.; Achour, A.; McInerney, G.M. Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation. PLoS Pathog. 2015, 11, e1004659. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Z.; Xu, R.; Zhang, J.; Zhou, Q.; Gao, R.; Lu, H.; Lan, Y.; Zhao, K.; He, H. The PERK/PKR-eIF2α pathway negatively regulates porcine hemagglutinating encephalomyelitis virus replication by attenuating global protein translation and facilitating stress granule formation. J. Virol. 2022, 96, e01695-21. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, A.P.; Mellor, L.E.; Pang, Y.F.; Kritsiligkou, P.; Needs, H.; Abou-Hamdan, H.; Désaubry, L.; Poulin, G.B.; Ashe, M.P.; Whitmarsh, A.J. Correction to: The mTOR-S6 kinase pathway promotes stress granule assembly. Cell Death Differ. 2020, 27, 1744. [Google Scholar] [CrossRef] [PubMed]
- Slaine, P.D.; Kleer, M.; Smith, N.K.; Khaperskyy, D.A.; McCormick, C. Stress granule-inducing eukaryotic translation initiation factor 4A inhibitors block influenza A virus replication. Viruses 2017, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, R.; Yan, S.; Pang, Z.; Li, H.; Yang, X.; Wang, K. Chiral Materials: Progress, Applications, and Prospects. Small 2023, 19, 2303059. [Google Scholar] [CrossRef]
- Kristensen, O. Crystal structure of the G3BP2 NTF2-like domain in complex with a canonical FGDF motif peptide. Biochem. Biophys. Res. Commun. 2015, 467, 53–57. [Google Scholar] [CrossRef]
- Cho, H.S.; Park, Y.H.; Moon, S.; Park, C.; Jung, H.S.; Namkoong, S. Targeting the NTF2-like domain of G3BP1: Novel modulators of intracellular granule dynamics. Biochem. Biophys. Res. Commun. 2024, 697, 149497. [Google Scholar] [CrossRef]
- Day, R.N.; Davidson, M.W. The fluorescent protein palette: Tools for cellular imaging. Chem. Soc. Rev. 2009, 38, 2887–2921. [Google Scholar] [CrossRef]
- Humeau, J.; Leduc, M.; Cerrato, G.; Loos, F.; Kepp, O.; Kroemer, G. Phosphorylation of eukaryotic initiation factor-2α (eIF2α) in autophagy. Cell Death Dis. 2020, 11, 433. [Google Scholar] [CrossRef]
- Kedersha, N.; Cho, M.R.; Li, W.; Yacono, P.W.; Chen, S.; Gilks, N.; Golan, D.E.; Anderson, P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell. Biol. 2000, 151, 1257–1268. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Vognsen, T.; Moller, I.R.; Kristensen, O. Crystal structures of the human G3BP1 NTF2-like domain visualize FxFG Nup repeat specificity. PLoS ONE 2013, 8, e80947. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucl. Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Liu-Yesucevitz, L.; Lin, A.Y.; Ebata, A.; Boon, J.Y.; Reid, W.; Xu, Y.F.; Kobrin, K.; Murphy, G.J.; Petrucelli, L.; Wolozin, B. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. J. Neurosci. 2014, 34, 4167–4174. [Google Scholar] [CrossRef]
