Bioceramics Enhance the Anti-Tumor Activity of Immune Cells in Adoptive Immunotherapy
Abstract
:1. Introduction
2. Results
2.1. Characterization of CPSB Ceramics
2.2. Cellular Responses of Mice Spleen Cells to CPSB Ceramics
2.3. Surface Zeta Potential of CPSB Ceramics
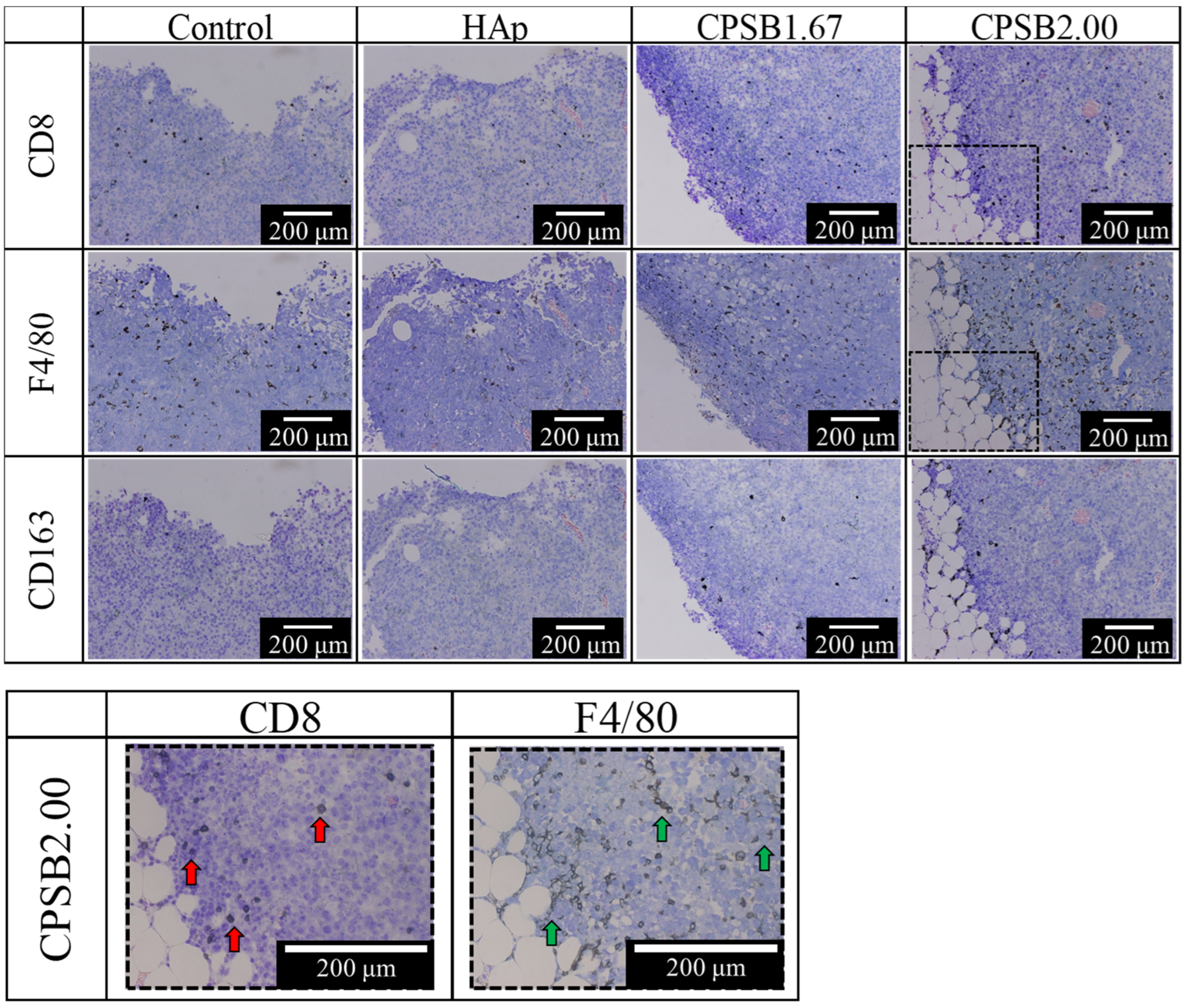
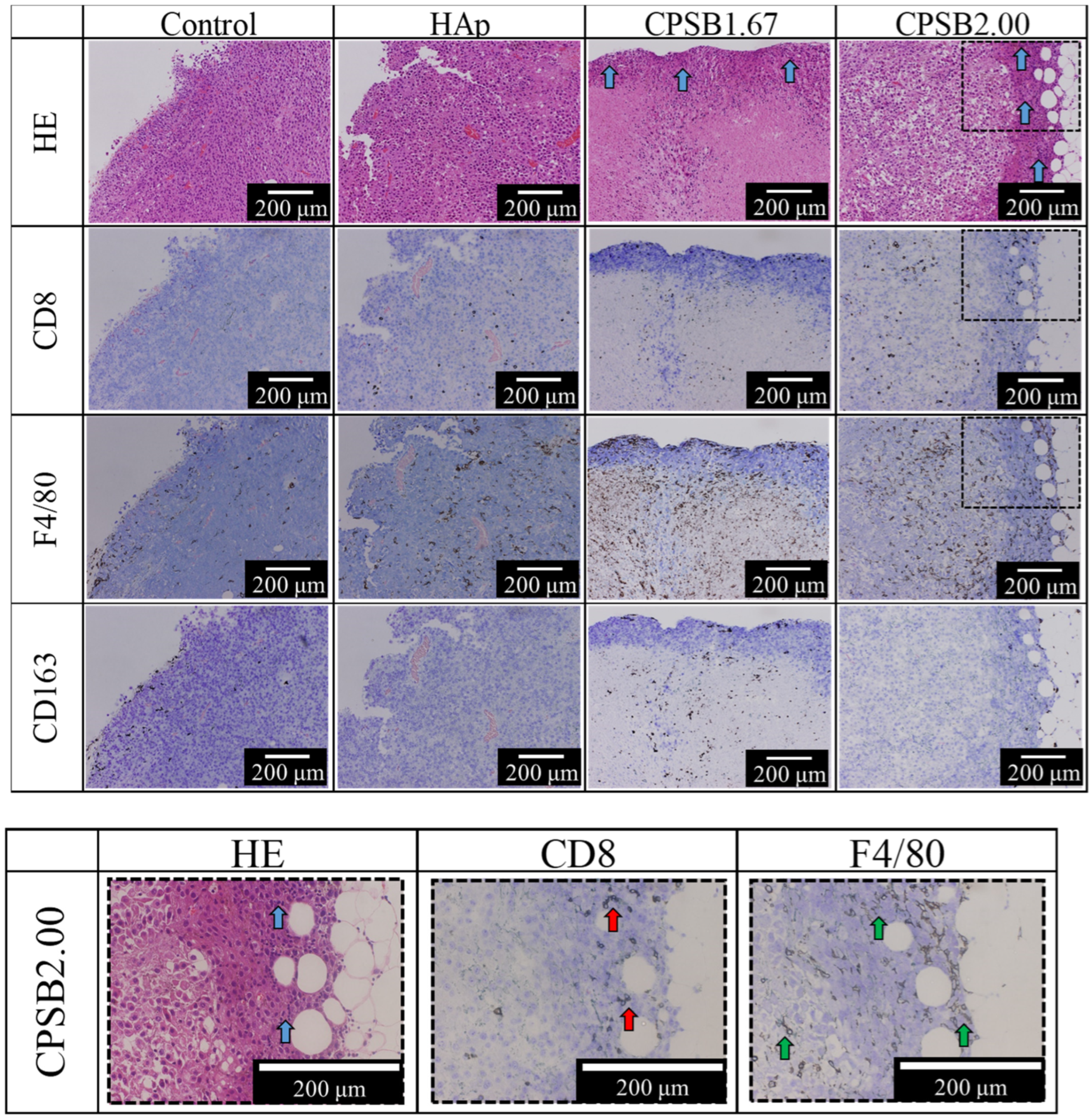
2.4. Anti-Tumor Effects In Vivo
3. Discussion
4. Materials and Methods
4.1. Fabrication of CPSB Ceramics by Sol–Gel Process and Their Characterization
4.2. Mice and Cell Culture
4.3. Flow Cytometry
4.4. Morphological Observation of Cells
4.5. Evaluation of the Interaction between Ceramic Surfaces and the Intracellular Sugar Chain by Model Experiments
4.6. Tumor Treatment Study in Mice
4.7. Histological Evaluation
