In Vitro Study of the Differential Anti-Inflammatory Activity of Dietary Phytochemicals upon Human Macrophage-like Cells as a Previous Step for Dietary Intervention
Abstract
1. Introduction
2. Results
2.1. Effect of Phytochemicals Sulforaphane, Sinalbin, Di-Caffeoylquinic Acid and Cyanidin-3,5-Diglucoside on Human Macrophage-like Cell Viability
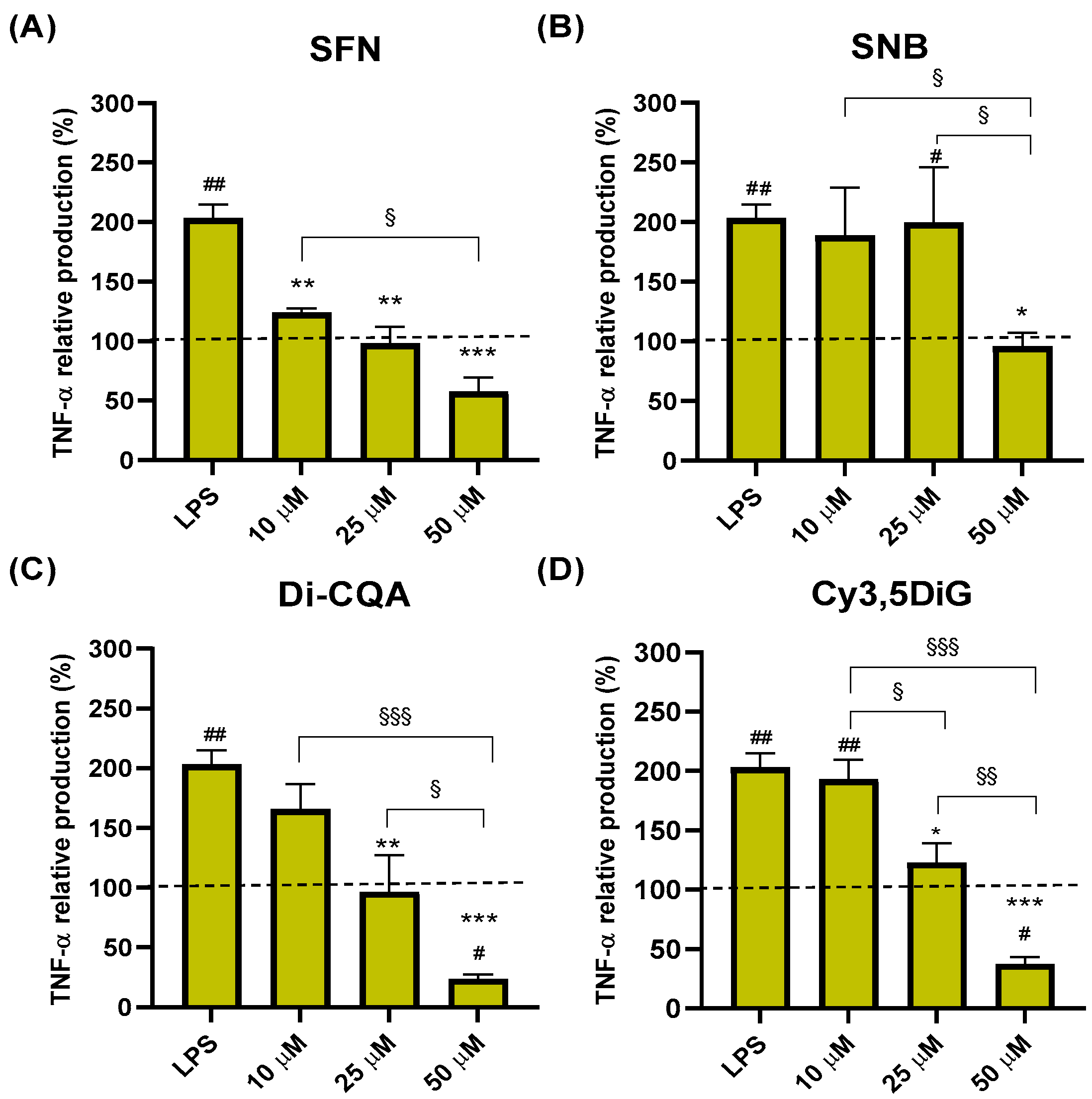
2.2. Effect of Phytochemicals Sulforaphane, Sinalbin, Di-Caffeoylquinic Acid, and Cyanidin-3,5-Diglucoside on TNF-α Production
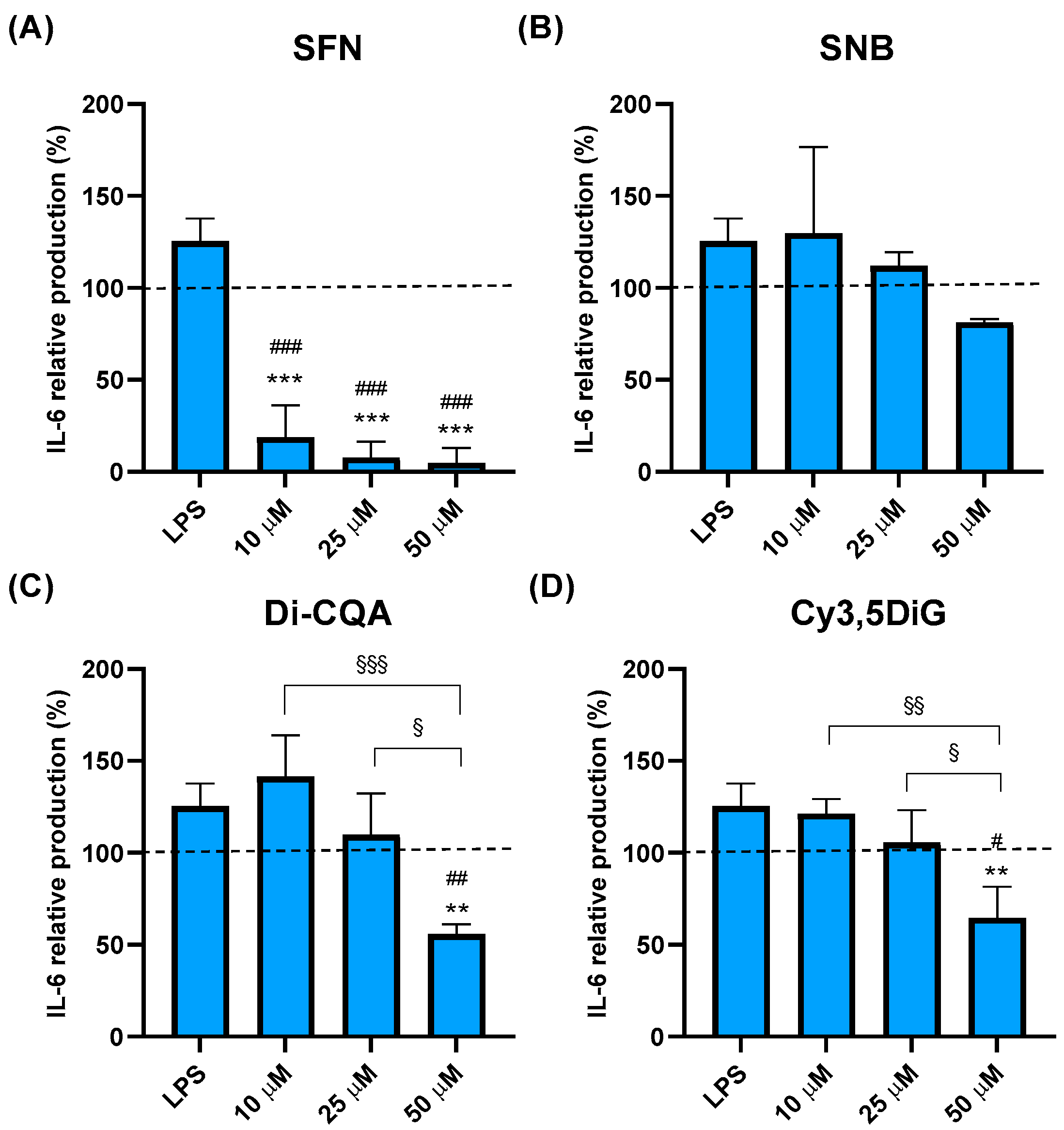
2.3. Effect of Phytochemicals Sulforaphane, Sinalbin, Di-Caffeoylquinic Acid, and Cyanidin-3,5-Diglucoside on IL-6 Production
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Compound Preparation for In Vitro Assays
4.3. Cell Culture
4.4. Cell Viability Assays
4.5. In Vitro Anti-Inflammatory Assays
4.6. Enzyme-Linked Immunosorbent Assays
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–60. [Google Scholar] [CrossRef]
- Knights, K.M.; Mangoni, A.A.; Miners, J.O. Defining the COX inhibitor selectivity of NSAIDs: Implications for understanding toxicity. Expert Rev. Clin. Pharmacol. 2010, 3, 769–776. [Google Scholar] [CrossRef]
- Fine, M. Quantifying the impact of NSAID-associated adverse events. Am. J. Manag. Care 2013, 19, s267–s272. [Google Scholar] [PubMed]
- Poyik, T.; Rasane, P.; Singh, J.; Kaur, S.; Kaur, J.; Gunjal, M.; Avinashe, H.; Dubey, N.; Mahato, D.S. Bioactive Compounds of Mustard, its Role in Consumer Health and in the Development of Potential Functional Foods. Curr. Nutr. Food Sci. 2023, 19, 950–960. [Google Scholar] [CrossRef]
- García-Ibáñez, P.; Núñez-Sánchez, M.A.; Oliva-Bolarín, A.; Martínez.Sánchez, M.A.; Ramos-Molina, B.; Ruiz-Alcaraz, A.J.; Moreno, D.A. Anti-inflammatory potential of digested Brassica sprout extracts in human macrophage-like HL-60 cells. Food Funct. 2023, 14, 112–121. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Hernandez-Canovas, L.; Abellan-Victorio, A.; Ben-Romdhane, O.; Moreno, D.A. The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency. Plants 2022, 11, 2961. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci.USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Gezer, C. Potential health effects if the popular compound of artichoke: Cyanarin. Prog. Nutr. 2017, 19, 5–9. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Protective effect of 3,5-dicaffeoyl-epi-quinic acid against UVB-induced photoaging in human HaCaT keratinocytes. Mol. Med. Rep. 2019, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, Y.; Yang, T.; Zhao, S.; Sun, N.; Tan, H.; Zhang, H.; Wang, C.; Fan, H. Effect of Chlorogenic Acid via Upregulating Resolvin D1 Inhibiting the NF-kappaB Pathway on Chronic Restraint Stress-Induced Liver Inflammation. J. Agric. Food Chem. 2022, 70, 10532–10542. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Martinez-Sanchez, M.A.; Garcia-Penarrubia, P.; Martinez-Esparza, M.; Ramos-Molina, B.; Moreno, D.A. Analysis of the anti-inflammatory potential of Brassica bioactive compounds in a human macrophage-like cell model derived from HL-60 cells. Biomed. Pharmacother. 2022, 149, 112804. [Google Scholar] [CrossRef]
- Wei, L.Y.; Zhang, J.K.; Zheng, L.; Chen, Y. The functional role of sulforaphane in intestinal inflammation: A review. Food Funct. 2022, 13, 514–529. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and meta-inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.D.; Gobbo-Neto, L.; Albarella, L.; de Souza, G.E.; Lopes, N.P. Analgesic activity of di-caffeoylquinic acids from roots of Lychnophora ericoides (Arnica da serra). J. Ethnopharmacol. 2005, 96, 545–549. [Google Scholar] [CrossRef]
- Siriwatanametanon, N.; Heinrich, M. The Thai medicinal plant Gynura pseudochina var. hispida: Chemical composition and in vitro NF-kappaB inhibitory activity. Nat. Prod. Commun. 2011, 6, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wu, T.; Liang, F.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Protective effects of di-caffeoylquinic acids from Artemisia selengensis Turcz leaves against monosodium urate-induced inflammation via the modulation of NLRP3 inflammasome and Nrf2 signaling pathway in THP-1 macrophages. J. Food Biochem. 