The Immobilization of an FGF2-Derived Peptide on Culture Plates Improves the Production and Therapeutic Potential of Extracellular Vesicles from Wharton’s Jelly Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Assessment of FGF2 Peptide Variants to Boost Proliferation and Exosome Yields in WJ MSCs
2.2. Investigating Features of Exosomes Produced by FP2
2.3. Coating with FP2 Elevated Expression Levels of Genes Contributing to Exosome Biogenesis
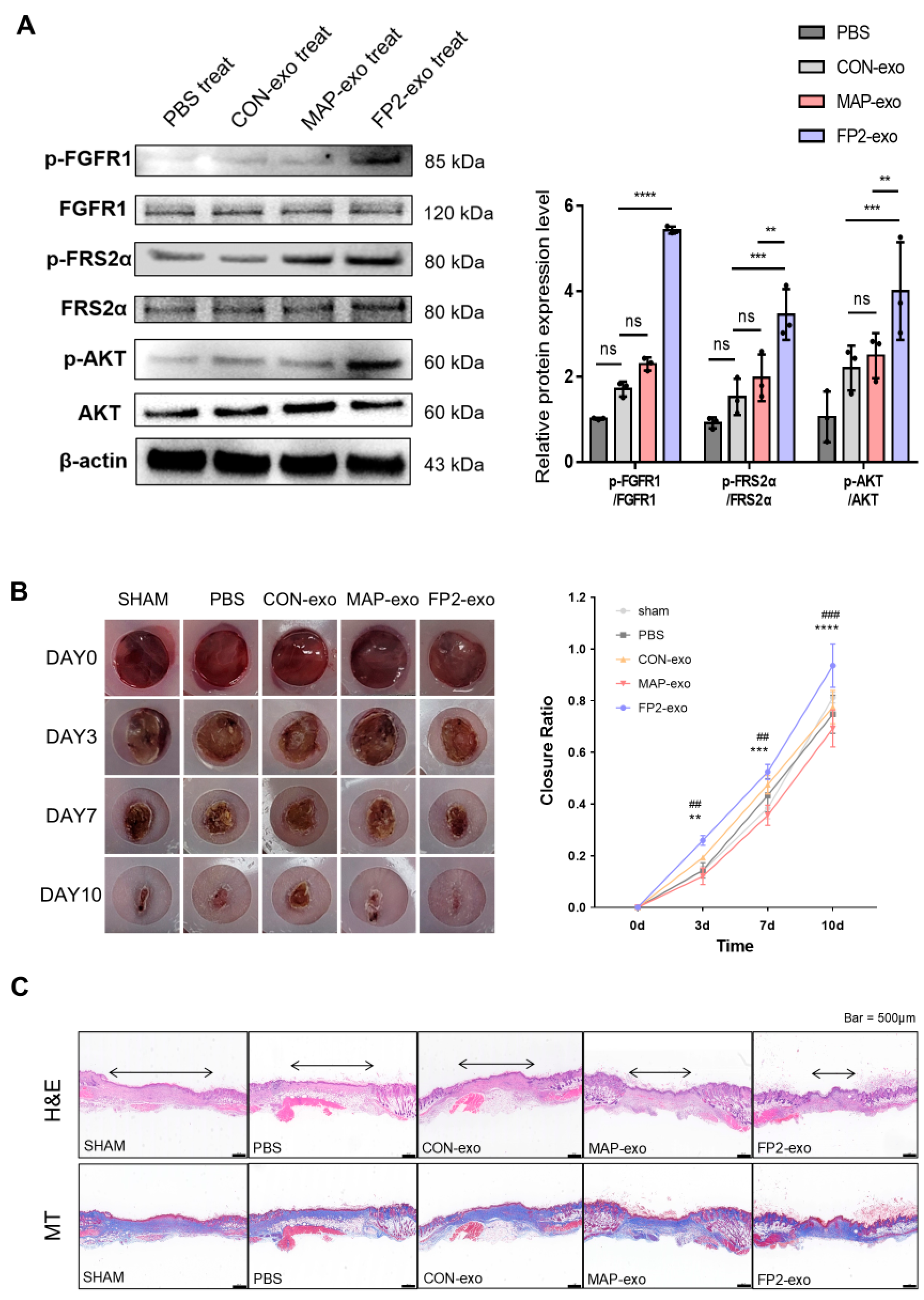
2.4. Confirming Migration-Promoting Effect of FP2-exo in Human Fibroblasts
2.5. FP2-exo Efficiently Reduced Expression of Pro-Inflammatory Factors Induced by Lipopolysaccharide (LPS)
2.6. FP2-exo Enhanced Healing of Skin Wounds, Acting via FGF2 Signal
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Peptide Preparation and Coating Method for 2D Culture
4.3. Protein Structure Prediction
4.4. Exosome Isolation
4.5. TEM
4.6. Western Blot Analysis
4.7. RT-qPCR
4.8. Cell Proliferation Assay
4.9. Transwell Migration Assay
4.10. Wound Closure Assay
4.11. NO Assay
4.12. Wound-Healing Mouse Model
4.13. Histological Analysis
4.14. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Wei, S.; Wang, W.; Li, L.; Meng, H.Y.; Feng, C.Z.; Dong, Y.Y.; Fang, X.C.; Dong, Q.Q.; Jiang, W.; Xin, H.L.; et al. Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs. Mil. Med. Res. 2021, 8, 18. [Google Scholar] [CrossRef]