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J. Control. Release 2012, 158, 286–292. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Yang, J.C.; Topalian, S.L.; Chang, A.E.; Schwartzentruber, D.J.; Aebersold, P.; Leitman, S.; Linehan, W.M.; Seipp, C.A. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst. 1993, 85, 622–632. [Google Scholar] [CrossRef]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef]
- Saito, M.; Takaku, F.; Hayashi, M.; Tanaka, I.; Abe, Y.; Nagai, Y.; Ishii, S. A role of valency of concanavalin A and its chemically modified derivatives in lymphocyte activation. Monovalent monomeric concanavalin A derivative can stimulate lymphocyte blastoid transformation. J. Biol. Chem. 1983, 258, 7499–7505. [Google Scholar] [CrossRef]
- Miyazaki, H.; Kikuchi, A.; Koyama, Y.; Okano, T.; Sakurai, Y.; Kataoka, K. Boronate-containing polymer as novel mitogen for lymphocytes. Biochem. Biophys. Res. Commun. 1993, 195, 829–836. [Google Scholar] [CrossRef]
- Miyazaki, H.; Kataoka, K.; Koyama, Y.; Okano, T.; Sakurai, Y. Water soluble polymer having phenylboronic acid moieties as synthetic mitogen. Jpn. J. Artif. Organs 1994, 23, 978–981. [Google Scholar] [CrossRef]
- Matsumoto, A.; Miyahara, Y. ‘Borono-lectin’ based engineering as a versatile platform for biomedical applications. Sci. Technol. Adv. Mater. 2018, 19, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Bourne, E.J.; Lees, E.M.; Weigel, H. Paper chromatography of carbohydrates and related compounds in the presence of benzeneboronic acid. J. Chromatogr. 1963, 11, 253–257. [Google Scholar] [CrossRef]
