Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality
Abstract
1. Introduction
2. Results
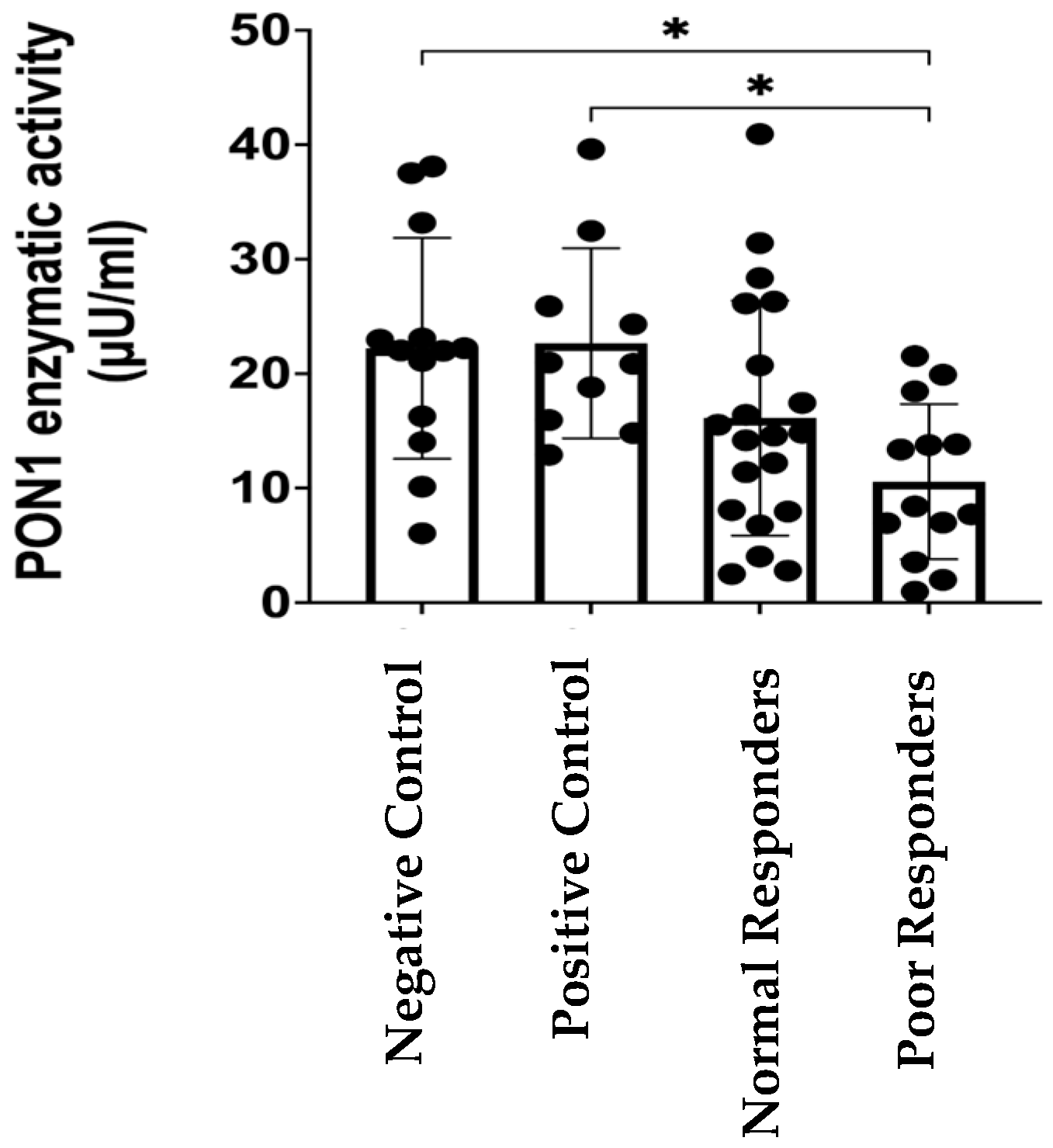
2.1. PON1 Enzymatic Activity
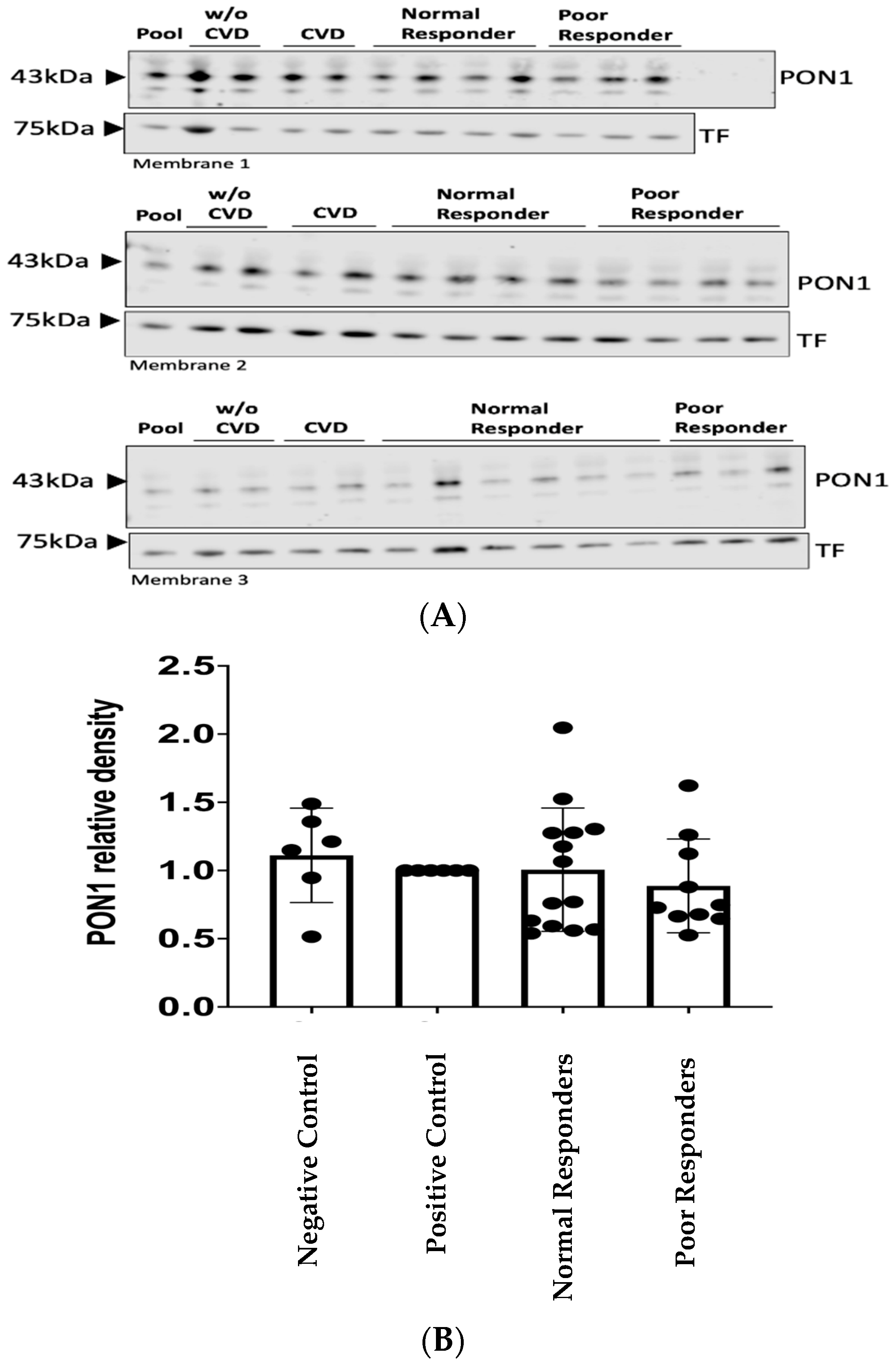
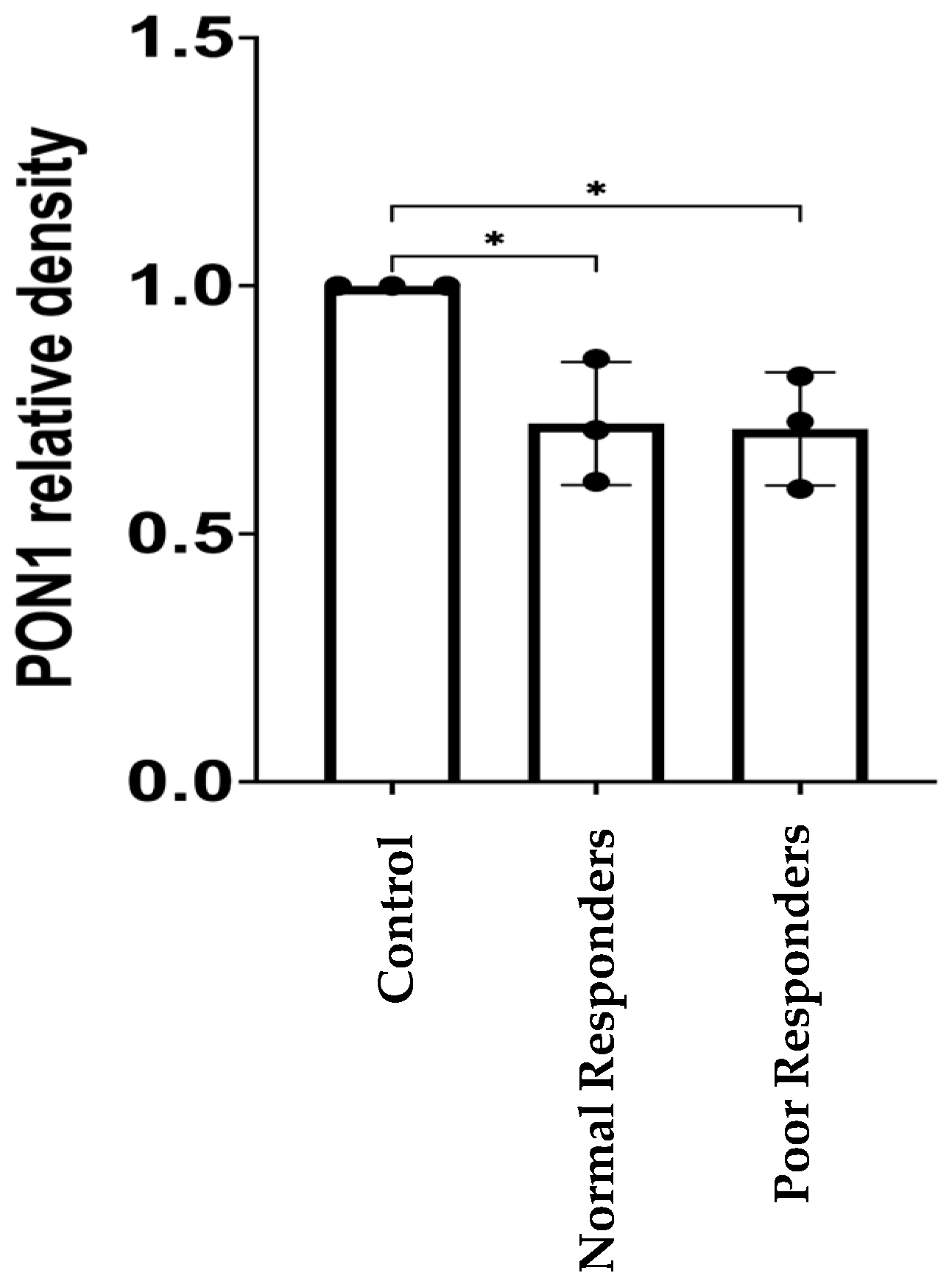
2.2. PON1 Relative Protein Abundance
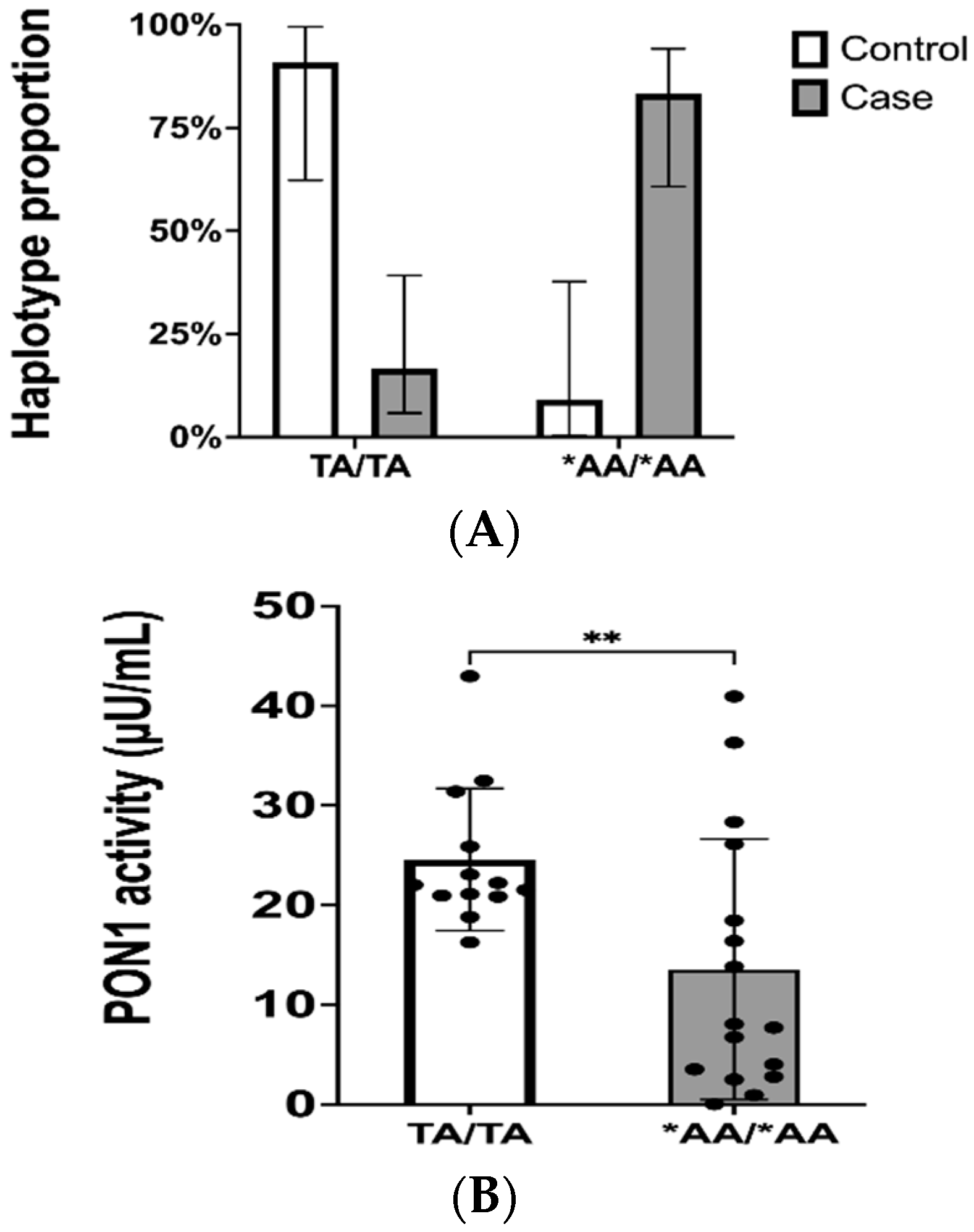
2.3. Genotypes and Haplotype Phasing
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Sample Processing
4.3. PON1 Function Assay
4.4. Western Blot Analysis
4.5. Genotyping Microarray
4.6. Haplotype Phasing for PON1 Gene
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. Highlights of Prescribing Information. 2016. Available online: www.fda.gov/medwatch (accessed on 27 August 2024).
- Gent, M. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar]
- Quinn, M.J.; Fitzgerald, D.J. Ticlopidine and Clopidogrel. Circulation 1999, 100, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-L.; Samant, S.; Lesko, L.J.; Schmidt, S. Clinical Pharmacokinetics and Pharmacodynamics of Clopidogrel. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef]
- Cavallari, L.H.; Lee, C.R.; Beitelshees, A.L.; Cooper-DeHoff, R.M.; Duarte, J.D.; Voora, D.; Kimmel, S.E.; McDonough, C.W.; Gong, Y.; Dave, C.V.; et al. Multisite Investigation of Outcomes with Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy after Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 181–191. [Google Scholar] [CrossRef] [PubMed Central]
- Garabedian, T.; Alam, S. High residual platelet reactivity on clopidogrel: Its significance and therapeutic challenges over-coming clopidogrel resistance. Cardiovasc. Diagn. Ther. 2013, 3, 23–37. [Google Scholar]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS J. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2016, 567, 12–21. [Google Scholar] [CrossRef]
- Serrato, M.; Marian, A.J. A variant of human paraoxonase/arylesterase (HUMPONA) gene is a risk factor for coronary artery disease. J. Clin. Investig. 1995, 96, 3005–3008. [Google Scholar] [CrossRef][Green Version]
- Mackness, B.; Mackness, M.I.; Arrol, S.; Turkie, W.; Durrington, P.N. Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br. J. Pharmacol. 1997, 122, 265–268. [Google Scholar] [CrossRef]