- Airola, M.V.; Shanbhogue, P.; Shamseddine, A.A.; Guja, K.E.; Senkal, C.E.; Maini, R.; Bartke, N.; Wu, B.X.; Obeid, L.M.; Garcia-Diaz, M.; et al. Structure of human nSMase2 reveals an interdomain allosteric activation mechanism for ceramide generation. Proc. Natl. Acad. Sci. USA 2017, 114, E5549–E5558. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Jo, H.; Brito, S.; Kwak, B.M.; Park, S.; Lee, M.G.; Bin, B.H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. [Google Scholar] [CrossRef]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef]
- Vidal, M.; Sainte-Marie, J.; Philippot, J.R.; Bienvenue, A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: Evidence precluding a role for “aminophospholipid translocase”. J. Cell. Physiol. 1989, 140, 455–462. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Waldenstrom, A.; Genneback, N.; Hellman, U.; Ronquist, G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS ONE 2012, 7, e34653. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem. Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef]
- Marofi, F.; Alexandrovna, K.I.; Margiana, R.; Bahramali, M.; Suksatan, W.; Abdelbasset, W.K.; Chupradit, S.; Nasimi, M.; Maashi, M.S. MSCs and their exosomes: A rapidly evolving approach in the context of cutaneous wounds therapy. Stem Cell Res. Ther. 2021, 12, 597. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, reviews3005.1. [Google Scholar] [CrossRef]