- Kitano, S.; Koyama, Y.; Kataoka, K.; Okano, T.; Sakurai, Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly (vinyl alcohol) and poly (N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J. Control. Release 1992, 19, 161–170. [Google Scholar] [CrossRef]
- Ito, A.; Aoki, H.; Akao, M.; Miura, N.; Otsuka, R.; Tsutsumi, S. Structure of borate groups in boron-containing apatite. J. Ceram. Soc. Jpn. 1988, 96, 707–709. [Google Scholar] [CrossRef]
- Nakagawa, D.; Nakamura, M.; Nagai, S.; Aizawa, M. Fabrications of boron-containing apatite ceramics via ultrasonic spray-pyrolysis route and their responses to immunocytes. J. Mater. Sci. Mater. Med. 2020, 31, 20. [Google Scholar] [CrossRef]
- Aizawa, M.; Itatani, K.; Howell, F.S.; Kinoshita, M.; Kishioka, A. Preparation and characterization of the composites in the CaO-P2O5-SiO2-B2O3 system by the sol-gel process. J. Ceram. Soc. Jpn. 1995, 103, 547–551. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardi, M.; Bianco, A.; Ravaglioli, A.; Montanaro, L. Sol-gel derived 45S5 bioglass: Synthesis, microstructural evolution and thermal behaviour. J. Mater. Sci. Mater. Med. 2012, 23, 1849–1866. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Kagami, S.; Kizukuri, R.; Nagai, S.; Aizawa, M. Responses of immune cells to hydroxyapatite ceramics loaded with immunostimulators. J. Soc. Inorg. Mater. Jpn. 2019, 26, 74–81. [Google Scholar]
- Kimm, M.A.; Tzoumas, S.; Glasl, S.; Omar, M.; Symvoulidis, P.; Olefir, I.; Rummeny, E.J.; Meier, R.; Ntziachristos, V. Longitudinal imaging of T cell-based immunotherapy with multi-spectral, multi-scale optoacoustic tomography. Sci. Rep. 2020, 10, 4903. