Improving the Prognostic and Predictive Value of Circulating Tumor Cell Enumeration: Is Longitudinal Monitoring the Answer?
Abstract
1. Introduction
2. The Current Status of CTC Research
2.1. Old and New Blood-Based Cancer Biomarkers for Screening and Progression
2.2. Biology and the Role of CTCs in the Metastatic Process
2.3. Molecular Characteristics of CTCs
2.4. Clinical Significance of CTC Enumeration and Profiling
2.5. The Shortcomings of a Single, Non-Recurrent CTC Analysis
2.5.1. The Methodology of CTC Detection Requires Standardization
2.5.2. Fluctuations in CTC Numbers Depend on Circadian Rhythm, Clinico-Pathological Features, and Therapeutic Interventions
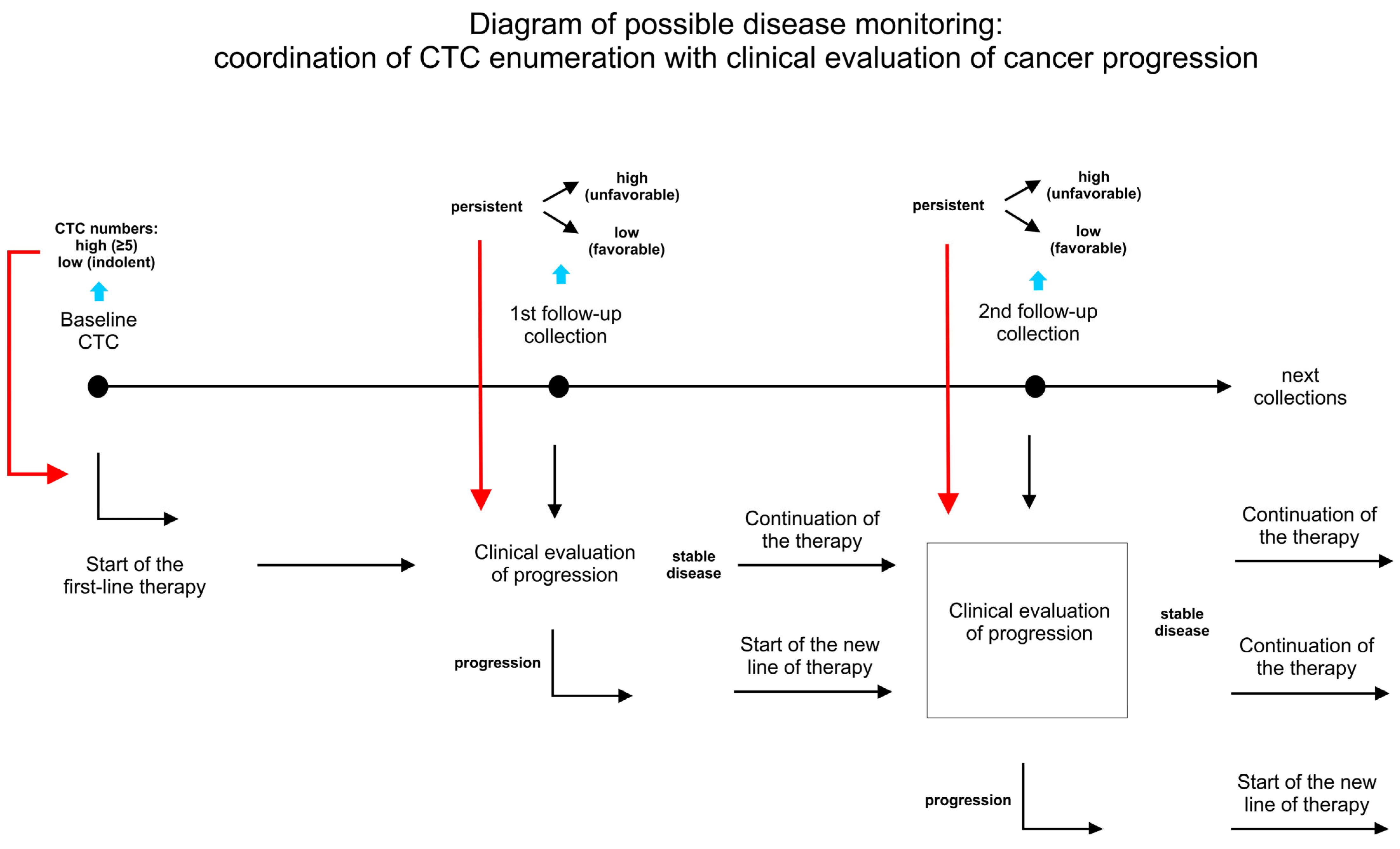
3. Principles and Advantages of Longitudinal Analysis
3.1. The Serial Monitoring of CTC Dynamics in a Single Patient Is Prognostically Superior to Single or Baseline/Follow-Up Blood Collection
3.2. Clinical Relevance of Long Monitoring in Relation to Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target Ther. 2024, 9, 132. [Google Scholar] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Uygur, M.M.; Gumus, M. The utility of serum tumor markers CEA and CA 15-3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat. Res. Commun. 2021, 28, 100402. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Matsuda, T. Limited usefulness of serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for gastrointestinal and whole-body cancer screening. Sci. Rep. 2020, 10, 18202. [Google Scholar] [CrossRef]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and limitation as a biomarker for breast cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Varzaru, V.B.; Eftenoiu, A.E.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Popescu, R.; Cobec, I.M. The Influence of Tumor-Specific Markers in Breast Cancer on Other Blood Parameters. Life 2024, 14, 458. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Crucial roles of circulating tumor cells in the metastatic cascade and tumor immune escape: Biology and clinical translation. J. Immunother. Cancer 2022, 10, e005615. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Atwal, S.K.; Spoerke, J.M.; Savage, H.; Pandita, A.; Yeh, R.F.; Pirzkall, A.; Fine, B.M.; Almer, L.C.; Chen, D.S.; et al. Molecular biomarker analyses using circulating tumor cells. PLoS ONE 2010, 5, e12517. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Haley, B.; Perkins, S.; Beithsch, P.; Khan, A.; Euhus, D.; Osborne, C.; et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad. Sci. USA 2004, 101, 9393–9398. [Google Scholar] [CrossRef]
- Li, J.; King, M.R. Adhesion receptors as therapeutic targets for circulating tumor cells. Front. Oncol. 2012, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, D.; Nteliopoulos, G.; Hastings, R.K.; Rushton, A.; Page, K.; Allsopp, R.C.; Ambasager, B.; Gleason, K.; Guttery, D.S.; Ali, S.; et al. Shallow WGS of individual CTCs identifies actionable targets for informing treatment decisions in metastatic breast cancer. Br. J. Cancer 2022, 127, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Strati, A.; Kallergi, G.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci. Rep. 2021, 11, 2313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guldner, I.H.; Golomb, S.M.; Sun, L.; Harris, J.A.; Lu, X.; Zhang, S. Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer. Nat. Commun. 2019, 10, 3817. [Google Scholar] [CrossRef]
- Jordan, N.V.; Bardia, A.; Wittner, B.S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T.K.; Licausi, J.A.; Desai, R.; et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016, 537, 102–106. [Google Scholar] [CrossRef]
- Franken, A.; Honisch, E.; Reinhardt, F.; Meier-Stiegen, F.; Yang, L.; Jaschinski, S.; Eposito, I.; Alberter, B.; Polzer, B.; Huebner, H.; et al. Detection of ESR1 Mutations in Single Circulating Tumor Cells on Estrogen Deprivation Therapy but Not in Primary Tumors from Metastatic Luminal Breast Cancer Patients. J. Mol. Diagn. 2020, 22, 111–121. [Google Scholar] [CrossRef]