- Koledova, Z.; Sumbal, J.; Rabata, A.; de La Bourdonnaye, G.; Chaloupkova, R.; Hrdlickova, B.; Damborsky, J.; Stepankova, V. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front. Cell Dev. Biol. 2019, 7, 331. [Google Scholar] [CrossRef]
- Dvorak, P.; Bednar, D.; Vanacek, P.; Balek, L.; Eiselleova, L.; Stepankova, V.; Sebestova, E.; Kunova Bosakova, M.; Konecna, Z.; Mazurenko, S.; et al. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol. Bioeng. 2018, 115, 850–862. [Google Scholar] [CrossRef]
- Dayem, A.A.; Won, J.; Goo, H.G.; Yang, G.M.; Seo, D.S.; Jeon, B.M.; Choi, H.Y.; Park, S.E.; Lim, K.M.; Jang, S.H.; et al. The immobilization of fibronectin- and fibroblast growth factor 2-derived peptides on a culture plate supports the attachment and proliferation of human pluripotent stem cells. Stem Cell Res. 2020, 43, 101700. [Google Scholar] [CrossRef]
- Manfe, V.; Kochoyan, A.; Bock, E.; Berezin, V. Peptides derived from specific interaction sites of the fibroblast growth factor 2-FGF receptor complexes induce receptor activation and signaling. J. Neurochem. 2010, 114, 74–86. [Google Scholar] [CrossRef]