2022, 46, e14252. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Medina, J.A.; Lopez Vaquera, S.R.; Dominguez-Uscanga, A.; Luna-Vital, D.; Garcia, N. Single anthocyanins effectiveness modulating inflammation markers in obesity: Dosage and matrix composition analysis. Front. Nutr. 2023, 10, 1255518. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef]
- Deepa, P.; Hong, M.; Sowndhararajan, K.; Kim, S. A Review of the Role of an Anthocyanin, Cyanidin-3-O-beta-glucoside in Obesity-Related Complications. Plants 2023, 12, 3889. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Long, Y. Cyanidin-3-O-Glucoside Attenuates Lipopolysaccharide-Induced Inflammation in Human Corneal Epithelial Cells by Inducing Let-7b-5p-Mediated HMGA2/PI3K/Akt Pathway. Inflammation 2020, 43, 1088–1096. [Google Scholar] [CrossRef]
- Molonia, M.S.; Occhiuto, C.; Muscara, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef]
- Molonia, M.S.; Quesada-Lopez, T.; Speciale, A.; Muscara, C.; Saija, A.; Villarroya, F.; Cimino, F. In Vitro Effects of Cyanidin-3-O-Glucoside on Inflammatory and Insulin-Sensitizing Genes in Human Adipocytes Exposed to Palmitic Acid. Chem. Biodivers. 2021, 18, e2100607. [Google Scholar] [CrossRef]
- Wu, C.F.; Wu, C.Y.; Lin, C.F.; Liu, Y.W.; Lin, T.C.; Liao, H.J.; Chang, G.R. The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. Pharmacother. 2022, 151, 113128. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, X.; Xu, Y.; Zhao, Y.; Song, F.; Tian, Z.; Zhao, M.; Liang, Y.; Ling, W.; Mao, Y.H.; et al. Cyanidin-3-O-beta-Glucoside Attenuates Platelet Chemokines and Their Receptors in Atherosclerotic Inflammation of ApoE(-/-) Mice. J. Agric. Food Chem. 2022, 70, 8254–8263. [Google Scholar] [CrossRef] [PubMed]
