3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Parasites
4.3. Growth Curves
4.4. Peritoneal Macrophage Extraction
4.5. In Vitro Assays
4.6. Prediction of ADMET Properties
4.7. Electron Microscopy
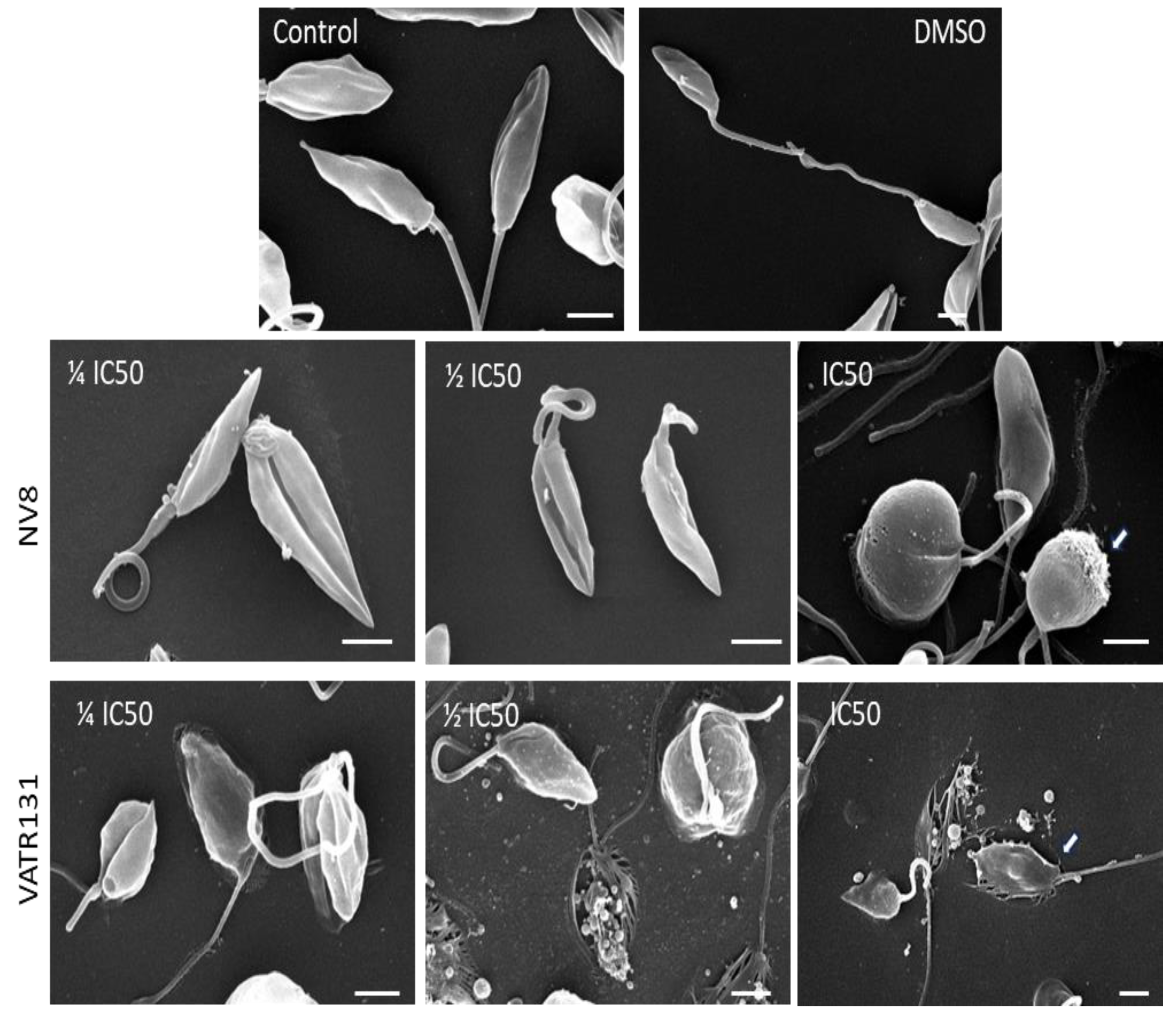
4.7.1. Scanning Electron Microscopy
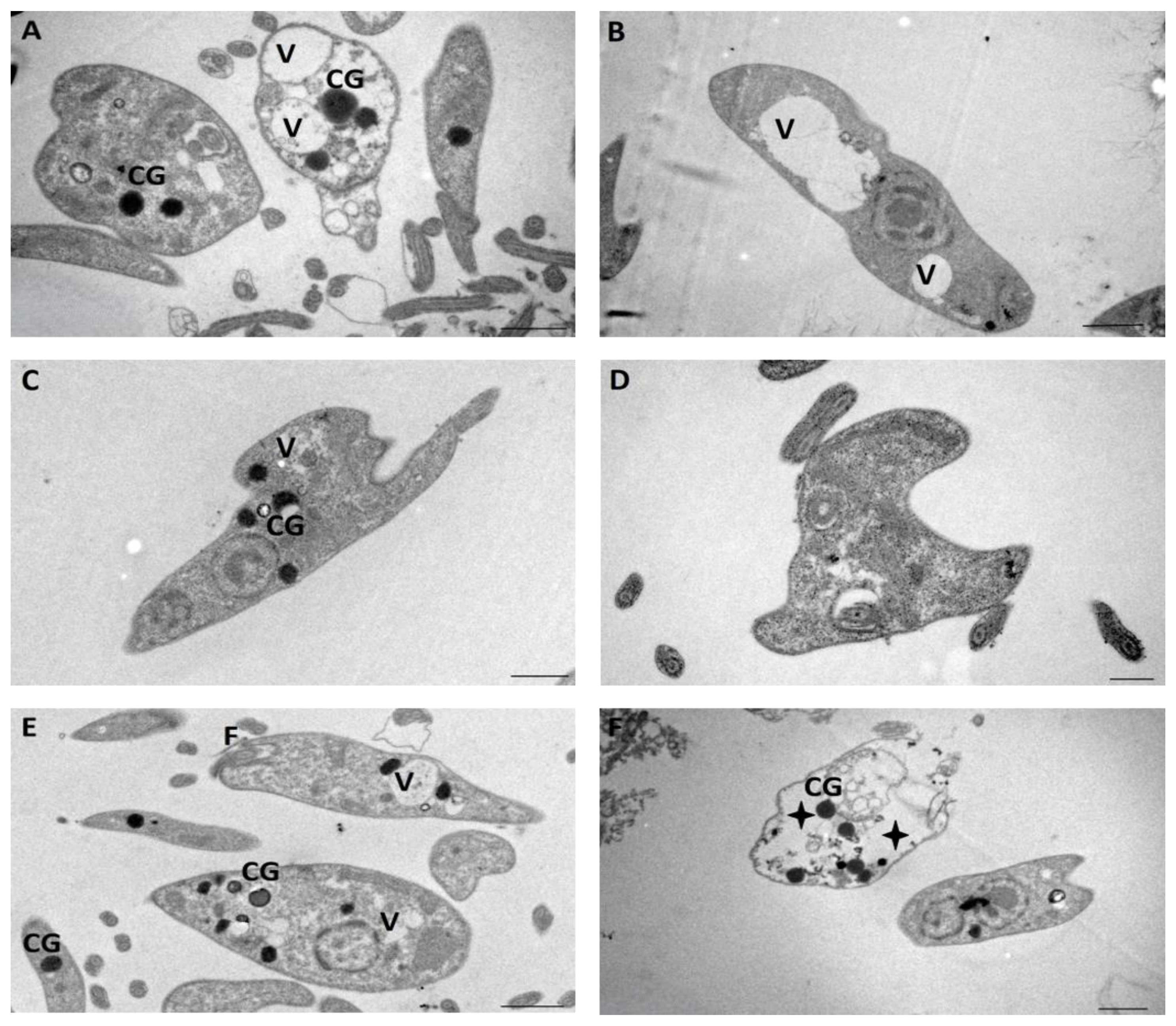
4.7.2. Transmission Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, F.C.; Ramos, H.; Antunes, I.F.; Curto, M.J.M.; Duarte, M.T.; Bento, I. Synthesis and structural characterization of 1-and 2-substituted indazoles: Ester and carboxylic acid derivatives. Molecules 2006, 11, 867–889. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 85, p. 67. Available online: https://shop.elsevier.com/books/advances-in-heterocyclic-chemistry/katritzky/978-0-12-020785-5 (accessed on 5 April 2024).