- Ruiz, J.; Morabia, A.; Blanche, H.; James, R.; Garin, M.-C.; Charpentier, G.; Passa, P.; Vaisse, C.; Froguel, P. Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet 1995, 346, 869–872. [Google Scholar] [CrossRef]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase Status in Coronary Heart Disease Are Activity and Concentration More Important Than Genotype? Arter. Thromb. Vasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef]
- Norris, E.T.; Wang, L.; Conley, A.B.; Rishishwar, L.; Mariño-Ramírez, L.; Valderrama-Aguirre, A.; Jordan, I.K. Genetic ancestry, admixture and health determinants in Latin America. BMC Genom. 2018, 19, 861. [Google Scholar] [CrossRef]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef]
- Bouman, H.J.; Schömig, E.; van Werkum, J.W.; Velder, J.; Hackeng, C.M.; Hirschhäuser, C.; Waldmann, C.; Schmalz, H.-G.; Berg, J.M.T.; Taubert, D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 2010, 17, 110–116, Erratum Nat. Med. 2011, 17, 1153. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Botton, M.R.; Scott, S.; Tomey, M.; Garcia, M.J.; Wiley, J.; Villablanca, P.; Melin, K.; Lopez-Candales, A.; Renta, J.Y.; et al. Pharmacogenetic association study on clopidogrel response in Puerto Rican Hispanics with cardiovascular disease: A novel characterization of a Caribbean population. Pharmacogenomics Pers. Med. 2018, 11, 95–106. [Google Scholar] [CrossRef]
- Santiago-Cartagena, E.; Cantres, Y.; Carrasquillo, K.; Moneró, M.; Meléndez, L.; Roche, A.; Duconge, J. Quantitative Proteomic Profile of Caribbean Hispanics with Resistance to Clopidogrel. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Mackness, B.; Turkie, W.; Mackness, M. Paraoxonase-1 (PON1) promoter region polymorphisms, serum PON1 status and coronary heart disease. Arch. Med. Sci. 2013, 1, 8–13. [Google Scholar] [CrossRef]
- Shunmoogam, N.; Naidoo, P.; Chilton, R. Paraoxonase (PON)-1: A brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc. Health Risk Manag. 2018, 14, 137–143. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organophosphatetoxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef]
- Mackness, B.; Hunt, R.; Durrington, P.N.; Mackness, M.I. Increased Immunolocalization of Paraoxonase, Clusterin, and Apolipoprotein A-I in the Human Artery Wall with the Progression of Atherosclerosis. Arter. Thromb. Vasc. Biol. 1997, 17, 1233–1238. [Google Scholar] [CrossRef]