- Hong, S.P.; Chan, T.E.; Lombardo, Y.; Corleone, G.; Rotmensz, N.; Bravaccini, S.; Rocca, A.; Pruneri, G.; McEwen, K.R.; Coombes, R.C.; et al. Single-cell transcriptomics reveals multi-step adaptations to endocrine therapy. Nat. Commun. 2019, 10, 3840. [Google Scholar] [CrossRef]
- He, W.; Hou, M.; Zhang, H.; Zeng, C.; He, S.; Chen, X.; Xu, M.; Sun, C.; Jiang, W.; Wang, H.; et al. Clinical significance of circulating tumor cells in predicting disease progression and chemotherapy resistance in patients with gestational choriocarcinoma. Int. J. Cancer 2019, 144, 1421–1431. [Google Scholar] [CrossRef]
- Rossi, T.; Gallerani, G.; Martinelli, G.; Maltoni, R.; Fabbri, F. Circulating Tumor Cells as a Tool to Untangle the Breast Cancer Heterogeneity Issue. Biomedicines 2021, 9, 1242. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Giret, T.M.; Cote, R.J. Circulating Tumor Cells from Enumeration to Analysis: Current Challenges and Future Opportunities. Cancers 2021, 13, 2723. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef]
- Ashworth, T.R. Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Med. J. Aust. 1869, 14, 146–147. [Google Scholar]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Cheung, K.J.; Padmanaban, V.; Silvestri, V.; Schipper, K.; Cohen, J.D.; Fairchild, A.N.; Gorin, M.A.; Verdone, J.E.; Pienta, K.J.; Bader, J.S.; et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. USA 2016, 113, E854–E863. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Wei, R.R.; Sun, D.N.; Yang, H.; Yan, J.; Zhang, X.; Zheng, X.L.; Fu, X.H.; Geng, M.Y.; Huang, X.; Ding, J. CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacol. Sin. 2018, 39, 1326–1337. [Google Scholar] [CrossRef]
- Larsson, A.M.; Jansson, S.; Bendahl, P.O.; Levin Tykjaer Jorgensen, C.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Ryden, L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Weber, T.S.; Serrano, A.; Vaillant, F.; Liu, K.; Pal, B.; Di Stefano, L.; Schreuder, J.; Lin, D.; Chen, Y.; et al. Barcoding reveals complex clonal behavior in patient-derived xenografts of metastatic triple negative breast cancer. Nat. Commun. 2019, 10, 766. [Google Scholar] [CrossRef]
- Mego, M.; Karaba, M.; Sedlackova, T.; Benca, J.; Repiska, G.; Krasnicanova, L.; Macuch, J.; Sieberova, G.; Jurisova, S.; Pindak, D.; et al. Circulating tumor cells and breast cancer-specific mutations in primary breast cancer. Mol. Clin. Oncol. 2020, 12, 565–573. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Han, L.; Tuo, X.; Ma, S.; Wang, Y.; Feng, Y.; Liang, D.; Sun, C.; Wang, Q.; et al. The Discordance of Gene Mutations between Circulating Tumor Cells and Primary/Metastatic Tumor. Mol. Ther. Oncolytics 2019, 15, 21–29. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Lovero, D.; Palmirotta, R.; Stucci, L.S.; Tucci, M.; Felici, C.; Cascardi, E.; Giardina, C.; Cafforio, P.; Silvestris, F. Dissection of major cancer gene variants in subsets of circulating tumor cells in advanced breast cancer. Sci. Rep. 2019, 9, 17276. [Google Scholar] [CrossRef]
- Genna, A.; Vanwynsberghe, A.M.; Villard, A.V.; Pottier, C.; Ancel, J.; Polette, M.; Gilles, C. EMT-Associated Heterogeneity in Circulating Tumor Cells: Sticky Friends on the Road to Metastasis. Cancers 2020, 12, 1632. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, W.; Yang, R.; Zhang, X.; Ye, Y.; Yu, J.; Ye, L.; Tang, W. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci. Rep. 2019, 9, 7084. [Google Scholar] [CrossRef]
- Vardas, V.; Politaki, E.; Pantazaka, E.; Georgoulias, V.; Kallergi, G. Epithelial-to-mesenchymal transition of tumor cells: Cancer progression and metastasis. Int. J. Dev. Biol. 2022, 66, 277–283. [Google Scholar] [CrossRef]