- Vidyacharan, S.; Murugan, A.; Sharada, D.S. C(sp2)-H Functionalization of 2H-indazoles at C3-position via palladium (II)-catalyzed isocyanide insertion strategy leading to diverse heterocycles. J. Org. Chem. 2016, 81, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.H.; Vidyacharan, S.; Sharada, D.S. BF3·OEt2 mediated metal-free one-pot sequential multiple annulation cascade (SMAC) synthesis of complex and diverse tetrahydroisoquinoline fused hybrid molecules. Org. Biomol. Chem. 2016, 14, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, S. Highly efficient one-pot three component synthesis of 2H-indazoles by consecutive condensation, C-N and N-N bond for-mations using Cu/Aminoclay/reduced grapheme oxide nanohybrid. J. Heterocycl. Chem. 2016, 54, 1863–1871. [Google Scholar] [CrossRef]
- Jayanthi, M.; Rajakumar, P. Synthesis, cell viability, and flow cytometric fluorescence pulse width analysis of dendrimers with indazoles surface unit. J. Heterocycl. Chem. 2017, 54, 3042–3050. [Google Scholar] [CrossRef]
- Popowycz, F.; Lavrard, H. Regioselective late-stage C-3 functionalization of pyrazolo[3,4-b]pyridines. Synthesis 2018, 50, 998–1006. [Google Scholar] [CrossRef]
- Bogonda, G.; Kim, H.Y.; Oh, K. Direct acyl radical addition to 2H-indazoles using ag-catalyzed decarboxylative cross-coupling of α-keto acids. Org. Lett. 2018, 20, 2711–2715. [Google Scholar] [CrossRef]
- Gao, M.; Xu, B. Transition metal-involving synthesis and utilization of N-containing heterocycles: Exploration of nitrogen sources. Chem. Rec. 2016, 16, 1701–1714. [Google Scholar] [CrossRef]
- Scott, L.J. Niraparib: First global approval. Drugs 2017, 77, 1029–1034. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Liang, C.-G.; Zhang, W.-H. Recent advances in indazole-containing derivatives: Synthesis and biological perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef]
- Boiani, L.; Gerpe, A.; Arán, V.J.; de Ortiz, S.T.; Serna, E.; de Bilbao, N.V.; Sanabria, L.; Yaluff, G.; Nakayama, H.; de Arias, A.R.; et al. In vitro and in vivo antitrypanosomatid activity of 5-nitroindazoles. Eur. J. Med. Chem. 2009, 44, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Arán, V.J.; Ochoa, C.; Boiani, L.; Buccino, P.; Cerecetto, H.; Gerpe, A.; González, M.; Montero, D.; Nogal, J.J.; Gómez-Barrio, A.; et al. Synthesis and biological properties of new 5-nitroindazole derivatives. Bioorg. Med. Chem. 2005, 13, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Gerpe, A.; Aguirre, G.; Kemmerling, U.; Piro, O.E.; Arán, V.J.; Maya, J.D.; Olea-Azar, C.; González, M.; Cerecetto, H. Study of 5-nitroindazoles’ anti-Trypanosoma cruzi mode of action: Electrochemical behaviour and ESR spectroscopic studies. Eur. J. Med. Chem. 2008, 44, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.C.; Rolón, M.; Montero-Torres, A.; Fonseca-Berzal, C.; Escario, J.A.; Gómez-Barrio, A.; Gálvez, J.; Marrero-Ponce, Y.; Arán, V.J. Synthesis, biological evaluation and chemometric analysis of indazole derivatives. 1,2-Disubstituted 5-nitroindazolinones, new prototypes of antichagasic drug. Eur. J. Med. Chem. 2012, 58, 214–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; Reviriego, F.; Cumella, J.; Morales, P.; Jagerovic, N.; Nogal-Ruiz, J.J.; Escario, J.A.; da Silva, P.B.; Soeiro, M.d.N.C.; et al. Antichagasic and trichomonacidal activity of 1-substituted 2-benzyl-5-nitroindazolin-3-ones and 3-alkoxy-2-benzyl-5-nitro-2H-indazoles. Eur. J. Med. Chem. 2016, 115, 295–310. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; Vela, N.; Cumella, J.; Nogal-Ruiz, J.J.; Escario, J.A.; da Silva, P.B.; Batista, M.M.; Soeiro, M.d.N.C.; Sifontes-Rodríguez, S.; et al. Antichagasic, Leismanicidal, and Trichomonacidal Activity of 2-Benzyl-5-nitroindazole-Derived Amines. ChemMedChem 2018, 13, 1246–1259. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; de Castro, S.; Escario, J.A.; Gómez-Barrio, A.; Arán, V.J. 5-Nitroindazole-based compounds: Further studies for activity optimization as anti-Trypanosoma cruzi agents. Acta Trop. 2022, 234, 106607. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A. Trichomonas Vaginalis: Corroboración Experimental de Modelos Virtuales de Cribado Farmacológico y Caracterización Biomolecular de Aislados. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2016. Available online: https://dialnet.unirioja.es/servlet/cittes?codigo=128489 (accessed on 3 June 2023).