- Gong, I.Y.; Crown, N.; Suen, C.M.; Schwarz, U.I.; Dresser, G.K.; Knauer, M.J.; Sugiyama, D.; DeGorter, M.K.; Woolsey, S.; Tirona, R.G.; et al. Clarifying the importance of CYP2C19 and PON1 in the mechanism of clopidogrel bioactivation and in vivo antiplatelet response. Eur. Heart J. 2012, 33, 2856–2864. [Google Scholar] [CrossRef]
- Hulot, J.S.; Collet, J.P.; Cayla, G.; Silvain, J.; Allanic, F.; Bellemain-Appaix, A.; Scott, S.A.; Montalescot, G. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ. Cardiovasc. Interv. 2011, 4, 422–428. [Google Scholar] [CrossRef]
- Lewis, J.P.; Fisch, A.S.; Ryan, K.; O’Connell, J.R.; Gibson, Q.; Mitchell, B.D.; Shen, H.; Tanner, K.; Horenstein, R.B.; Pakzy, R.; et al. Paraoxonase 1 (PON1) Gene Variants Are Not Associated with Clopidogrel Response. Clin. Pharmacol. Ther. 2011, 90, 568–574. [Google Scholar] [CrossRef]
- Sibbing, D.; Koch, W.; Massberg, S.; Byrne, R.A.; Mehilli, J.; Schulz, S.; Mayer, K.; Bernlochner, I.; Schömig, A.; Kastrati, A. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur. Hear. J. 2011, 32, 1605–1613. [Google Scholar] [CrossRef]
- Tresukosol, D.; Suktitipat, B.; Hunnangkul, S.; Kamkaew, R.; Poldee, S.; Tassaneetrithep, B.; Likidlilid, A. Effects of Cytochrome P450 2C19 and Paraoxonase 1 Polymorphisms on Antiplatelet Response to Clopidogrel Therapy in Patients with Coronary Artery Disease. PLoS ONE 2014, 9, e110188. [Google Scholar] [CrossRef]
- Leviev, I.; Negro, F.; James, R.W. Two Alleles of the Human Paraoxonase Gene Produce Different Amounts of mRNA: An Explanation for Differences in Serum Concentrations of Paraoxonase Associated with the (Leu-Met54) Polymorphism. Arte-Rioscler. Thromb. Vasc. Biol. 1997, 17, 2935–2939. [Google Scholar] [CrossRef]
- Altman, N.; Krzywinski, M. Association, correlation and causation. Nat. Methods 2015, 12, 899–900. [Google Scholar] [CrossRef]
- Claudio-Campos, K.I.; González-Santiago, P.; Renta, J.Y.; Rodríguez, J.; Carrasquillo, K.; Gaedigk, A.; Roche, A.; Ducongé, J. CYP2C9*61, a rare missense variant identified in a Puerto Rican patient with low warfarin dose requirements. Pharmacogenomics 2019, 20, 3–8. [Google Scholar] [CrossRef]