- Gires, O.; Stoecklein, N.H. Dynamic EpCAM expression on circulating and disseminating tumor cells: Causes and consequences. Cell Mol. Life Sci. 2014, 71, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, C.; Corra, F.; Minotti, L.; Baldassari, F.; Crudele, F.; Cook, W.J.J.; Di Leva, G.; d’Adamo, A.P.; Gasparini, P.; Volinia, S. Heterogeneity in Circulating Tumor Cells: The Relevance of the Stem-Cell Subset. Cancers 2019, 11, 1632. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Schiedmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, X.; Zheng, J.; Dong, D. DNA methylome profiling of circulating tumor cells in lung cancer at single base-pair resolution. Oncogene 2021, 40, 1884–1895. [Google Scholar] [CrossRef]
- Markou, A.; Londra, D.; Tserpeli, V.; Kollias, I.; Tsaroucha, E.; Vamvakaris, I.; Tsaroucha, E.; Vamvakaris, I.; Potaris, K.; Pateras, I.; et al. DNA methylation analysis of tumor suppressor genes in liquid biopsy components of early stage NSCLC: A promising tool for early detection. Clin. Epigenetics 2022, 14, 61. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Li, B.; Lu, X.; Wang, R.; Li, H.; Yan, B.; Gu, A.; Wang, W.; Huang, A.; et al. Whole-Exome Sequencing on Circulating Tumor Cells Explores Platinum-Drug Resistance Mutations in Advanced Non-small Cell Lung Cancer. Front. Genet. 2021, 12, 722078. [Google Scholar] [CrossRef]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Si, J.; Huang, B.; Lan, G.; Zhang, B.; Wei, J.; Deng, Z.; Li, Y.; Qin, Y.; Li, B.; Lu, Y.; et al. Comparison of whole exome sequencing in circulating tumor cells of primitive and metastatic nasopharyngeal carcinoma. Transl. Cancer Res. 2020, 9, 4080–4092. [Google Scholar] [CrossRef]
- Szostakowska, M.; Trebinska-Stryjewska, A.; Grzybowska, E.A.; Fabisiewicz, A. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019, 173, 489–497. [Google Scholar] [CrossRef]
- Jeselsohn, R.; Buchwalter, G.; De Angelis, C.; Brown, M.; Schiff, R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 573–583. [Google Scholar] [CrossRef]
- Mack, P.C.; Banks, K.C.; Espenschied, C.R.; Burich, R.A.; Zill, O.A.; Lee, C.E.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: Analysis of over 8000 cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.K.; Lee, E.; Ng, C.C.; Yang, V.S.; Farid, M.; Teh, B.T.; Chan, J.Y.; Somasundaram, N. Circulating Tumor DNA Mutations in Progressive Gastrointestinal Stromal Tumors Identify Biomarkers of Treatment Resistance and Uncover Potential Therapeutic Strategies. Front. Oncol. 2022, 12, 840843. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.M.; van Marion, R.; Atmodimedjo, P.N.; de Jonge, E.; Mathijssen, R.H.J.; Paats, M.S.; de Bruijn, P.; Koolen, S.L.; von der Thusen, J.K.; Aerts, J.; et al. Clinical Utility of Circulating Tumor DNA in Patients with Advanced KRAS(G12C)-Mutated NSCLC Treated with Sotorasib. J. Thorac. Oncol. 2024, 19, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Rothe, F.; Venet, D.; Peeters, D.; Rouas, G.; Rediti, M.; Smeets, D.; Dupont, F.; Campbell, P.; Lambrechts, D.; Dirix, L.; et al. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. NPJ Breast Cancer 2022, 8, 79. [Google Scholar] [CrossRef]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.Y.; Taran, F.A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.C.; Friedl, T.W.; et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef]
- Goodman, C.R.; Seagle, B.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018, 4, e180163. [Google Scholar] [CrossRef]
- Bidard, F.C.; Michiels, S.; Riethdorf, S.; Mueller, V.; Esserman, L.J.; Lucci, A.; Naume, B.; Horiguchi, J.; Gisbert-Criado, R.; Sleijfer, S.; et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 560–567. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef]
- Smerage, J.B.; Barlow, W.E.; Hortobagyi, G.N.; Winer, E.P.; Leyland-Jones, B.; Srkalovic, G.; Tejwani, S.; Schott, A.F.; O’Rourke, M.K.; Lew, D.L.; et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014, 32, 3483–3489. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Jacot, W.; Dureau, S.; Brain, E.; Bachelot, T.; Bourgeois, H.; Gonclaves, A.; Ladoire, S.; Naman, H.; Dalenc, F.; et al. Abstract GS3-07: Clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2− metastatic breast cancer: Results of the phase III STIC CTC trial. Cancer Res. 2019, 79 (Suppl. S4), GS3-07. [Google Scholar] [CrossRef]