- Ibáñez-Escribano, A.; Reviriego, F.; Vela, N.; Fonseca-Berzal, C.; Nogal-Ruiz, J.J.; Arán, V.J.; Escario, J.A.; Gómez-Barrio, A. Promising hit compounds against resistant trichomoniasis: Synthesis and antiparasitic activity of 3-(ω-aminoalkoxy)-1-benzyl-5-nitroindazoles. Bioorg. Med. Chem. Lett. 2021, 37, 127843. [Google Scholar] [CrossRef]
- Mollineda-Diogo, N.; de Oca, C.S.C.-M.; Sifontes-Rodríguez, S.; Espinosa-Buitrago, T.; Monzote-Fidalgo, L.; Meneses-Marcel, A.; Morales-Helguera, A.; Perez-Castillo, Y.; Arán-Redó, V. Antileishmanial activity of 5-nitroindazole derivatives. Ther. Adv. Infect. Dis. 2023, 10, 20499361231208294. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Marín, C.; Ramírez-Macías, I.; Rosales, M.J.; Muro, B.; Reviriego, F.; Navarro, P.; Arán, V.J.; Sánchez-Moreno, M. In vitro leishmanicidal activity of 1,3-disubstituted 5-nitroindazoles. Acta Trop. 2015, 148, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.A.; de Almeida, L.; Passalacqua, T.G.; Reis, J.S.; Torres, F.A.E.; Martinez, I.; Peccinini, R.G.; Chin, C.M.; Chegaev, K.; Guglielmo, S.; et al. Leishmanicidal activities of novel synthetic furoxan and benzofuroxan derivatives. Antimicrob. Agents Chemother. 2014, 58, 4837–4847. [Google Scholar] [CrossRef] [PubMed]
- Scariot, D.B.; Britta, E.A.; Moreira, A.L.; Falzirolli, H.; Silva, C.C.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Nakamura, C.V. Induction of Early Autophagic Process on Leishmania amazonensis by Synergistic Effect of Miltefosine and Innovative Semi-synthetic Thiosemicarbazone. Front. Microbiol. 2017, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, E.S.; Antinarelli, L.M.; Silva, N.P.; Souza, I.O.; Meinel, R.S.; Rocha, M.N.; Soares, R.P.; da Silva, A.D. Quinoline derivatives: Synthesis, leishmanicidal activity and involvement of mitochondrial oxidative stress as mechanism of action. Chem. Interact. 2016, 260, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Antinarelli, L.M.R.; Souza, I.d.O.; Glanzmann, N.; Almeida, A.d.C.; Porcino, G.N.; Vasconcelos, E.G.; da Silva, A.D.; Coimbra, E.S. Aminoquinoline compounds: Effect of 7-chloro-4-quinolinylhydrazone derivatives against Leishmania amazonensis. Exp. Parasitol. 2016, 171, 10–16. [Google Scholar] [CrossRef]