- Brophy, V.H.; Jampsa, R.L.; Clendenning, J.B.; McKinstry, L.A.; Jarvik, G.P.; Furlong, C.E. Effects of 5′ Regulatory-Region Polymorphisms on Paraoxonase-Gene (PON1) Expression. Am. J. Hum. Genet. 2001, 68, 1428–1436. [Google Scholar] [CrossRef]
- Leviev, I.; James, R.W. Promoter Polymorphisms of Human Paraoxonase PON1 Gene and Serum Paraoxonase Activities and Concentrations. Arter. Thromb. Vasc. Biol. 2000, 20, 516–521. [Google Scholar] [CrossRef]
- Zhou, C.; Simpson, K.L.; Lancashire, L.J.; Walker, M.J.; Dawson, M.J.; Unwin, R.D.; Rembielak, A.; Price, P.; West, C.; Dive, C.; et al. Statistical Considerations of Optimal Study Design for Human Plasma Proteomics and Biomarker Discovery. J. Proteome Res. 2012, 11, 2103–2113. [Google Scholar] [CrossRef]
- Handler, D.C.; Pascovici, D.; Mirzaei, M.; Gupta, V.; Salekdeh, G.H.; Haynes, P.A. The Art of Validating Quantitative Proteomics Data. Proteomics 2018, 18, e1800222. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Mölleken, C.; Sitek, B.; Henkel, C.; Poschmann, G.; Sipos, B.; Wiese, S.; Warscheid, B.; Broelsch, C.; Reiser, M.; Friedman, S.L.; et al. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology 2009, 49, 1257–1266. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Abu-Saleh, N.; Aviram, M.; Hayek, T. Aqueous or lipid components of atherosclerotic lesion increase macrophage oxidation and lipid accumulation. Life Sci. 2016, 154, 1–14. [Google Scholar] [CrossRef]
- Keaney Jr, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham study. Arte-Rioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Meisinger, C.; Freuer, D.; Bub, A.; Linseisen, J. Association between inflammatory markers and serum paraoxonase and arylesterase activities in the general population: A cross-sectional study. Lipids Health Dis. 2021, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Inoue, M.; Suehiro, T.; Arii, K.; Kumon, Y.; Hashimoto, K. Low human paraoxonase predicts cardiovascular events in Japanese patients with type 2 diabetes. Acta Diabetol. 2009, 46, 239–242. [Google Scholar] [CrossRef]