- Rudenko, O.; Tkach, V.; Berezin, V.; Bock, E. Effects of FGF receptor peptide agonists on animal behavior under normal and pathological conditions. Neurosci. Res. 2010, 68, 35–43. [Google Scholar] [CrossRef]
- Olivieri, M.P.; Baier, R.E.; Loomis, R.E. Surface properties of mussel adhesive protein component films. Biomaterials 1992, 13, 1000–1008. [Google Scholar] [CrossRef]
- Lee, S.B.; Abdal Dayem, A.; Kmiecik, S.; Lim, K.M.; Seo, D.S.; Kim, H.T.; Kumar Biswas, P.; Do, M.; Kim, D.H.; Cho, S.G. Efficient improvement of the proliferation, differentiation, and anti-arthritic capacity of mesenchymal stem cells by simply culturing on the immobilized FGF2 derived peptide, 44-ERGVVSIKGV-53. J. Adv. Res. 2024, 62, 119–141. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Hamalainen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 2002, 62, 698–704. [Google Scholar] [CrossRef]
- Matsumura, M.; Kakishita, H.; Suzuki, M.; Banba, N.; Hattori, Y. Dexamethasone suppresses iNOS gene expression by inhibiting NF-kappaB in vascular smooth muscle cells. Life Sci. 2001, 69, 1067–1077. [Google Scholar] [CrossRef]
- Lee, S.H.; Jin, S.Y.; Song, J.S.; Seo, K.K.; Cho, K.H. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef]
- Schlosser, S.; Dennler, C.; Schweizer, R.; Eberli, D.; Stein, J.V.; Enzmann, V.; Giovanoli, P.; Erni, D.; Plock, J.A. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc. Res. 2012, 83, 267–275. [Google Scholar] [CrossRef]