- Jacot, W.; Cottu, P.; Berger, F.; Dubot, C.; Venat-Bouvet, L.; Lortholary, A.; Bourgeois, H.; Bollet, M.; Servent, V.; Luporsi, E.; et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: The CirCe T-DM1 trial. Breast Cancer Res. 2019, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Polasik, A.; Schramm, A.; Friedl, T.W.; Rack, B.K.; Trapp, E.K.; Fasching, P.A.; Taran, F.A.; Hartkopf, A.D.; Schneeweiss, A.; Mueller, V.; et al. The DETECT study concept: Individualized therapy of metastatic breast cancer. J. Clin. Oncol. 2016, 34 (Suppl. S15), TPS634. [Google Scholar] [CrossRef]
- Stoecklein, N.H.; Oles, J.; Franken, A.; Neubauer, H.; Terstappen, L.; Neves, R.P.L. Clinical application of circulating tumor cells. Med. Genet. 2023, 35, 237–250. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wang, P.; Peng, J.; Wang, X.; Zhu, Y.W.; Shen, N. Meta-analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci. Rep. 2017, 7, 905. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Buchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Xu, H.; Wang, Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015, 15, 202. [Google Scholar] [CrossRef]
- Krebs, M.G.; Renehan, A.G.; Backen, A.; Gollins, S.; Chau, I.; Hasan, J.; Valle, J.W.; Morris, K.; Beech, J.; Ashcroft, L.; et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin. Color. Cancer 2015, 14, 115–122.e2. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.; Priest, L.; Lancashire, L.; Hou, J.M.; Greystoke, A.; Ward, T.H.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef]
- Kapeleris, J.; Kulasinghe, A.; Warkiani, M.E.; Vela, I.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front. Oncol. 2018, 8, 311. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.M.; Hughes, B.G.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: Association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 2012, 18, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, Y.; Zhao, J.; Chen, M.; Xu, Y.; Zhong, W.; Xing, J.; Wang, M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016, 21, 519–525. [Google Scholar] [CrossRef]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- De Souza, L.M.; Robertson, B.M.; Robertson, G.P. Future of circulating tumor cells in the melanoma clinical and research laboratory settings. Cancer Lett. 2017, 392, 60–70. [Google Scholar] [CrossRef]
- Aktar, S.; Baghaie, H.; Islam, F.; Gopalan, V.; Lam, A.K. Current Status of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: A Review. Otolaryngol. Head Neck Surg. 2023, 168, 988–1005. [Google Scholar] [CrossRef]
- Rack, B.; Schindlbeck, C.; Juckstock, J.; Andergassen, U.; Hepp, P.; Zwingers, T.; Friedl, T.W.; Lorenz, R.; Tesch, H.; Fasching, P.A.; et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst. 2014, 106, dju066. [Google Scholar] [CrossRef]
- Massard, C.; Borget, I.; Farace, F.; Aspeslagh, S.; Le Deley, M.C.; Le Tourneau, C.; Bidard, F.C.; Pierga, J.Y.; Dieras, V.; Hofman, P.; et al. RECIST response and variation of circulating tumour cells in phase 1 trials: A prospective multicentric study. Eur. J. Cancer. 2017, 83, 185–193. [Google Scholar] [CrossRef]
- Muller, V.; Riethdorf, S.; Rack, B.; Janni, W.; Fasching, P.A.; Solomayer, E.; Aktas, B.; Kasimir-Bauer, S.; Pantel, K.; Fehm, T.; et al. Prognostic impact of circulating tumor cells assessed with the CellSearch System and AdnaTest Breast in metastatic breast cancer patients: The DETECT study. Breast Cancer Res. 2012, 14, R118. [Google Scholar] [CrossRef]
- Templeman, A.; Miller, M.C.; Cooke, M.J.; O’Shannessy, D.J.; Gurung, Y.; Pereira, T.; Peters, S.G.; Piano, M.; Teo, M.; Khazan, N.; et al. Analytical performance of the FDA-cleared Parsortix((R)) PC1 system. J. Circ. Biomark. 2023, 12, 26–33. [Google Scholar] [CrossRef]