- Trinei, M.; Carpi, A.; Menabo, R.; Storto, M.; Fornari, M.; Marinelli, A.; Minardi, S.; Riboni, M.; Casciaro, F.; DiLisa, F.; et al. Dietary intake of cyanidin-3-glucoside induces a long-lasting cardioprotection from ischemia/reperfusion injury by altering the microbiota. J. Nutr. Biochem. 2022, 101, 108921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Wan, T.; Huang, Y.; Pang, N.; Jiang, X.; Gu, Y.; Zhang, Z.; Luo, J.; Yang, L. Cyanidin-3-O-beta-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-kappaB signaling pathway. Free. Radic. Biol. Med. 2020, 160, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.U.H.; Ahmed, A.; Rasool, F. The inhibitory potential of chemical constituents of Ficus carica targeting interleukin-6 (IL-6) mediated inflammation. Cell Biochem. Funct. 2023, 41, 573–589. [Google Scholar] [CrossRef]
- Bamias, G.; Corridoni, D.; Pizarro, T.T.; Cominelli, F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine 2012, 59, 451–459. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Boscaro, V.; Boffa, L.; Binello, A.; Amisano, G.; Fornasero, S.; Cravotto, G.; Gallicchio, M. Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts. Molecules 2018, 23, 3004. [Google Scholar] [CrossRef]
- Natella, F.; Maldini, M.; Leoni, G.; Scaccini, C. Glucosinolates redox activities: Can they act as antioxidants? Food Chem. 2014, 149, 226–232. [Google Scholar] [CrossRef]
- Guirado, A.; Lopez Sanchez, J.I.; Ruiz-Alcaraz, A.J.; Bautista, D.; Galvez, J. Synthesis and biological evaluation of 4-alkoxy-6,9-dichloro[1,2,4]triazolo[4,3-a]quinoxalines as inhibitors of TNF-alpha and IL-6. Eur. J. Med. Chem. 2012, 54, 87–94. [Google Scholar] [CrossRef]
| Dose (µM) | |||
|---|---|---|---|
| Compound | 10 | 25 | 50 |
| SFN | 117.0 ± 13.1 | 101.8 ± 11.4 | 69.6 ± 9.51 * |
| SNB | 123.9 ± 13.5 | 122.9 ± 8.8 | 126.6 ± 10.2 |
| Di-CQA | 138.5 ± 4.4 | 130.9 ± 8.4 | 120.2 ± 8.7 |
| Cy3,5DiG | 117.9 ± 5.7 | 106.5 ± 7.1 | 95.5 ± 6.8 |
| Compound | Type | Abbreviation | Formula | Structure | Natural/Food Source |
|---|---|---|---|---|---|
| Sulforaphane | Aliphatic Isothiocyanate | SFN | C6H11NOS2 | 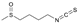 | Glucoraphanin from broccoli, broccolini, cabbage, etc. |
| Sinalbin | Aromatic Isothiocyanate | SNB | C14H18NO10S2K | 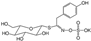 | Glucosinalbin from mustards, horseradish, watercress, etc. |
