SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes
Abstract
1. Introduction
2. Renal Medullary Hypoxia, Diabetes and the Pathogenesis of CAN
3. SGLT2is and Renal Oxygenation: Aggravating Renal Medullary Hypoxia
4. Clinical Studies Indicating Reduced Risk of CAN in Patients on SGLT2is Undergoing Coronary Interventions
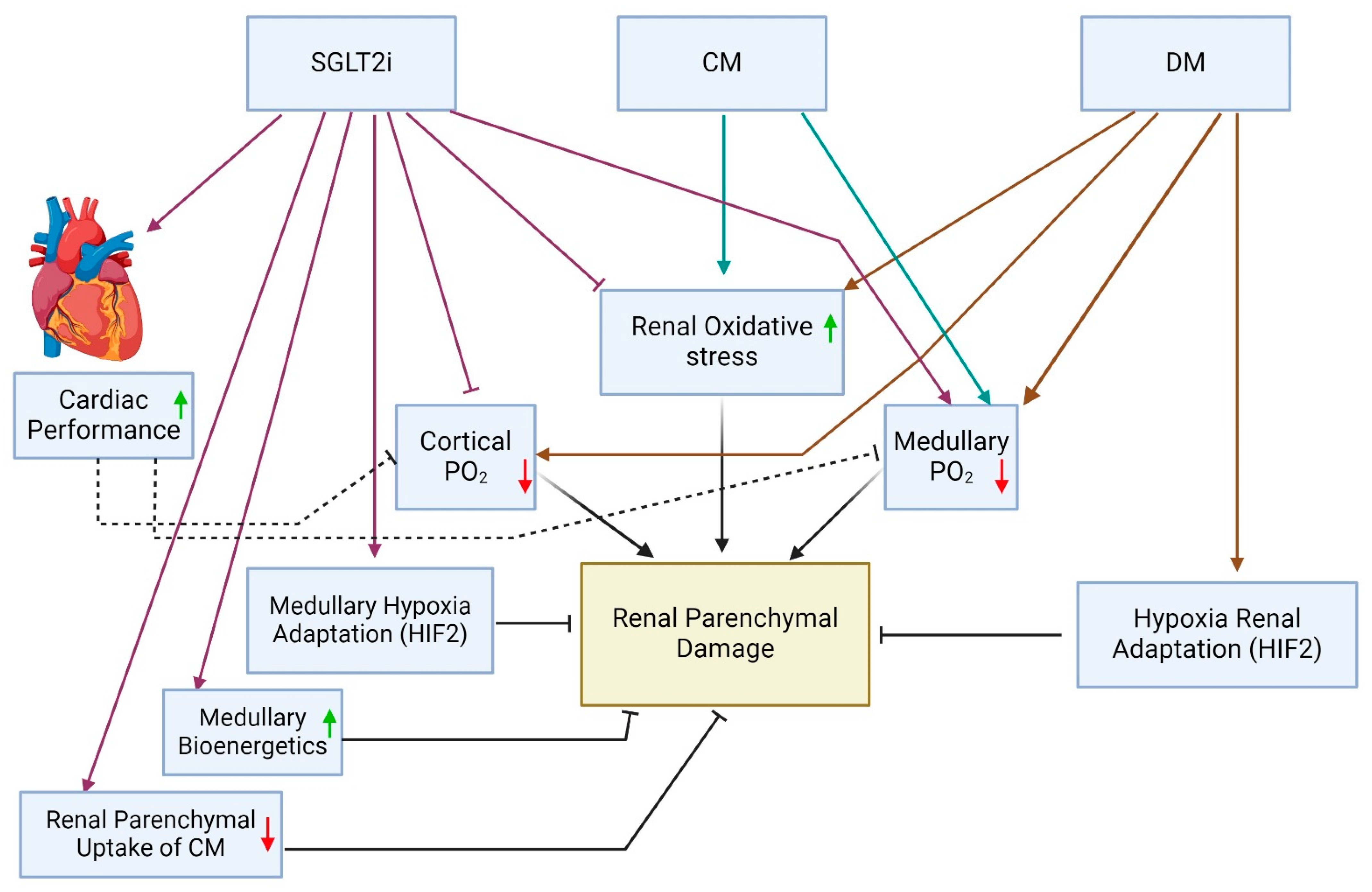
5. Contradictory Clinical and Anticipated Outcomes Regarding the Renal Safety of SGLT2is during CM Studies: Plausible Explanations
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Melzer-Cohen, C.; Pollack, R.; Chodick, G.; Shalev, V. Acute renal outcomes with sodium-glucose co-transporter-2 inhibitors: Real-world data analysis. Diabetes Obes. Metab. 2019, 21, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Ajiro, Y.; Uchida, Y.; Jujo, K.; Iwade, K.; Tanaka, N.; Shimamoto, K.; Tsurumi, Y.; Kawana, M.; Hagiwara, N. Contrast-Induced Nephropathy and Oxygen Pretreatment in Patients With Impaired Renal Function. Kidney Int. Rep. 2018, 3, 65–72. [Google Scholar] [CrossRef]
- Szalat, A.; Perlman, A.; Muszkat, M.; Khamaisi, M.; Abassi, Z.; Heyman, S.N. Can SGLT2 Inhibitors Cause Acute Renal Failure? Plausible Role for Altered Glomerular Hemodynamics and Medullary Hypoxia. Drug Saf. 2018, 41, 239–252. [Google Scholar] [CrossRef]
- Heyman, S.N.; Khamaisi, M.; Rosen, S.; Rosenberger, C.; Abassi, Z. Potential Hypoxic Renal Injury in Patients With Diabetes on SGLT2 Inhibitors: Caution Regarding Concomitant Use of NSAIDs and Iodinated Contrast Media. Diabetes Care 2017, 40, e40–e41. [Google Scholar] [CrossRef]
- Perlman, A.; Heyman, S.N.; Matok, I.; Stokar, J.; Muszkat, M.; Szalat, A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1108–1113. [Google Scholar] [CrossRef]
- Hua, R.; Ding, N.; Guo, H.; Wu, Y.; Yuan, Z.; Li, T. Contrast-Induced Acute Kidney Injury in Patients on SGLT2 Inhibitors Undergoing Percutaneous Coronary Interventions: A Propensity-Matched Analysis. Front. Cardiovasc. Med. 2022, 9, 918167. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Gragnano, F.; Gallinoro, E.; Cesaro, A.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; Sansonetti, A.; et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol. Res. 2023, 187, 106597. [Google Scholar] [CrossRef]
- Kultursay, B.; Yilmaz, C.; Guven, B.; Mutlu, D.; Karagoz, A. Potential renoprotective effect of SGLT2 inhibitors against contrast-induced AKI in diabetic STEMI patients undergoing primary PCI. Kardiol. Pol. 2024, 82, 29–36. [Google Scholar] [CrossRef]