- Patel, A.A.; Mohamed, A.H.; Rizaev, J.; Mallick, A.K.; Qasim, M.T.; Abdulmonem, W.A.; Jamal, A.; Hattiwale, H.M.; Kamal, M.A.; Ahmad, F. Application of mesenchymal stem cells derived from the umbilical cord or Wharton’s jelly and their extracellular vesicles in the treatment of various diseases. Tissue Cell 2024, 89, 102415. [Google Scholar] [CrossRef]
- Debbi, L.; Guo, S.; Safina, D.; Levenberg, S. Boosting extracellular vesicle secretion. Biotechnol. Adv. 2022, 59, 107983. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Menck, K.; Sonmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Soriano, O.; Alcon-Perez, M.; Vicente-Manzanares, M.; Castellano, E. The Crossroads between RAS and RHO Signaling Pathways in Cellular Transformation, Motility and Contraction. Genes 2021, 12, 819. [Google Scholar] [CrossRef]
- Sexton, R.E.; Mpilla, G.; Kim, S.; Philip, P.A.; Azmi, A.S. Ras and exosome signaling. Semin. Cancer Biol. 2019, 54, 131–137. [Google Scholar] [CrossRef]
- Auer, M.; Hausott, B.; Klimaschewski, L. Rho GTPases as regulators of morphological neuroplasticity. Ann. Anat. 2011, 193, 259–266. [Google Scholar] [CrossRef]
- Poujade, M.; Grasland-Mongrain, E.; Hertzog, A.; Jouanneau, J.; Chavrier, P.; Ladoux, B.; Buguin, A.; Silberzan, P. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl. Acad. Sci. USA 2007, 104, 15988–15993. [Google Scholar] [CrossRef]