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Fujii, S.; Tanaka, M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 2011, 34, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Aoki, H.; Akao, M.; Miura, N.; Otsuka, R.; Tsutsumi, S. Flux growth and crystal structure of boron-containing apatite. J. Ceram. Soc. Jpn. 1988, 96, 305–309. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Hu, F.; Huang, D.; Luo, Y.; Zhou, P.; Lv, C.; Wang, K.; Weng, Q.; Liu, X.; Guan, Y.; Geng, Y.; et al. Hematopoietic lineage-converted T cells carrying tumor-associated antigen-recognizing TCRs effectively kill tumor cells. J. Immunother. Cancer 2020, 8, e000498. [Google Scholar] [CrossRef]
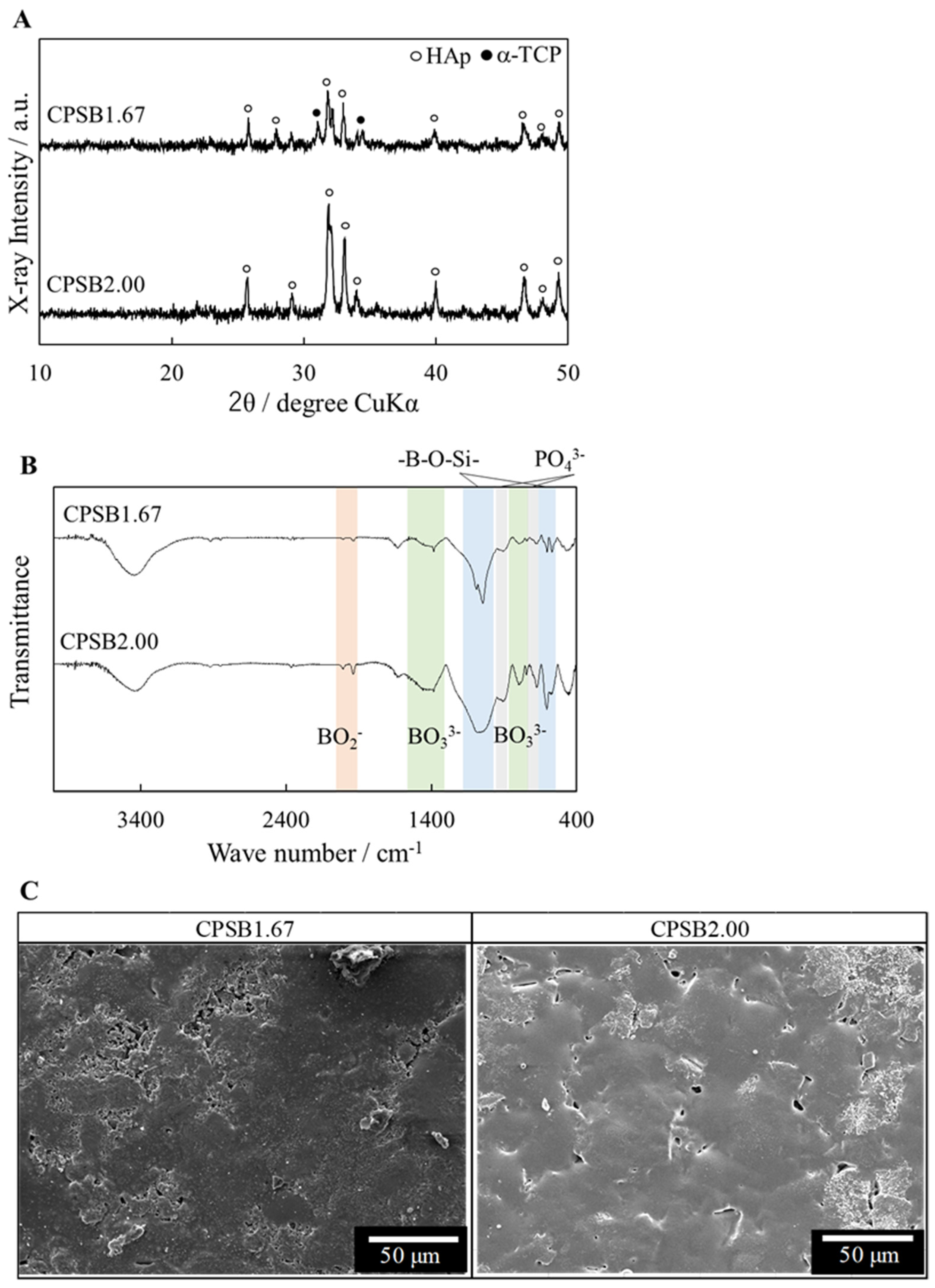
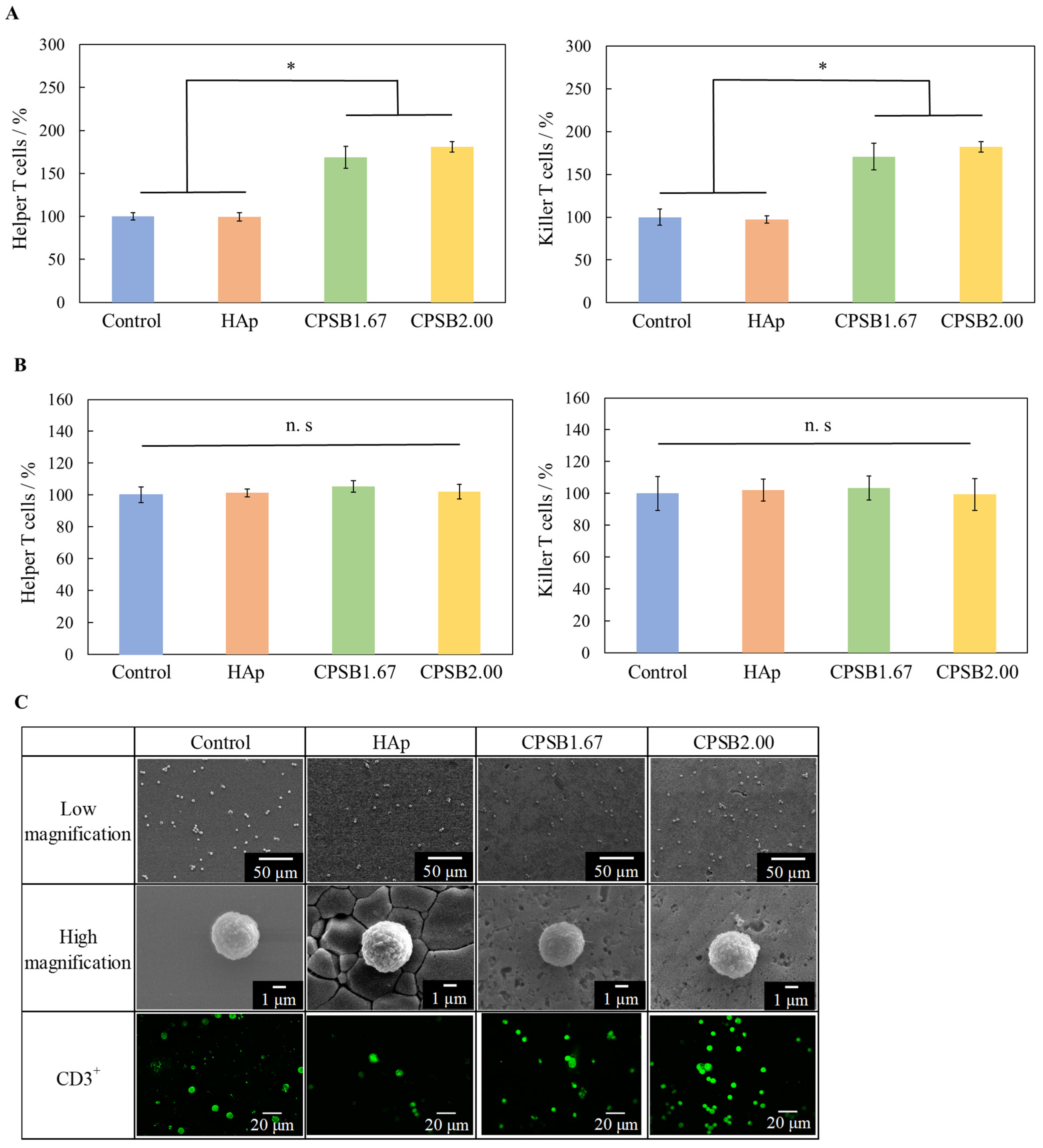
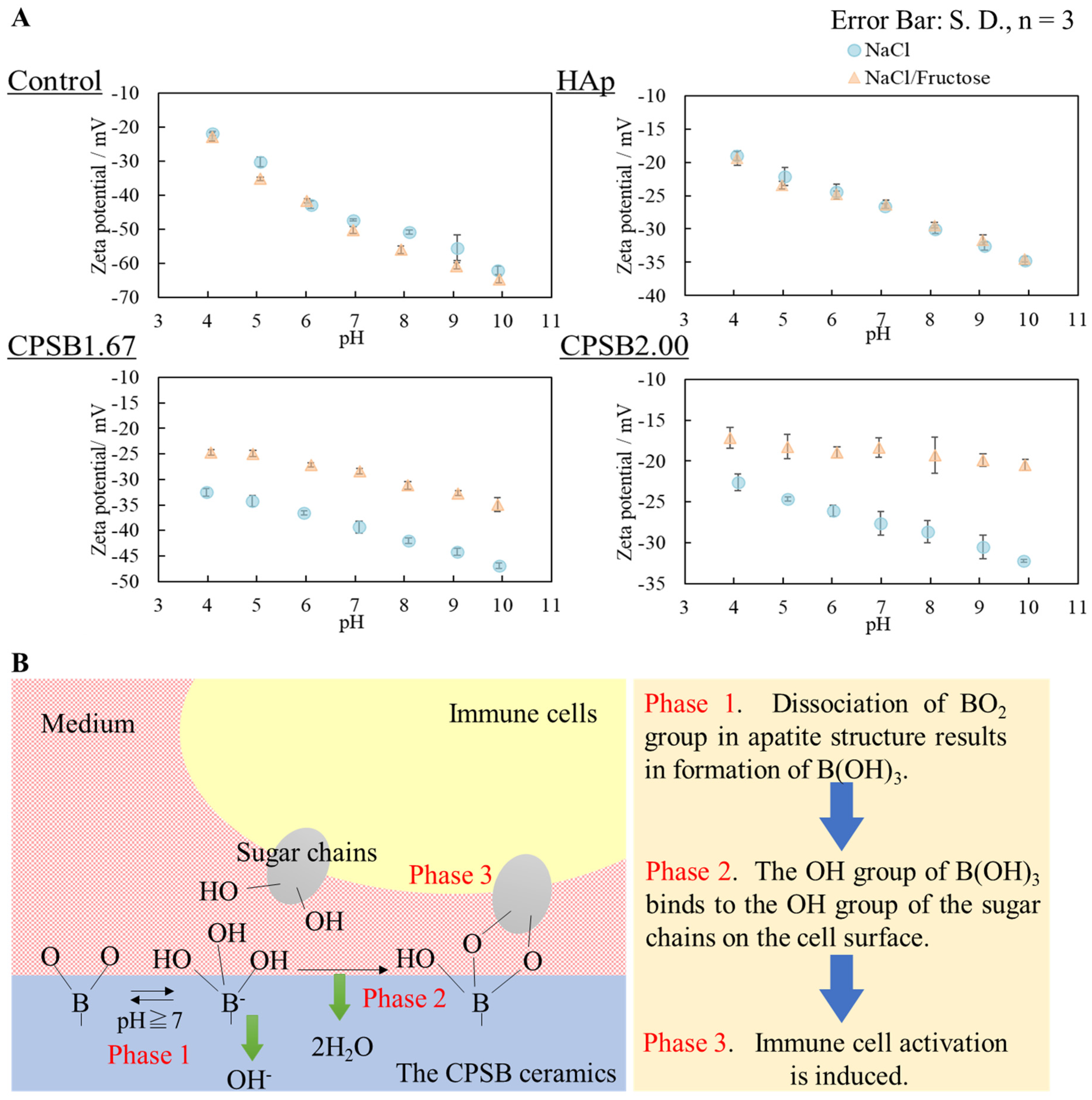
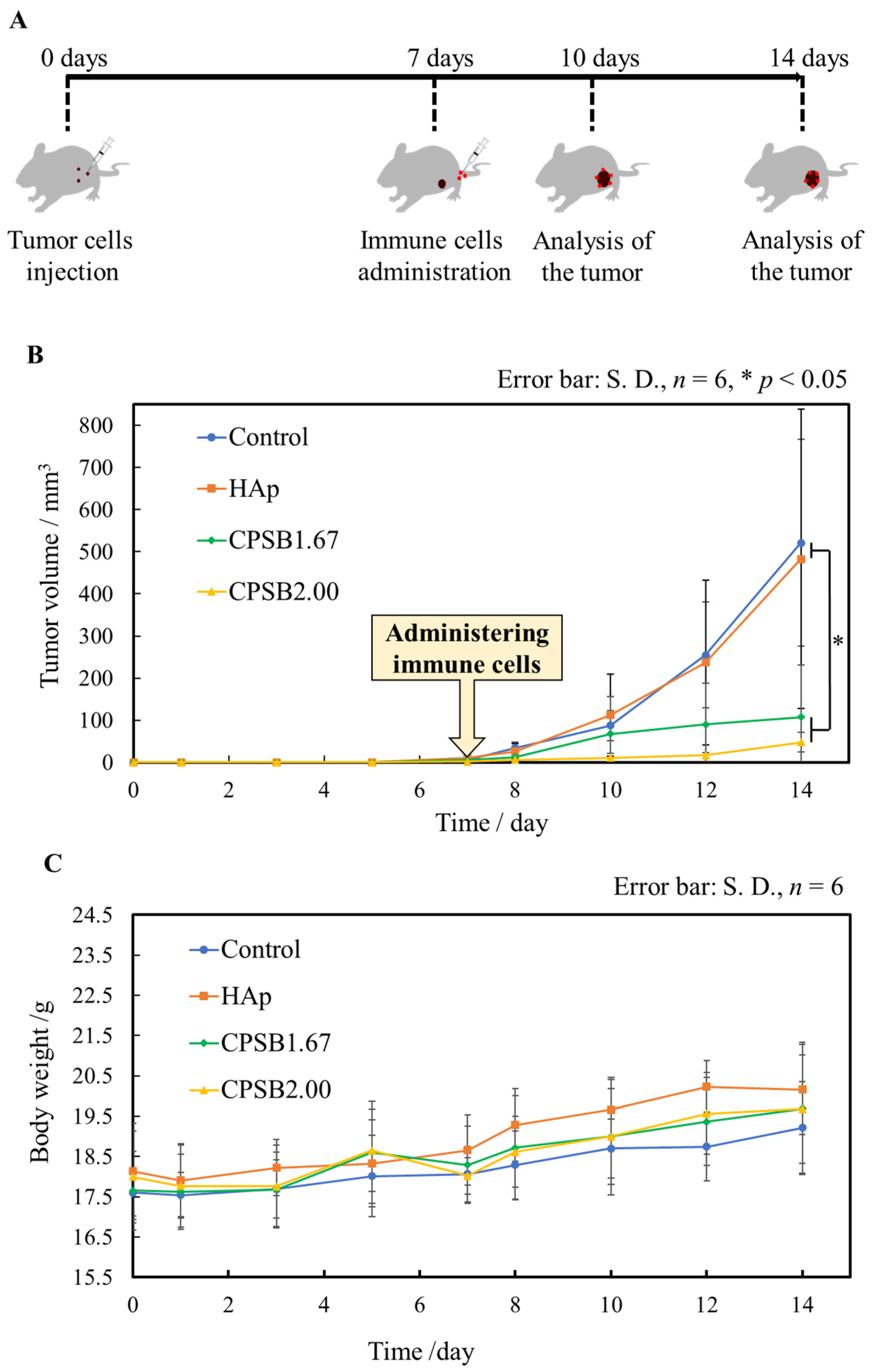