- Feitosa, M.P.M.; Lima, E.G.; Abizaid, A.A.C.; Mehran, R.; Lopes, N.H.M.; de Assis Fischer Ramos, T.; Hideo-Kajita, A.; Filho, R.K.; Junior, C.V.S. The safety of SGLT-2 inhibitors in diabetic patients submitted to elective percutaneous coronary intervention regarding kidney function: SAFE-PCI pilot study. Diabetol. Metab. Syndr. 2023, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, U.; Gurdogan, M. The Effect of SGLT2 Inhibitors on the Development of Contrast-Induced Nephropathy in Diabetic Patients with Non-ST Segment Elevation Myocardial Infarction. Medicina 2023, 59, 505. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Palamara, G.; Requena-Ibanez, J.A.; Vargas, A.P.; Mohebi, R.; Abascal, V.; Moreno, P.; Badimon, J.J. Pretreatment with Sglt2 Inhibitors Ameliorates Contrast-Induced Nephropathy. J. Am. Coll. Cardiol. 2020, 75, 1405. [Google Scholar] [CrossRef]
- Meregildo-Rodriguez, E.D.; Asmat-Rubio, M.G.; Vasquez-Tirado, G.A. SGLT-2 inhibitors and prevention of contrast-induced nephropathy in patients with diabetes undergoing coronary angiography and percutaneous coronary interventions: Systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1307715. [Google Scholar] [CrossRef]
- Brezis, M.; Rosen, S. Hypoxia of the renal medulla--its implications for disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef]
- Heyman, S.N.; Khamaisi, M.; Zorbavel, D.; Rosen, S.; Abassi, Z. Role of Hypoxia in Renal Failure Caused by Nephrotoxins and Hypertonic Solutions. Semin. Nephrol. 2019, 39, 530–542. [Google Scholar] [CrossRef]
- Brezis, M.; Agmon, Y.; Epstein, F.H. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am. J. Physiol. 1994, 267, F1059–F1062. [Google Scholar] [CrossRef]
- Heyman, S.N.; Brezis, M.; Greenfeld, Z.; Rosen, S. Protective role of furosemide and saline in radiocontrast-induced acute renal failure in the rat. Am. J. Kidney Dis. 1989, 14, 377–385. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosen, S.; Epstein, F.H.; Spokes, K.; Brezis, M.L. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994, 45, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Rosenberger, C.; Heyman, S.N.; Eckardt, K.U. Regulation of hypoxia-inducible factor in kidney disease. Clin. Exp. Pharmacol. Physiol. 2013, 40, 148–157. [Google Scholar] [CrossRef]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Fasching, A.; Pihl, L.; Patinha, D.; Franzén, S.; Palm, F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am. J. Physiol. Ren. Physiol. 2015, 309, F227–F234. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.; Khamaisi, M.; Abassi, Z.; Shilo, V.; Weksler-Zangen, S.; Goldfarb, M.; Shina, A.; Zibertrest, F.; Eckardt, K.U.; Rosen, S.; et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008, 73, 34–42. [Google Scholar] [CrossRef]
- Vinovskis, C.; Li, L.P.; Prasad, P.; Tommerdahl, K.; Pyle, L.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.; Rewers, M.; Pragnell, M.; et al. Relative Hypoxia and Early Diabetic Kidney Disease in Type 1 Diabetes. Diabetes 2020, 69, 2700–2708. [Google Scholar] [CrossRef]