- Silva, S.T.D.M.; Visbal, G.; Godinho, J.L.P.; Urbina, J.A.; De Souza, W.; Rodrigues, J.C.F. In vitro antileishmanial activity of ravuconazole, a triazole antifungal drug, as a potential treatment for leishmaniasis. J. Antimicrob. Chemother. 2018, 73, 2360–2373. [Google Scholar] [CrossRef]
- Oliveira, V.G.; Faiões, V.d.S.; Goncalves, G.B.R.; Lima, M.F.O.; Boechat, F.C.S.; Cunha, A.C.; de Andrade-Neto, V.V.; Silva, F.d.C.d.; Torres-Santos, E.C.; de Souza, M.C.B.V. Design, Synthesis and Antileishmanial Activity of Naphthotriazolyl-4-Oxoquinolines. Curr. Top. Med. Chem. 2018, 18, 1454–1464. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Mesa, C.V.; Muñoz, D.L.; Echeverry, M.; Vélez, I.D.; Robledo, S.M. Susceptibilidad in vitro a infección por Leishmania y sensibilidad a medicamentos difiere según tipo de macrófagos. Salud UIS 2010, 42, 200–211. [Google Scholar]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef]
- Vannier-Santos, M.; De Castro, S. Electron microscopy in antiparasitic chemotherapy: A (close) view to a kill. Curr. Drug Targets 2009, 10, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Sifontes-Rodríguez, S.; Mollineda-Diogo, N.; Monzote-Fidalgo, L.; Escalona-Montaño, A.R.; García-Trevijano, J.A.E.; Aguirre-García, M.M.; Meneses-Marcel, A. In Vitro and In Vivo Antileishmanial Activity of Thioridazine. Acta Parasitol. 2023, 69, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Rolón, M.; Vega, C.; Escario, J.A.; Gómez-Barrio, A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2006, 99, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bodley, A.L.; McGarry, M.W.; Shapiro, T.A. Drug cytotoxicity assay for African Trypanosomes and Leishmania Species. J. Infect. Dis. 1995, 172, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ketkar, R.; Tao, P. ADMETboost: A web server for accurate ADMET prediction. J. Mol. Model. 2022, 28, 408. [Google Scholar] [CrossRef]
- Sander, T. OSIRIS Property Explorer. Org. Chem. Portal 2001, 49, 232–246. [Google Scholar] [CrossRef]
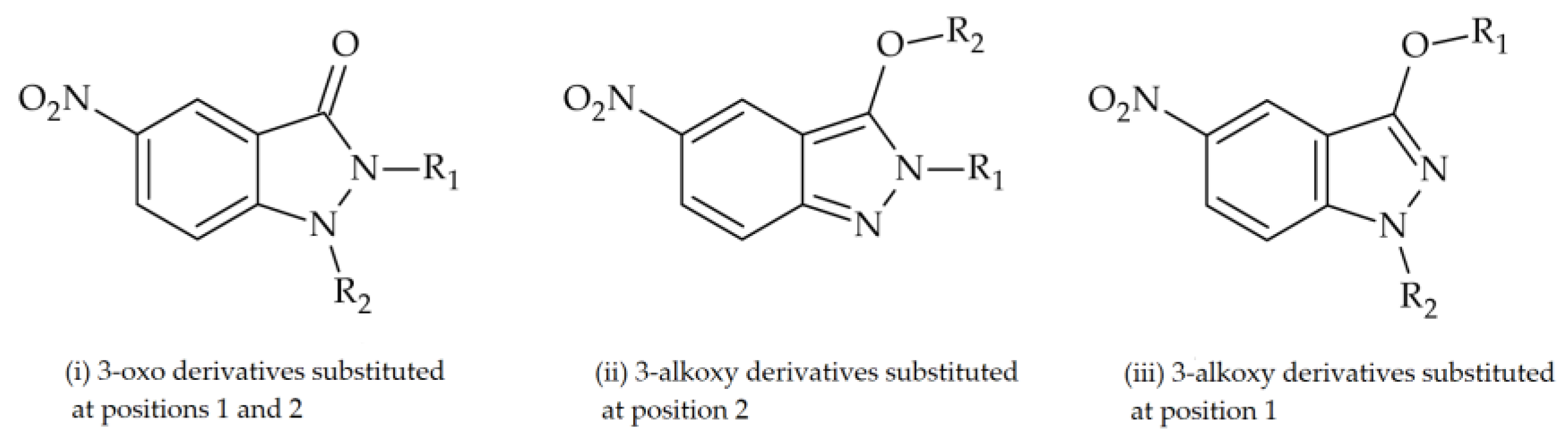
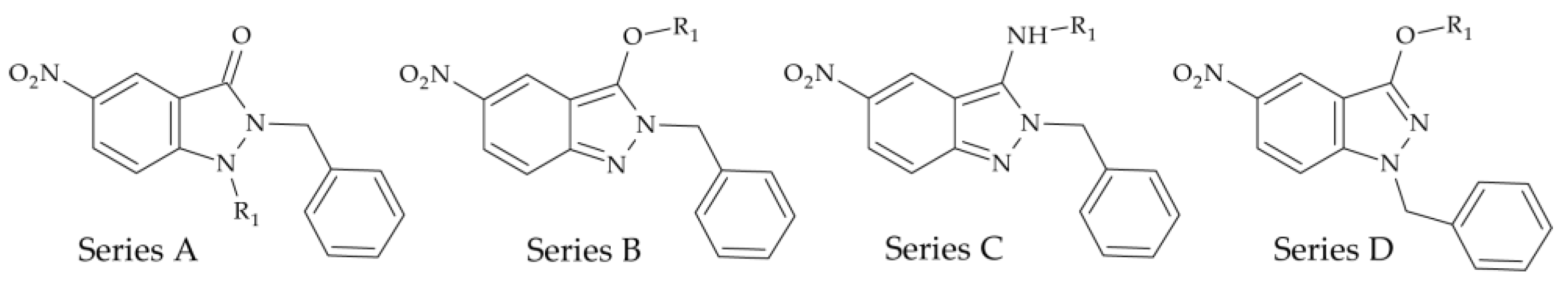


| Base Structure | Product | Substituent R1 | CC50 ± SD (µM) |
|---|---|---|---|
 | NV4 | (CH2)2-N(CH3)2 | 67.1 ± 8.9 |
| NV6 | (CH2)3-N(CH3)2 | 61 ± 6.9 | |
| NV7 | (CH2)2-Piperidine | 24.4 ± 6.7 | |