- Wishart, G.; Templeman, A.; Hendry, F.; Miller, K.; Pailhes-Jimenez, A.S. Molecular Profiling of Circulating Tumour Cells and Circulating Tumour DNA: Complementary Insights from a Single Blood Sample Utilising the Parsortix((R)) System. Curr. Issues Mol. Biol. 2024, 46, 773–787. [Google Scholar] [CrossRef]
- Zavridou, M.; Mastoraki, S.; Strati, A.; Koutsodontis, G.; Klinakis, A.; Psyrri, A.; Lianidou, E. Direct comparison of size-dependent versus EpCAM-dependent CTC enrichment at the gene expression and DNA methylation level in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 6551. [Google Scholar] [CrossRef] [PubMed]
- Braso-Maristany, F.; Griguolo, G.; Pascual, T.; Pare, L.; Nuciforo, P.; Llombart-Cussac, A.; Bermejo, B.; Oliveira, M.; Morales, S.; Martinez, N.; et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat. Commun. 2020, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of circulating tumor cells: Opportunities and challenges. Biomark. Res. 2022, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef] [PubMed]
- Edd, J.F.; Mishra, A.; Smith, K.C.; Kapur, R.; Maheswaran, S.; Haber, D.A.; Toner, M. Isolation of circulating tumor cells. iScience 2022, 25, 104696. [Google Scholar] [CrossRef]
- Mathias, T.J.; Chang, K.T.; Martin, S.S.; Vitolo, M.I. Gauging the Impact of Cancer Treatment Modalities on Circulating Tumor Cells (CTCs). Cancers 2020, 12, 743. [Google Scholar] [CrossRef]
- Donato, C.; Kunz, L.; Castro-Giner, F.; Paasinen-Sohns, A.; Strittmatter, K.; Szczerba, B.M.; Scherrer, R.; Di Maggio, N.; Heusermann, W.; Biehlmaier, O.; et al. Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells. Cell Rep. 2020, 32, 108105. [Google Scholar] [CrossRef]
- Zhu, X.; Suo, Y.; Fu, Y.; Zhang, F.; Ding, N.; Pang, K.; Xie, C.; Weng, X.; Tian, M.; He, H.; et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light Sci. Appl. 2021, 10, 110. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwartz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Aceto, N. Fluctuating numbers of circulating tumor cells in cancer patients and the meaning of zero counts. Oncotarget 2019, 10, 2658–2659. [Google Scholar] [CrossRef]
- Juratli, M.A.; Siegel, E.R.; Nedosekin, D.A.; Sarimollaoglu, M.; Jamshidi-Parsian, A.; Cai, C.; Menyaev, Y.A.; Suen, J.Y.; Galanzha, E.I.; Zhareov, V.P. In Vivo Long-Term Monitoring of Circulating Tumor Cells Fluctuation during Medical Interventions. PLoS ONE 2015, 10, e0137613. [Google Scholar] [CrossRef] [PubMed]
- Gall, T.M.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014, 149, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Xiao, W.; Dong, S.L.; Liang, H.F.; Zhang, Z.W.; Zhang, B.X.; Huang, Z.Y.; Chen, Y.F.; Zhang, G.W.; Luo, H.P.; et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer 2018, 18, 835. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Onagi, A.; Koguchi, T.; Hoshi, S.; Ogawa, S.; Akaihata, H.; Hata, J.; Hiraki, H.; Honda, R.; Tanji, R.; et al. Perioperative Detection of Circulating Tumor Cells in Radical or Partial Nephrectomy for Renal Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 1272–1281. [Google Scholar] [CrossRef]
- Camara, O.; Kavallaris, A.; Noschel, H.; Rengsberger, M.; Jorke, C.; Pachmann, K. Seeding of epithelial cells into circulation during surgery for breast cancer: The fate of malignant and benign mobilized cells. World J. Surg. Oncol. 2006, 4, 67. [Google Scholar] [CrossRef]
- Pang, S.; Li, H.; Xu, S.; Feng, L.; Ma, X.; Chu, Y.; Zou, B.; Wang, S.; Zhou, G. Circulating tumour cells at baseline and late phase of treatment provide prognostic value in breast cancer. Sci. Rep. 2021, 11, 13441. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Russell, P.A.; Cox, R.A.; Ivashkevich, A.; Swierczak, A.; Doherty, J.P.; Jacobs, D.H.; Smith, J.; Siva, S.; et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 395–403. [Google Scholar] [CrossRef]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R.; Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 303–307. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Narayan, K.; MacManus, M.P. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat. Rev. Clin. Oncol. 2017, 14, 32–44. [Google Scholar] [CrossRef]