- González, F.E.M.; Ponce-Ruíz, N.; Rojas-García, A.E.; Bernal-Hernández, Y.Y.; Mackness, M.; Ponce-Gallegos, J.; Cardoso-Saldaña, G.; Jorge-Galarza, E.; Torres-Tamayo, M.; Medina-Díaz, I.M. PON1 concentration and high-density lipoprotein characteristics as cardiovascular biomarkers. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, Y.; Fang, Y.; Liu, L.; Wu, S.; Fu, D.; Wang, X. Association between PON1 activity and coronary heart disease risk: A meta-analysis based on 43 studies. Mol. Genet. Metab. 2012, 105, 141–148. [Google Scholar] [CrossRef]
- Chen, H.; Ding, S.; Zhou, M.; Wu, X.; Liu, X.; Liu, J.; Wu, Y.; Liu, D. PON1 L55M and Q192R gene polymorphisms and CAD risks in patients with hyperlipidemia: Clinical study of possible associations. Herz 2018, 43, 642–648. [Google Scholar] [CrossRef]
- Luo, J.; Ren, H.; Liu, M.; Fang, P.; Xiang, D. European versus Asian differences for the associations between paraoxonase-1 genetic polymorphisms and susceptibility to type 2 diabetes mellitus. J. Cell. Mol. Med. 2018, 22, 1720–1732. [Google Scholar] [CrossRef]
- Macharia, M.; Kengne, A.P.; Blackhurst, D.M.; Erasmus, R.T.; Matsha, T.E. Paraoxonase1 Genetic Polymorphisms in a Mixed Ancestry African Population. Mediat. Inflamm. 2014, 2014, 217019. [Google Scholar] [CrossRef]
- Garin, M.C.; James, R.W.; Dussoix, P.; Blanché, H.; Passa, P.; Froguel, P.; Ruiz, J. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Investig. 1997, 99, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Furlong, C.; Bastaki, M.; Richter, R.; Bradman, A.; Huen, K.; Beckman, K.; Eskenazi, B. Paraoxonase polymorphisms, haplotypes, and enyzme activity in Latino mothers and newborns. Environ. Health Perspect. 2006, 114, 985–991. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of Paraoxonase 1 (PON1) Gene Polymorphisms and Functional Activity With Systemic Oxidative Stress and Cardiovascular Risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef]
- Kumru, S.; Aydin, S.; Aras, A.; Gursu, M.; Gulcu, F. Effects of Surgical Menopause and Estrogen Replacement Therapy on Serum Paraoxonase Activity and Plasma Malondialdehyde Concentration. Gynecol. Obstet. Investig. 2005, 59, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Taşkiran, P.; Cam, S.F.; Sekuri, C.; Tüzün, N.; Alioğlu, E.; Altintaş, N.; Berdeli, A. The relationship between paraoxanase gene Leu-Met (55) and Gln-Arg (192) polymorphisms and coronary artery disease. Turk. Kardiyol. Dern. Ars. 2009, 37, 473–478. [Google Scholar] [PubMed]