| NV8 | (CH2)3-Piperidine | 23.2 ± 5.0 | |
| NV9 | (CH2)3-NH2 | 47.9 ± 10.4 | |
| NV10 | (CH2)2-NH2 | 46.7 ± 7.7 | |
| NV11 | (CH2)2-Br | 4.5 ± 1.0 | |
| NV12 | (CH2)5-Br | 4.1 ± 0.6 | |
| NV13 | (CH2)2-NHCH3 | 61.4 ± 9.4 | |
| NV14 | (CH2)5-N(CH3)2 | 2.5 ± 0.7 | |
| NV15 | (CH2)5-NHCH3 | 5.6 ± 2.5 | |
| NV16 | (CH2)5-Piperidine | 21.1 ± 7.1 | |
| NV17 | (CH2)5-NH2 | 7.74 ± 3.0 | |
| AmB | 5.8 ± 0.5 |
| PROMASTIGOTES | ||||||
| Species | L. amazonensis | L. infantum | L. mexicana | |||
| Products | IC50 ± SD (µM) | SIp | IC50 ± SD (µM) | SIp | IC50 ± SD (µM) | SIp |
| NV4 | 0.4 ± 0.1 | 127 | 0.52 ± 0.05 | 127 | 2.3 ± 0.4 | 32 |
| NV6 | 0.43 ± 0.007 | 61 | 0.69 ± 0.063 | 45 | 0.28 ± 0.04 | 121 |
| NV7 | 0.04 ± 0.01 | 653 | 0.33 ± 0.021 | 74 | 0.2 ± 0.08 | 14 |
| NV8 | 0.007 ± 0.001 | 3467 | 0.22 ± 0.003 | 104 | 0.11 ± 0.04 | 208 |
| NV9 | 0.81 ± 0.17 | 58 | 0.59 ± 0.16 | 87 | 2.8 ± 0003 | 17 |
| NV10 | 0.99 ± 0.14 | 54 | 0.93 ± 0.27 | 51 | 4.5 ± 0.5 | 12 |
| NV11 | 20.7 ± 1.38 | 2 | 20.4 ± 1.3 | 0.2 | 9.38 ± 0.13 | 0.5 |
| NV12 | 2.88 ± 1.47 | 2 | 32.96 ± 0.25 | 0.1 | 2.8 ± 1.1 | 1 |
| NV13 | 0.82 ± 0.27 | 74 | 0.29 ± 0.1 | 223 | 0.3 ± 0.04 | 223 |
| NV14 | 0.5 ± 0.22 | 6 | 0.93 ± 0.18 | 3 | 0.05 ± 0.01 | 55 |
| NV15 | 1.88 ± 1.34 | 4 | 1.27 ± 0.06 | 4.4 | 0.1 ± 0.01 | 80 |
| NV16 | 0.3 ± 0.22 | 49 | 1.41 ± 0.03 | 15 | 0.5 ± 0.1 | 51 |
| NV17 | 1.01 ± 0.39 | 8 | 0.20 ± 0.003 | 38 | 2.9 ± 0.1 | 3 |
| AmB | 0.03 ± 0.01 | 193 | 0.28 ± 0.01 | 21 | 0.046 ± 0.012 | 126 |
| AMASTIGOTES | ||||||
| Species | L. amazonensis | L. infantum | L. mexicana | |||
| Products | EC50 ± SD (µM) | SIa | EC50 ± SD (µM) | SIa | EC50 ± SD (µM) | SIa |
| NV4 | 12.3 ± 0.7 | 5 | 3.42 ± 1.8 | 20 | NE | |
| NV6 | 0.43 ± 0.049 | 71 | 3.79 ± 1.46 | 16 | 2.2 ± 0.05 | 14 |
| NV7 | 5.61 ± 0.69 | 5 | 12.8 ± 0.13 | 2 | 3.1 ± 1.05 | 8 |
| NV8 | 1.00 ± 0.48 | 23 | 1.26 ± 0.5 | 18 | 1.0 ± 0.1 | 23 |
| NV9 | 1.13 ± 0.98 | 42 | 9.71 ± 0.4 | 5 | NE | |
| NV10 | 4.35 ± 0.9 | 11 | 2.4 ± 0.34 | 194 | NE | |
| NV11 | NE | NE | NE | |||
| NV12 | NE | NE | NE | |||
| NV13 | 5.66 ± 0.53 | 11 | 16.2± 0.54 | 4 | 11.6 ± 0.7 | 5 |
| NV14 | NE | NE | 8.2 ± 0.2 | 0.3 | ||
| NV15 | NE | NE | 1.53 ± 0.13 | 4 | ||
| NV16 | 0.17 ± 0.042 | 129 | NE | 4.29 ± 0.48 | 5 | |
| NV17 | NE | 11.8 ± 1.04 | 1 | NE | ||