- Fenteany, G.; Janmey, P.A.; Stossel, T.P. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 2000, 10, 831–838. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Bosca, L.; Zeini, M.; Traves, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Kone, B.C.; Kuncewicz, T.; Zhang, W.; Yu, Z.Y. Protein interactions with nitric oxide synthases: Controlling the right time, the right place, and the right amount of nitric oxide. Am. J. Physiol. Renal. Physiol. 2003, 285, F178–F190. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Dominguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, L.; Cheng, Z.; Peng, Y.; Cao, Z.; Chen, B.; Duan, Y.; Wang, Y. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J. Nanobiotechnol. 2022, 20, 239. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Xiong, Y.; Wang, L.; Zhang, H.; He, F.; Zhao, J.; Liu, S.; Gao, L.; Guo, Y.; et al. Enhancing Cutaneous Wound Healing Based on Human Induced Neural Stem Cell-derived Exosomes. Int. J. Nanomed. 2022, 17, 5991–6006. [Google Scholar] [CrossRef]
- Rose, P.W.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dimitropoulos, D.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Prlic, A.; Quesada, M.; et al. The RCSB Protein Data Bank: New resources for research and education. Nucleic Acids Res. 2013, 41, D475–D482. [Google Scholar] [CrossRef]
- Lim, K.M.; Dayem, A.A.; Choi, Y.; Lee, Y.; An, J.; Gil, M.; Lee, S.; Kwak, H.J.; Vellingirl, B.; Shin, H.J.; et al. High Therapeutic and Esthetic Properties of Extracellular Vesicles Produced from the Stem Cells and Their Spheroids Cultured from Ocular Surgery-Derived Waste Orbicularis Oculi Muscle Tissues. Antioxidants 2021, 10, 1292. [Google Scholar] [CrossRef]
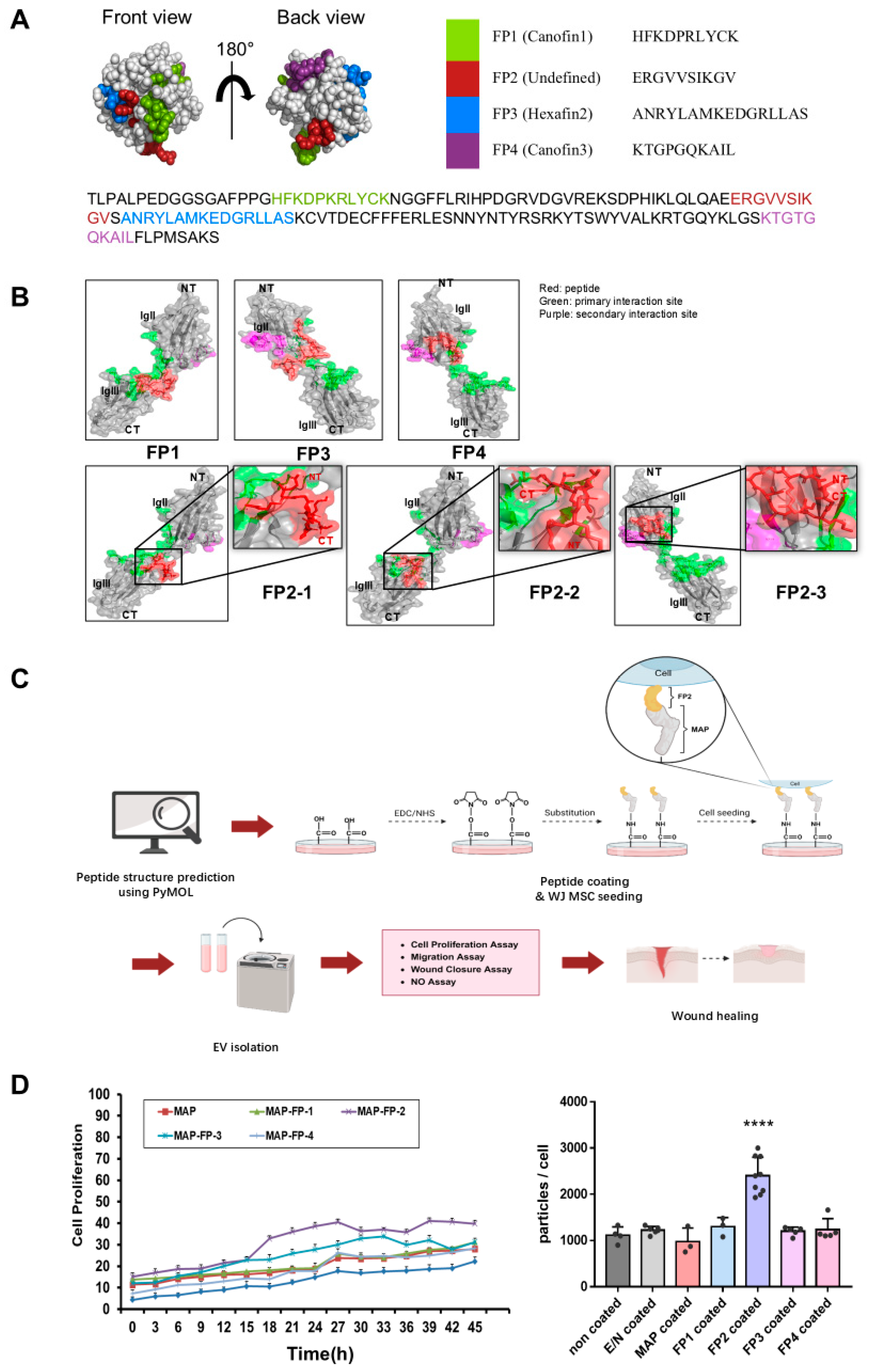
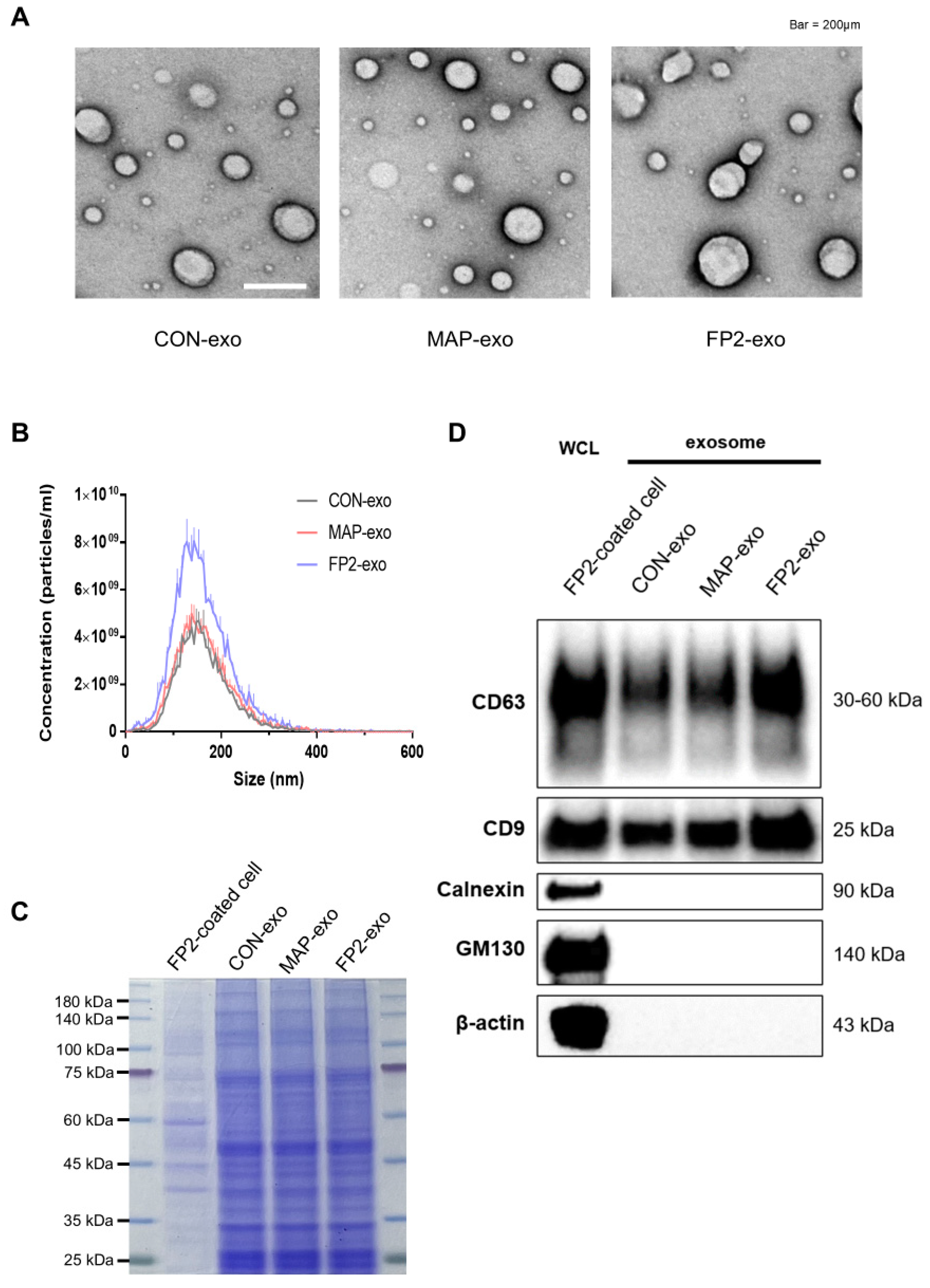

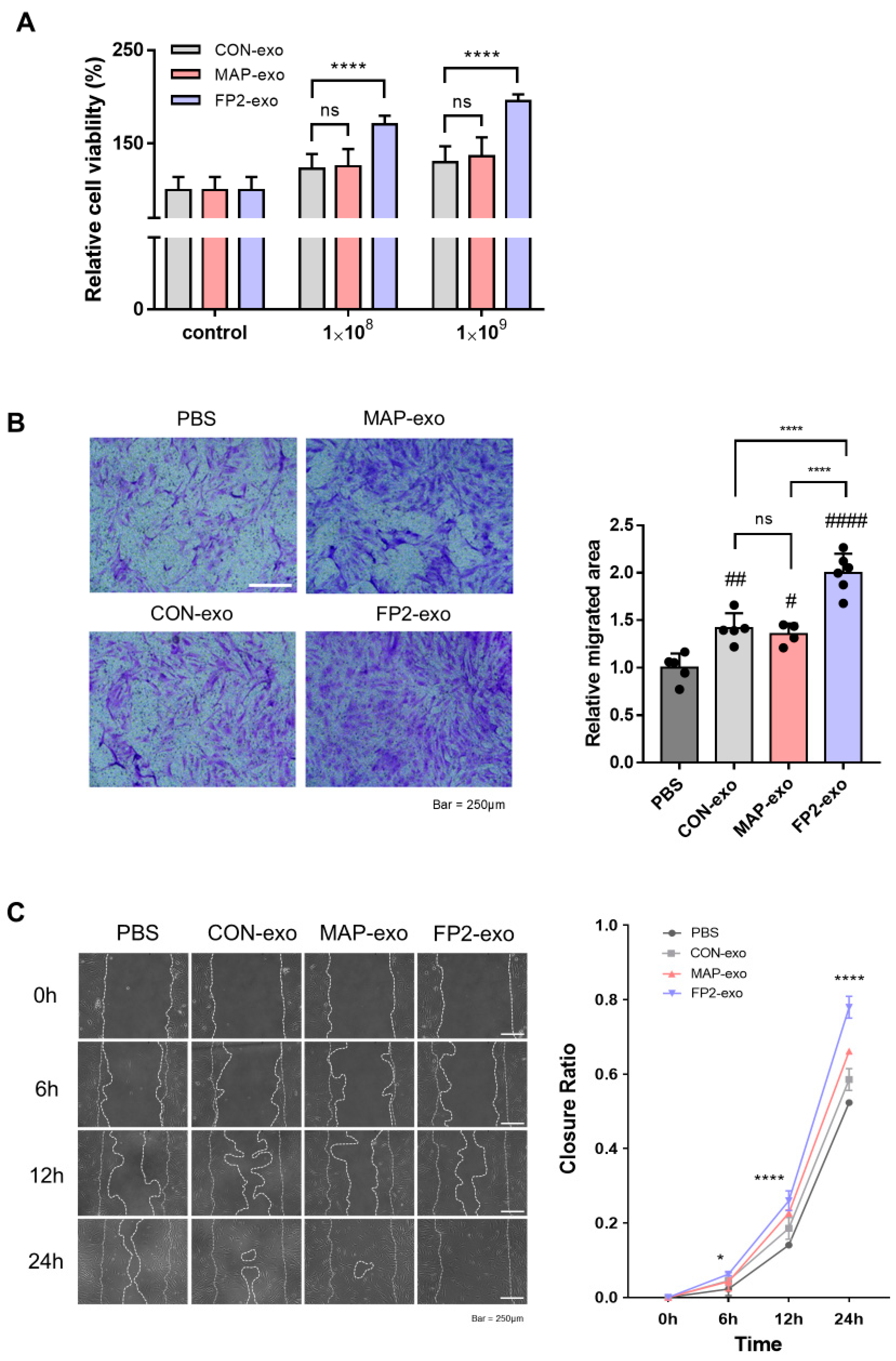
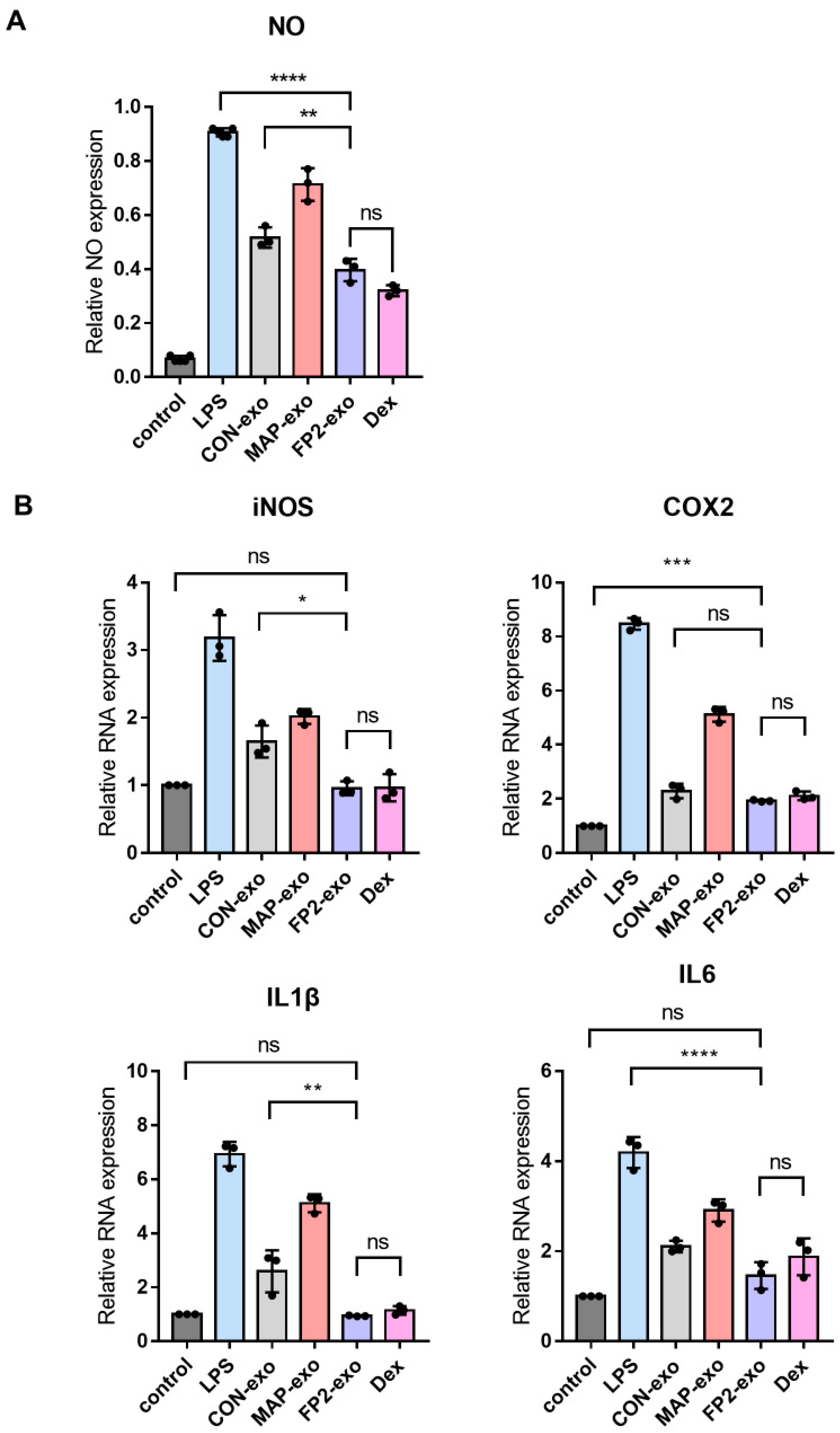
| Gene | Forward Primer Sequence (5′ to 3′) | Reverse Primer Sequence (5′ to 3′) |
|---|---|---|
| hCD63 | CAA CCA CAC TGC TTC GAT CCT G | GAC TCG GTT CTT CGA CAT GGA AG |
| hCD9 | TCG CCA TTG AAA TAG CTG CGG C | CGC ATA GTG GAT GGC TTT CAG C |
| hCDC42 | TGA CAG ATT ACG ACC GCT GAG TT | GGA GTC TTT GGA CAG TGG TGA G |
| hnSMase | GAA GCA CAC CTC AGG ACC AAA G | CAG CCA GTC CTG AAG CAG GTC |
| hRab27b | TAG ACT TTC GGG AAA AAC GTG TG | AGA AGC TCT GTT GAC TGG TGA |
| hRab35 | CAG CCC ATC TTA CTG CAA GCA G | GCT GAC AAC CTG TCG GAG AGA A |
| miNOS | GAG ACA GGG AAG TCT GAA GCA C | CCA GCA GTA GTT GCT CCT CTT C |
| mCOX2 | CTC ACG AAG GAA CTC AGC AC | GGA TTG GAA CAG CAA GGA TTT G |
| mIL1β | TGG ACC TTC CAG GAT GAG GAC A | GTT CAT CTC GGA GCC TGT AGT G |