- Watzinger, N.; Schmidt, H.; Schumacher, M.; Schmidt, R.; Eber, B.; Fruhwald, F.; Zweiker, R.; Kostner, G.; Klein, W. Human Paraoxonase1 Gene Polymorphisms and the Risk of Coronary Heart Disease: A Community-Based Study. Cardiology 2002, 98, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Barres, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute Exercise Remodels Promoter Methylation in Human Skeletal Muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Luijk, R.; Zhernakova, D.; Moed, M.; Deelen, P.; Vermaat, M.; Van Iterson, M.M.; Van Dijk, F.; Van Galen, M.; Bot, J.; et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat. Genet. 2017, 49, 131–138. [Google Scholar] [CrossRef]
- Breton, C.V.; Byun, H.-M.; Wenten, M.; Pan, F.; Yang, A.; Gilliland, F.D. Prenatal Tobacco Smoke Exposure Affects Global and Gene-specific DNA Methylation. Am. J. Respir. Crit. Care Med. 2009, 180, 462–467. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Gamboa, R.; Zamora, J.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Cardoso, G.; Posadas-Romero, C.; Vargas-Alarcón, G. Distribution of paraoxonase PON1 gene polymorphisms in Mexican populations. Its role in the lipid profile. Exp. Mol. Pathol. 2006, 80, 85–90. [Google Scholar] [CrossRef]
- Ginsberg, G.; Neafsey, P.; Hattis, D.; Guyton, K.Z.; Johns, D.O.; Sonawane, B. Genetic Polymorphism in Paraoxonase 1 (PON1): Population Distribution of PON1 Activity. J. Toxicol. Environ. Health Part B 2009, 12, 473–507. [Google Scholar] [CrossRef]
- Abcam. ab241044 Paraoxonase 1 Activity Assay Kit. 2018. Available online: https://www.abcam.com/en-us/products/assay-kits/paraoxonase-1-activity-assay-kit-ab241044#tab=support (accessed on 27 August 2024).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklarb, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Delaneau, O.; Zagury, J.-F.; Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods 2013, 10, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Medina, H.J.; Monero, M.; Torres, L.M.; Leal, E.; Gonzalez-Sepulveda, L.; Mayor, M.; Renta, J.Y.; González-García, E.R.; González, A.; Melin, K.; et al. Implementing a pharmacogenomic-driven algorithm to guide antiplatelet therapy among Caribbean Hispanics: A non-randomised clinical trial. BMJ Open 2024, 14, e084119. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 36) | Normal Responders (n = 20) | Poor Responders (n = 16) | p Value | Negative Controls (n = 13) | Positive Controls (n = 11) |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 67 ± 10 | 67 ± 9 | 68 ± 10 | 0.9817 | 48 ± 11 | 53 ± 18 |