| AmB | 0.034 ± 0.006 | 170 | 0.6 ± 0.21 | 10 | 0.036 ± 0.008 | 725 |
| Molecule Property | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| Molecular Weight | kg/mol | 340.15 | 354.17 | 394.2 | 326.14 | 312.12 | 422.23 | 901.56 |
| Number of Heteroatoms | 7 | 7 | 7 | 7 | 7 | 7 | 14 | |
| Number of Rotatable Bonds | 7 | 8 | 8 | 7 | 6 | 10 | 3 | |
| Number of Rings | 3 | 3 | 4 | 3 | 3 | 4 | 3 | |
| Number of HA | 6 | 6 | 6 | 6 | 6 | 6 | 14 | |
| Number of HD | 0 | 0 | 0 | 1 | 1 | 0 | 9 | |
| log KOW | log-ratio | 2.93 | 3.32 | 4.25 | 2.72 | 2.33 | 5.03 | 4.51 |
| Absorption | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| Caco-2 Permeability | log(cm/s) | −5.05 | −5.04 | −5.09 | −5.35 | −5.36 | −5.2 | −5.45 |
| HIA | % | 75.42 | 73.4 | 75.73 | 75.39 | 76.06 | 75.7 | 66.16 |
| Pgp Inhibition | % | 46.26 | 50.9 | 52.58 | 48.08 | 46.75 | 51.01 | 41.51 |
| log D7.4 | log-ratio | 1.99 | 2 | 1.92 | 1.68 | 1.74 | 1.91 | 1.65 |
| Aqueous Solubility | log(mol/L) | −4.25 | −4.4 | −4.41 | −4.1 | −4.03 | −4.41 | −4.16 |
| Oral Bioavailability | % | 50.3 | 48.42 | 41.73 | 49.73 | 50.45 | 35.75 | 38.02 |
| Distribution | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| BBB | % | 39.3 | 40.39 | 39.87 | 38.68 | 38.44 | 38.8 | 26.41 |
| PPBR | % | 49.51 | 50.1 | 50.93 | 49.34 | 48.56 | 50.92 | 41.18 |
| VDss | L/kg | 3.64 | 3.64 | 4.15 | 3.33 | 3.36 | 4.14 | 3.85 |
| Metabolism | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| CYP2C9 Inhibition | % | 78.25 | 80 | 67.88 | 78.18 | 70.38 | 74.12 | 50.16 |
| CYP2D6 Inhibition | % | 95.53 | 97.23 | 97.64 | 89.45 | 91.47 | 96.98 | 96.33 |
| CYP3A4 Inhibition | % | 31.87 | 33.97 | 29.14 | 33.95 | 32.39 | 31.28 | 30.95 |
| CYP2C9 Substrate | % | 31.29 | 32.76 | 30.06 | 29.4 | 29.32 | 30.68 | 31.7 |
| CYP2D6 Substrate | % | 59.39 | 64.41 | 53.41 | 60.29 | 62.1 | 52.28 | 57.32 |
| CYP3A4 Substrate | % | 38.43 | 39.3 | 36.13 | 35.46 | 35.79 | 36.86 | 33.09 |
| Excretion | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| Half Life | h | 78.88 | 92.59 | 79.39 | 80.43 | 81.42 | 79.75 | 118.71 |
| CL-Hepa | uL∙min−1(106 cells)−1 | 44.17 | 48.83 | 45.61 | 57.02 | 52.74 | 44.73 | 41.66 |
| CL-Micro | mL∙min−1 g−1 | 42.42 | 43.52 | 39.64 | 43.65 | 41.2 | 41.8 | 46.05 |