- Ito, Y.; Kobuchi, S.; Kawakita, A.; Tosaka, K.; Matsunaga, Y.; Yoshioka, S.; Jonan, S.; Amagase, K.; Hashimoto, K.; Kanda, M.; et al. Mobilization of Circulating Tumor Cells after Short- and Long-Term FOLFIRINOX and GEM/nab-PTX Chemotherapy in Xenograft Mouse Models of Human Pancreatic Cancer. Cancers 2023, 15, 5482. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Otero, N.; Marshall, J.R.; Lash, B.; King, M.R. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 2020, 20, 873. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.; Landin, J.; Szczerba, B.M.; Castro-Giner, F.; Gkountela, S.; Donato, C.; Krol, I.; Scherrer, R.; Balmelli, C.; Malinovska, A.; et al. Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res. 2018, 20, 141. [Google Scholar] [CrossRef]
- Bendahl, P.O.; Belting, M.; Gezelius, E. Longitudinal Assessment of Circulating Tumor Cells and Outcome in Small Cell Lung Cancer: A Sub-Study of RASTEN-A Randomized Trial with Low Molecular Weight Heparin. Cancers 2023, 15, 3176. [Google Scholar] [CrossRef]
- Lozano, R.; Lorente, D.; Aragon, I.M.; Romero-Laorden, N.; Nombela, P.; Mateo, J.; Reid, A.H.M.; Cendon, Y.; Bianchini, D.; Llacer, C.; et al. Value of Early Circulating Tumor Cells Dynamics to Estimate Docetaxel Benefit in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients. Cancers 2021, 13, 2334. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Costa, C.; Muinelo-Romay, L.; Cebey-Lopez, V.; Pereira-Veiga, T.; Martinez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Leston, R.M.; Abuin, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Zhang, Q.; Basile, D.; Rossi, G.; Strickland, K.; Franzoni, A.; Allergi, L.; Mu, Z.; Zhang, Y.; et al. Longitudinal Dynamics of Circulating Tumor Cells and Circulating Tumor DNA for Treatment Monitoring in Metastatic Breast Cancer. JCO Precis Oncol. 2021, 5, 943–952. [Google Scholar] [CrossRef]
- Szostakowska-Rodzos, M.; Fabisiewicz, A.; Wakula, M.; Tabor, S.; Szafron, L.; Jagiello-Gruszfeld, A.; Grzybowska, E.A. Longitudinal analysis of circulating tumor cell numbers improves tracking metastatic breast cancer progression. Sci. Rep. 2024, 14, 12924. [Google Scholar] [CrossRef]
- Forsare, C.; Bendahl, P.O.; Moberg, E.; Levin Tykjaer Jorgensen, C.; Jansson, S.; Larsson, A.M.; Aaltonen, K.; Ryden, L. Evolution of Estrogen Receptor Status from Primary Tumors to Metastasis and Serially Collected Circulating Tumor Cells. Int. J. Mol. Sci. 2020, 21, 2885. [Google Scholar] [CrossRef]
- Cohen, E.N.; Jayachandran, G.; Gao, H.; Peabody, P.; McBride, H.B.; Alvarez, F.D.; Kai, M.; Song, J.; Shen, Y.; Willey, J.S.; et al. Phenotypic Plasticity in Circulating Tumor Cells Is Associated with Poor Response to Therapy in Metastatic Breast Cancer Patients. Cancers 2023, 15, 1616. [Google Scholar] [PubMed]
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive liquid biopsy analysis as a tool for the early detection of minimal residual disease in breast cancer. Sci. Rep. 2023, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.; Dall, K.; Brandt, B.; Geisen, R.; Roder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Longitudinal Analysis of Circulating Tumor Cells in Colorectal Cancer Patients by a Cytological and Molecular Approach: Feasibility and Clinical Application. Front. Oncol. 2021, 11, 646885. [Google Scholar] [CrossRef]
- Ko, J.M.; Vardhanabhuti, V.; Ng, W.T.; Lam, K.O.; Ngan, R.K.; Kwong, D.L.; Lee, V.H.; Lui, Y.H.; Yau, C.C.; Kwan, C.K.; et al. Clinical utility of serial analysis of circulating tumour cells for detection of minimal residual disease of metastatic nasopharyngeal carcinoma. Br. J. Cancer 2020, 123, 114–125. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Hendrix, L.H.; Hyslop, T.; Barry, W.T.; Winer, E.P.; Hudis, C.; Toppmeyer, D.; Carey, L.A.; Partridge, A.; Pierga, J.Y.; et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. J. Natl. Cancer Inst. 2021, 113, 443–452. [Google Scholar]