| Sex (%, n) | 0.5152 | |||||
| Female | 56% (20) | 50% (10) | 63% (10) | 53.8% (7) | 54.5% (6) | |
| Male | 44% (16) | 50% (10) | 38% (6) | 46.2% (6) | 45.5% (5) | |
| BMI | 30.9 ± 5.9 | 29.6 ± 5.2 | 32.6 ± 6.5 | 0.1291 | 28.9 ± 6.2 | 30.1 ± 5.5 |
| Current Smoker (%, n) | 19% (7) | 20% (4) | 19% (3) | >0.9999 | 15.4% (2) | 18.2% (2) |
| Diabetes Mellitus (%, n) | 92% (33) | 90% (18) | 94% (15) | >0.9999 | 91% (10) | |
| Dyslipidemia (%, n) | 92% (33) | 95% (19) | 88% (14) | 0.5742 | 91% (10) | |
| Hypertension (%, n) | 97% (35) | 95% (19) | 100% (16) | >0.9999 | 100% (11) | |
| Clopidogrel Indication (%, n) | ||||||
| Stable CAD or ACS | 72.2% (26) | 75% (15) | 68.75% (11) | |||
| PAD | 27.78% (10) | 25% (5) | 31.25% (5) | 0.7225 | ||
| MI, STEMI, and NSTEMI (%, n) | 2.77% (1) | 0% (0) | 6.25% (1) | 0.4444 | ||
| Coronary Artery Stents (%, n) # | 36.1% (13) | 40% (8) | 31.3% (5) | 0.7314 | ||
| Aspirin Users (%, n) | 64% (23) | 60% (12) | 69% (11) | 0.7314 | 0% (0) | |
| CCB Users (%, n) | 31% (11) | 35% (7) | 25% (4) | 0.7182 | 27.3% (3) | |
| Cilostazol Users (%, n) | 14% (5) | 15% (3) | 13% (2) | >0.999 | ||
| PPI Users (%, n) | 28% (10) | 20% (4) | 38% (6) | 0.2853 | 18.2% (2) | |
| Statins Users (%, n) | 81% (29) | 90% (18) | 69% (11) | 0.2036 | 82% (9) | |
| CYP2C19*2 Status (MAF, %, n) * | 13.9% (10) | 12.5% (5) | 15.6% (5) | 0.7426 | 15.4% (4) | 13.6% (3) |
| Variable | Negative Controls (n = 13) | Positive Controls (n = 11) | Normal Responders (n = 20) | Poor Responders (n = 16) |
|---|---|---|---|---|
| rs662 (p.Q192R) | ||||
| 4 (31%) | 5 (45.5%) | 3 (15%) | 2 (12.5%) | |
| QR | 7 (54%) | 4 (36.4%) | 11 (55%) | 11 (68.75%) |
| RR | 2 (15%) | 2 (18.1%) | 6 (30%) | 3 (18.75%) |
| MAF | 42.31% | 36.4% | 57.5% | 53.12% |
| 95%CI | 19.33–68.05 | 13.81–60.94 | 39.07–73.50 | 34.21–74.18 |
| rs854560 (p.L55M) | ||||
| LL | 9 (69.2%) | 6 (54.5%) | 10 (50%) | 10 (62.5%) |
| LM | 4 (30.8%) | 2 (18.2%) | 7 (35%) | 6 (37.5%) |
| MM | 0 (0%) | 3 (27.3%) | 3 (15%) | 0 (0%) |
| MAF | 15.38% | 36.4% | 32.5% | 18.75% |