| Sample Name | Relative Density/% | Lattice Constant/nm | Surface Roughness/μm | |
|---|---|---|---|---|
| a(b) Axis | c Axis | |||
| HAp | 96.6 | 0.942 | 0.688 | 0.067 |
| CPSB1.67 | 90.3 | 0.943 | 0.693 | 0.064 |
| CPSB2.00 | 93.3 | 0.941 | 0.695 | 0.057 |
| Sample Name | CaO/mol% | P2O5/mol% | SiO2/mol% | B2O3/mol% | Ca/P Molar Ratio |
|---|---|---|---|---|---|
| CPSB1.67 | 30.78 | 9.22 | 50.00 | 10.00 | 1.67 |
| CPSB2.00 | 32.00 | 8.00 | 50.00 | 10.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nose, M.; Nitta, A.; Zheng, Y.; Kizukuri, R.; Nagao, Y.; Nagai, S.; Aizawa, M. Bioceramics Enhance the Anti-Tumor Activity of Immune Cells in Adoptive Immunotherapy. Int. J. Mol. Sci. 2024, 25, 10567. https://doi.org/10.3390/ijms251910567
Nose M, Nitta A, Zheng Y, Kizukuri R, Nagao Y, Nagai S, Aizawa M. Bioceramics Enhance the Anti-Tumor Activity of Immune Cells in Adoptive Immunotherapy. International Journal of Molecular Sciences. 2024; 25(19):10567. https://doi.org/10.3390/ijms251910567
Chicago/Turabian StyleNose, Masato, Aiko Nitta, Yundi Zheng, Rihoko Kizukuri, Yuki Nagao, Shigenori Nagai, and Mamoru Aizawa. 2024. "Bioceramics Enhance the Anti-Tumor Activity of Immune Cells in Adoptive Immunotherapy" International Journal of Molecular Sciences 25, no. 19: 10567. https://doi.org/10.3390/ijms251910567
APA StyleNose, M., Nitta, A., Zheng, Y., Kizukuri, R., Nagao, Y., Nagai, S., & Aizawa, M. (2024). Bioceramics Enhance the Anti-Tumor Activity of Immune Cells in Adoptive Immunotherapy. International Journal of Molecular Sciences, 25(19), 10567. https://doi.org/10.3390/ijms251910567