- Magbanua, M.J.M.; Savenkov, O.; Asmus, E.J.; Ballman, K.V.; Scott, J.H.; Park, J.W.; Dickler, M.; Partridge, A.; Carey, L.A.; Winer, E.P.; et al. Clinical Significance of Circulating Tumor Cells in Hormone Receptor-positive Metastatic Breast Cancer Patients who Received Letrozole with or without Bevacizumab. Clin. Cancer Res. 2020, 26, 4911–4920. [Google Scholar] [CrossRef]
- Ko, J.M.Y.; Lam, K.O.; Kwong, D.L.W.; Wong, I.Y.; Chan, F.S.; Wong, C.L.; Chan, K.K.; Law, T.T.; Chiu, K.W.H.; Lam, C.C.S.; et al. Circulating Tumor Cell Enumeration for Serial Monitoring of Treatment Outcomes for Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers 2023, 15, 832. [Google Scholar] [CrossRef]
| Cancer Type | Authors | Year | No. of Patients Included in Analysis | No. of Collections | CTC Detection Method | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Breast Cancer | Wang C, Mu Z, Chervoneva I, …, Cristofanilli M, Yang, H. | 2017 | 128 | 3 | CellSearch | CTC clusters added additional prognostic values to CTC enumeration alone, and a larger-size CTC cluster conferred a higher risk of death in MBC patients. | [32] |
| Breast Cancer | Larsson AM, Jansson S, Bendahl PO, …, Rydén L. | 2018 | 152 | 4 | CellSearch | A longitudinal evaluation of CTC and CTC clusters improved prognostication and monitoring in patients with MBC starting first-line systemic therapy. Changes in CTC count throughout treatment were significantly correlated with survival, and the prognostic value was more prominent at later time points. High CTC counts and the presence of clusters were identified as prognostic factors for OS and PFS. | [31] |
| Breast Cancer | Forsare C, Bendahl PO, Moberg E, …, Rydén L. | 2020 | 147 | 3 | CellSearch | A shift in ER status from PT to DM/CTCs was demonstrated. A retained ER positivity of CTCs after the initiation of systemic therapy was associated with a better prognosis for PFS. This effect was observed only for follow-up samples, highlighting the importance of CTC phenotyping during treatment. | [110] |
| Nasopharyngeal Carcinoma (NPC) | Ko JMY, Vardhanabhuti VV, Ng WT, …, Lung ML. | 2020 | 21 | 4 | CTChip®FR1 | CTCs were characterized as a more sensitive biomarker for MRD, when compared with imaging. Longitudinal changes in CTCs and EBV DNA along with CT treatment for mNPC were found to be predictive for disease relapse. | [114] |
| Breast Cancer | Magbanua MJM, Hendrix LH, Hyslop T, …, Rugo HS. | 2021 | 469 | ≥3 | CellSearch | The authors used the CTC trajectory model, which divided patients into groups, predicting a consistent trend for negative CTCs, low CTCs, mid CTCs, and high CTCs. The mid and high tCTC groups were identified with a higher risk of early progression and shorter PFS and OS. | [115] |
| Breast Cancer | Magbanua MJM, Savenkov O, Asmus EJ, …, Rugo HS. | 2021 | 294 | 4 | CellSearch | CTC-positive patients at the baseline were identified with a worse PFS and OS than CTC-negative patients. CTC positivity during treatment or baseline was identified as a risk factor for PFS and OS. Patients that became CTC-positive in the 1st follow-up had a poorer prognosis for OS than patients that stayed CTC-negative or patients that remained CTC-positive since baseline. Patients who stayed CTC-positive had a poorer PFS and OS than patients who stayed CTC-negative since baseline. | [116] |
| Breast Cancer | Gerratana L, Davis AA, Zhang Q, …, Cristofanilli M. | 2021 | 107 | 3 | CellSearch | The ctDNA analysis revealed that mutant allele frequency (MAF) changes followed the response to treatment, while CTC numbers increased only at the time of clinical progression. Conclusion: MAF could be more suitable for real-time disease monitoring, while CTCs could be more likely linked to metastatic biology. | [108] |