| 95%CI | 2.96–44.80 | 19.33–68.05 | 17.93–50.66 | 8.07–41.60 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Caribbean Hispanics residing in Puerto Rico. Both sexes (i.e., males/females). Age ≥ 21 years-old. Receiving clopidogrel (75 mg/day) for therapeutic indications (ACS, stable CAD, PAD) over at least 6 months. No clinically active hepatic abnormality. The ability to understand the requirements of the study. The ability to comply with the study procedures and protocols. A female patient is eligible to enter the study if she is of child-bearing potential and not pregnant or nursing, or not of child-bearing potential. | Non-Hispanic patients Currently enrolled in another active research protocols Blood Urea Nitrogen (BUN) > 30 and creatinine > 2.0 mg/dL Hematocrit (Hct) ≤ 25% Nasogastric or enteral feedings Acute illness (e.g., sepsis, infection, anemia) HIV/AIDS, Hepatitis B patients Alcoholism and drug abuse Patients with any cognitive and mental health impairment Sickle cell patients Active malignancy Patients taking another antiplatelet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monero-Paredes, M.; Santiago, E.; Carrasquillo-Carrion, K.; Renta, J.Y.; Rogozin, I.B.; Roche-Lima, A.; Duconge, J. Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality. Int. J. Mol. Sci. 2024, 25, 10657. https://doi.org/10.3390/ijms251910657
Monero-Paredes M, Santiago E, Carrasquillo-Carrion K, Renta JY, Rogozin IB, Roche-Lima A, Duconge J. Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality. International Journal of Molecular Sciences. 2024; 25(19):10657. https://doi.org/10.3390/ijms251910657
Chicago/Turabian StyleMonero-Paredes, Mariangeli, Ednalise Santiago, Kelvin Carrasquillo-Carrion, Jessicca Y. Renta, Igor B. Rogozin, Abiel Roche-Lima, and Jorge Duconge. 2024. "Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality" International Journal of Molecular Sciences 25, no. 19: 10657. https://doi.org/10.3390/ijms251910657
APA StyleMonero-Paredes, M., Santiago, E., Carrasquillo-Carrion, K., Renta, J. Y., Rogozin, I. B., Roche-Lima, A., & Duconge, J. (2024). Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality. International Journal of Molecular Sciences, 25(19), 10657. https://doi.org/10.3390/ijms251910657