- Grabocka, E.; Bar-Sagi, D. Mutant KRAS Enhances Tumor Cell Fitness by Upregulating Stress Granules. Cell 2016, 167, 1803–1813.e12. [Google Scholar] [CrossRef]
- Namkoong, S.; Kim, T.J.; Jang, I.S.; Kang, K.W.; Oh, W.K.; Park, J. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-kappaB pathways in lung tumor cells. Biol. Pharm. Bull 2011, 34, 203–208. [Google Scholar] [CrossRef]


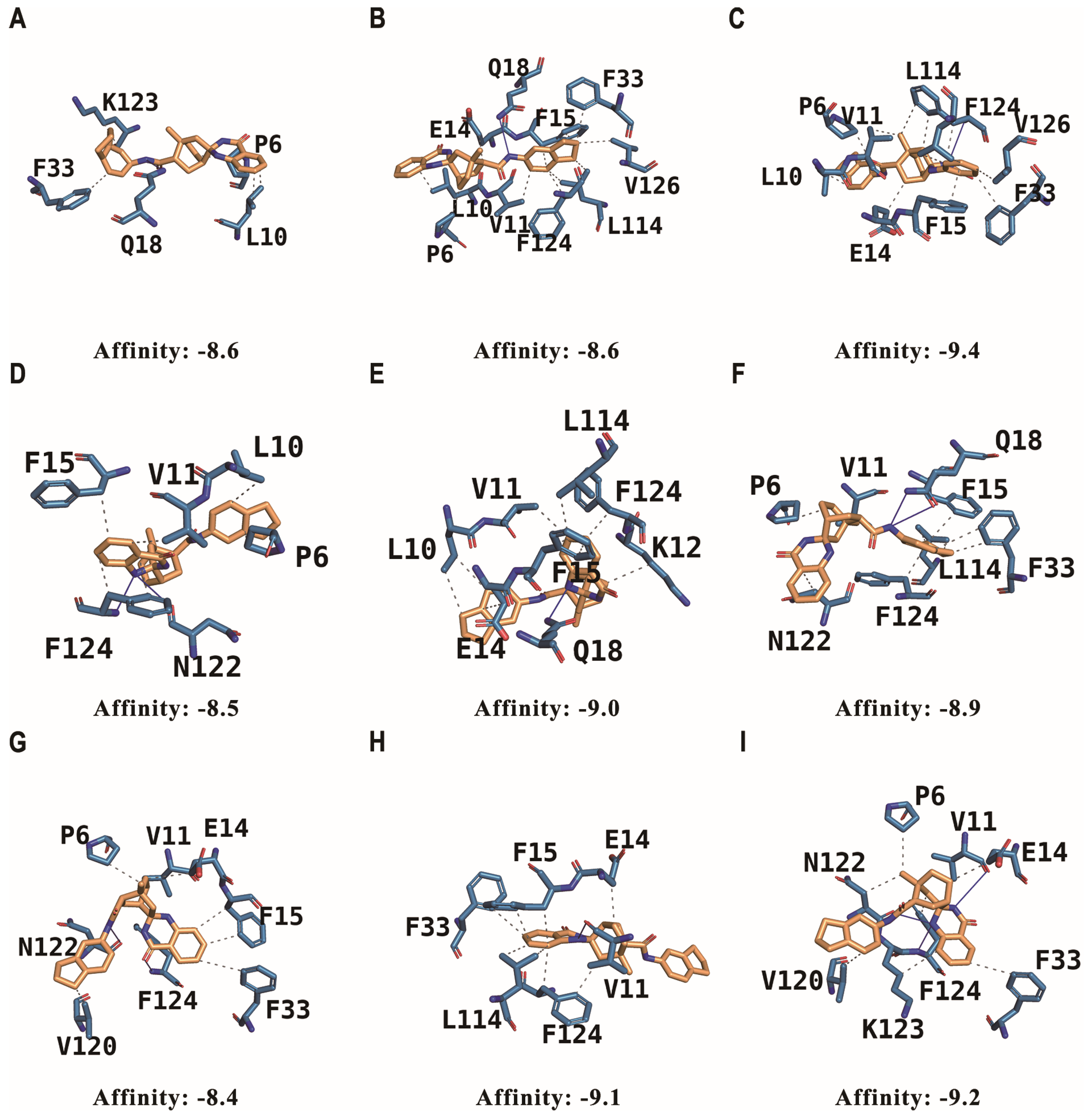
| Conformation Number | Affinity (kcal/mol) | Hydrophobic Interaction | Hydrogen Interaction |
|---|---|---|---|
| (1) | −8.6 | P9, L10, F33, K123 | Q18 |
| (2) | −8.6 | P6, L10, V11, E14, F15, F33, L114, F124, V126 | Q18 |
| (3) | −9.4 | P6, L10, V11, E14, F15, F33, L114, F124, V126 | F124 |
| (4) | −8.5 | P6, L10, V11, F15, F124 | N122, F124 |
| (5) | −9.0 | L10, V11, E14, F15 L114, K123, F124 | Q18 |
| (6) | −8.9 | P6, V11, F15, F33, L114, N122, F124 | Q18 |
| (7) | −8.4 | P6, V11, E14, F15, F33, V120 | N122, F124 |
| (8) | −9.1 | E14, F15, F33, L114, F124 | V11 |
| (9) | −9.2 | P6, E14, F33, N122, K123, F124 | V11, N122, F124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.H.; Cho, H.S.; Moon, S.; Namkoong, S.; Jung, H.S. Enhancement of Stress Granule Formation by a Chiral Compound Targeting G3BP1 via eIF2α Phosphorylation. Int. J. Mol. Sci. 2024, 25, 10571. https://doi.org/10.3390/ijms251910571
Park YH, Cho HS, Moon S, Namkoong S, Jung HS. Enhancement of Stress Granule Formation by a Chiral Compound Targeting G3BP1 via eIF2α Phosphorylation. International Journal of Molecular Sciences. 2024; 25(19):10571. https://doi.org/10.3390/ijms251910571
Chicago/Turabian StylePark, Yoon Ho, Hyun Suh Cho, Sungjin Moon, Sim Namkoong, and Hyun Suk Jung. 2024. "Enhancement of Stress Granule Formation by a Chiral Compound Targeting G3BP1 via eIF2α Phosphorylation" International Journal of Molecular Sciences 25, no. 19: 10571. https://doi.org/10.3390/ijms251910571
APA StylePark, Y. H., Cho, H. S., Moon, S., Namkoong, S., & Jung, H. S. (2024). Enhancement of Stress Granule Formation by a Chiral Compound Targeting G3BP1 via eIF2α Phosphorylation. International Journal of Molecular Sciences, 25(19), 10571. https://doi.org/10.3390/ijms251910571