| Breast Cancer | Pang S, Li H, Xu S, …, Zhou G. | 2021 | 164 | 4 | IMNs (immunomagnetic nanospheres) | Surgery led to an increase in the number and prevalence of CTCs on the first day after surgery, and they did not return to the preoperative level until 14 days after surgery. CTC prevalence at the baseline and end point follow-up visits was related to PFS and OS, while the CTCs detected before chemotherapy were only related to PFS. | [96] |
| Colorectal Cancer | Hendricks A, Dall K, Brandt B, …, Sebens S. | 2021 | 47 | 5 | NYONE, RT-PCR | Surgery did not have any statistically significant effect on the quantity of CTCs detected by the cytological approach utilizing the cell imager NYONE. In one of the patients, a constant increase in CTCs detected via both methods (9 months after the surgery) occurred before the local clinical recurrence (13 months after surgery). | [113] |
| Breast Cancer | Stergiopoulou D, Markou A, Strati A, …, Lianidou E. | 2023 | 13 | ≥10 | CellSearch | The molecular characteristics of CTCs were highly different even for the same patient at different time points, and they always increased before the clinical relapse. Rapid increases in CTC numbers in months 74 and 122, were associated with metastatic disease documented by biopsy 6 months earlier. | [112] |
| Breast Cancer | Cohen EN, Jayachandran G, Gao H, …, Reuben JM. | 2023 | 184 | 9 | MCA (microactivity array) | This study reaffirmed the cut-off of ≥5 CTCs for an inferior prognosis of patients with MBC. It also highlighted that epithelial CTC counts were prognostic before the initiation of therapy and early in therapy, whereas a shift towards mesenchymal CTC phenotypes as detected by gene expression was associated with disease progression. | [111] |
| Esophageal Squamous Cell Carcinoma | Ko JMY, Lam KO, Kwong DLW, …, Lung ML | 2023 | 88 | 12 | CTChip®FR1 | The changes in CTC status pre-surgery and 1 or 3 months after surgery and pT staging after resection are independent prognostic factors of poor prognosis for locally advanced ESCC patients receiving surgical treatment, as well as the presence of CTC clusters, unfavorable CTC status at baseline, and 1 month and 3 months post-surgery. | [117] |
| Small Cell Lung Cancer | Bendahl PO, Belting M, Gezelius E. | 2023 | 42 | 2 | CellSearch | CTC presence at the baseline was identified as a poor prognostic factor for the survival of patients. A persistent CTC presence at 2-month follow-up and baseline was associated with a significantly higher HR for OS. | [104] |
| Breast Cancer | Szostakowska-Rodzos M, Fabisiewicz A, Wakula M, …, Grzybowska EA. | 2024 | 135 | 3 | CytoTrack | A high CTC count was an independent poor prognosis marker for PFS and OS, regardless of the time of enumeration. Consistently low CTCs numbers during treatment were revealed to be favorable prognostic markers for PFS and OS. Rising values of CTC counts were identified as predictors for rapid progression. | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabisiewicz, A.; Szostakowska-Rodzos, M.; Grzybowska, E.A. Improving the Prognostic and Predictive Value of Circulating Tumor Cell Enumeration: Is Longitudinal Monitoring the Answer? Int. J. Mol. Sci. 2024, 25, 10612. https://doi.org/10.3390/ijms251910612
Fabisiewicz A, Szostakowska-Rodzos M, Grzybowska EA. Improving the Prognostic and Predictive Value of Circulating Tumor Cell Enumeration: Is Longitudinal Monitoring the Answer? International Journal of Molecular Sciences. 2024; 25(19):10612. https://doi.org/10.3390/ijms251910612
Chicago/Turabian StyleFabisiewicz, Anna, Malgorzata Szostakowska-Rodzos, and Ewa A. Grzybowska. 2024. "Improving the Prognostic and Predictive Value of Circulating Tumor Cell Enumeration: Is Longitudinal Monitoring the Answer?" International Journal of Molecular Sciences 25, no. 19: 10612. https://doi.org/10.3390/ijms251910612
APA StyleFabisiewicz, A., Szostakowska-Rodzos, M., & Grzybowska, E. A. (2024). Improving the Prognostic and Predictive Value of Circulating Tumor Cell Enumeration: Is Longitudinal Monitoring the Answer? International Journal of Molecular Sciences, 25(19), 10612. https://doi.org/10.3390/ijms251910612