| Toxicity | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| hERG Blockers | % | 42.2 | 44.89 | 46.81 | 43.1 | 42.02 | 47.91 | 42.12 |
| DILI | % | 52.83 | 51.13 | 47.9 | 53.34 | 53.44 | 49.41 | 52.26 |
| LD50 | −log(mol/kg) | 2.35 | 2.28 | 2.21 | 2.24 | 2.37 | 2.11 | 2.75 |
| OSIRIS Property Explorer | Unit | NV4 | NV6 | NV8 | NV9 | NV10 | NV16 | AmB |
| Mutagenic | No | No | No | No | No | No | No | |
| Tumorigenic | No | No | No | No | No | No | No | |
| Irritant | No | No | No | No | No | No | No | |
| Reproductive effects | No | No | No | No | No | No | No | |
| Drug score | 0.68 | 0.56 | 0.37 | 0.42 | 0.43 | 0.31 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollineda-Diogo, N.; Sifontes-Rodríguez, S.; Aguirre-García, M.M.; Escalona-Montaño, A.R.; Espinosa-Buitrago, T.; Mondragón-Flores, R.; Mondragón-Castelán, M.E.; Meneses-Marcel, A.; Pérez-Olvera, O.; Sánchez-Almaraz, D.A.; et al. 3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds. Int. J. Mol. Sci. 2024, 25, 10582. https://doi.org/10.3390/ijms251910582
Mollineda-Diogo N, Sifontes-Rodríguez S, Aguirre-García MM, Escalona-Montaño AR, Espinosa-Buitrago T, Mondragón-Flores R, Mondragón-Castelán ME, Meneses-Marcel A, Pérez-Olvera O, Sánchez-Almaraz DA, et al. 3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds. International Journal of Molecular Sciences. 2024; 25(19):10582. https://doi.org/10.3390/ijms251910582
Chicago/Turabian StyleMollineda-Diogo, Niurka, Sergio Sifontes-Rodríguez, María Magdalena Aguirre-García, Alma Reyna Escalona-Montaño, Teresa Espinosa-Buitrago, Ricardo Mondragón-Flores, Mónica Edith Mondragón-Castelán, Alfredo Meneses-Marcel, Ofelia Pérez-Olvera, Daniel Andrés Sánchez-Almaraz, and et al. 2024. "3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds" International Journal of Molecular Sciences 25, no. 19: 10582. https://doi.org/10.3390/ijms251910582
APA StyleMollineda-Diogo, N., Sifontes-Rodríguez, S., Aguirre-García, M. M., Escalona-Montaño, A. R., Espinosa-Buitrago, T., Mondragón-Flores, R., Mondragón-Castelán, M. E., Meneses-Marcel, A., Pérez-Olvera, O., Sánchez-Almaraz, D. A., Perez-Castillo, Y., & Arán-Redó, V. (2024). 3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds. International Journal of Molecular Sciences, 25(19), 10582. https://doi.org/10.3390/ijms251910582