| mIL6 | TAC CAC TTC ACA AGT CGG AGG C | CTG CAA GTG CAT CAT CGT TGT TC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lim, K.-M.; Bong, H.; Lee, S.-B.; Jeon, T.-I.; Lee, S.-Y.; Park, H.-S.; Kim, J.-Y.; Song, K.; Kang, G.-H.; et al. The Immobilization of an FGF2-Derived Peptide on Culture Plates Improves the Production and Therapeutic Potential of Extracellular Vesicles from Wharton’s Jelly Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 10709. https://doi.org/10.3390/ijms251910709
Lee Y, Lim K-M, Bong H, Lee S-B, Jeon T-I, Lee S-Y, Park H-S, Kim J-Y, Song K, Kang G-H, et al. The Immobilization of an FGF2-Derived Peptide on Culture Plates Improves the Production and Therapeutic Potential of Extracellular Vesicles from Wharton’s Jelly Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2024; 25(19):10709. https://doi.org/10.3390/ijms251910709
Chicago/Turabian StyleLee, Youngseo, Kyung-Min Lim, Hanbit Bong, Soo-Bin Lee, Tak-Il Jeon, Su-Yeon Lee, Hee-Sung Park, Ji-Young Kim, Kwonwoo Song, Geun-Ho Kang, and et al. 2024. "The Immobilization of an FGF2-Derived Peptide on Culture Plates Improves the Production and Therapeutic Potential of Extracellular Vesicles from Wharton’s Jelly Mesenchymal Stem Cells" International Journal of Molecular Sciences 25, no. 19: 10709. https://doi.org/10.3390/ijms251910709