| Di-caffeoylquinic acid | Hydroxycinnamic acid (phenolic acid) | Di-CQA | C25H24O12 | 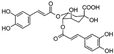 | Chlorogenic acids in mustards, artichoke, eggplant, bay leaf, etc. |
| Cyanidin-3,5-diglucoside | Anthocyanin (flavonoid) | Cy3,5DiG | C27H31O16Cl |  | Natural pigments in berries, beans, sprouts, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Alcaraz, A.J.; Baquero, L.; Pérez-Munar, P.M.; Oliva-Bolarín, A.; Sánchez-Martínez, M.A.; Ramos-Molina, B.; Núñez-Sánchez, M.A.; Moreno, D.A. In Vitro Study of the Differential Anti-Inflammatory Activity of Dietary Phytochemicals upon Human Macrophage-like Cells as a Previous Step for Dietary Intervention. Int. J. Mol. Sci. 2024, 25, 10728. https://doi.org/10.3390/ijms251910728
Ruiz-Alcaraz AJ, Baquero L, Pérez-Munar PM, Oliva-Bolarín A, Sánchez-Martínez MA, Ramos-Molina B, Núñez-Sánchez MA, Moreno DA. In Vitro Study of the Differential Anti-Inflammatory Activity of Dietary Phytochemicals upon Human Macrophage-like Cells as a Previous Step for Dietary Intervention. International Journal of Molecular Sciences. 2024; 25(19):10728. https://doi.org/10.3390/ijms251910728
Chicago/Turabian StyleRuiz-Alcaraz, Antonio J., Lorena Baquero, Paula Martínez Pérez-Munar, Alba Oliva-Bolarín, María A. Sánchez-Martínez, Bruno Ramos-Molina, María A. Núñez-Sánchez, and Diego A. Moreno. 2024. "In Vitro Study of the Differential Anti-Inflammatory Activity of Dietary Phytochemicals upon Human Macrophage-like Cells as a Previous Step for Dietary Intervention" International Journal of Molecular Sciences 25, no. 19: 10728. https://doi.org/10.3390/ijms251910728
APA StyleRuiz-Alcaraz, A. J., Baquero, L., Pérez-Munar, P. M., Oliva-Bolarín, A., Sánchez-Martínez, M. A., Ramos-Molina, B., Núñez-Sánchez, M. A., & Moreno, D. A. (2024). In Vitro Study of the Differential Anti-Inflammatory Activity of Dietary Phytochemicals upon Human Macrophage-like Cells as a Previous Step for Dietary Intervention. International Journal of Molecular Sciences, 25(19), 10728. https://doi.org/10.3390/ijms251910728