- Sorensen, S.S.; Gullaksen, S.; Vernstrom, L.; Ringgaard, S.; Laustsen, C.; Funck, K.L.; Laugesen, E.; Poulsen, P.L. Evaluation of renal oxygenation by BOLD-MRI in high-risk patients with type 2 diabetes and matched controls. Nephrol. Dial. Transplant. 2023, 38, 691–699. [Google Scholar] [CrossRef]
- Rosenberger, C.; Khamaisi, M.; Goldfarb, M.; Shina, A.; Shilo, V.; Zilbertrest, F.; Rosen, S.; Heyman, S.N. Acute kidney injury in the diabetic rat: Studies in the isolated perfused and intact kidney. Am. J. Nephrol. 2008, 28, 831–839. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosen, S.; Rosenberger, C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 288–296. [Google Scholar] [CrossRef]
- Heyman, S.N.; Brezis, M.; Epstein, F.H.; Spokes, K.; Silva, P.; Rosen, S. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991, 40, 632–642. [Google Scholar] [CrossRef]
- Liss, P.; Nygren, A.; Erikson, U.; Ulfendahl, H.R. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int. 1998, 53, 698–702. [Google Scholar] [CrossRef][Green Version]
- Hofmann, L.; Simon-Zoula, S.; Nowak, A.; Giger, A.; Vock, P.; Boesch, C.; Frey, F.J.; Vogt, B. BOLD-MRI for the assessment of renal oxygenation in humans: Acute effect of nephrotoxic xenobiotics. Kidney Int. 2006, 70, 144–150. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosen, S.; Khamaisi, M.; Idee, J.M.; Rosenberger, C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest. Radiol. 2010, 45, 188–195. [Google Scholar] [CrossRef]
- Heyman, S.N.; Brezis, M.; Reubinoff, C.A.; Greenfeld, Z.; Lechene, C.; Epstein, F.H.; Rosen, S. Acute renal failure with selective medullary injury in the rat. J. Clin. Investig. 1988, 82, 401–412. [Google Scholar] [CrossRef]
- Brezis, M.; Heyman, S.N.; Dinour, D.; Epstein, F.H.; Rosen, S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J. Clin. Investig. 1991, 88, 390–395. [Google Scholar] [CrossRef]
- Agmon, Y.; Peleg, H.; Greenfeld, Z.; Rosen, S.; Brezis, M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J. Clin. Investig. 1994, 94, 1069–1075. [Google Scholar] [CrossRef]
- Brezis, M.; Greenfeld, Z.; Shina, A.; Rosen, S. Angiotensin II augments medullary hypoxia and predisposes to acute renal failure. Eur. J. Clin. Investig. 1990, 20, 199–207. [Google Scholar] [CrossRef]
- Sen, T.; Ju, W.; Nair, V.; Ladd, P.; Menon, R.; Otto, E.A.; Pyle, L.; Vigers, T.; Nelson, R.G.; Arnott, C.; et al. Sodium glucose co-transporter 2 inhibition increases epidermal growth factor expression and improves outcomes in patients with type 2 diabetes. Kidney Int. 2023, 104, 828–839. [Google Scholar] [CrossRef]
- Li, Y.; Ren, K. The Mechanism of Contrast-Induced Acute Kidney Injury and Its Association with Diabetes Mellitus. Contrast Media Mol. Imaging 2020, 2020, 3295176. [Google Scholar] [CrossRef]
- McCullough, P.A.; Wolyn, R.; Rocher, L.L.; Levin, R.N.; O’Neill, W.W. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am. J. Med. 1997, 103, 368–375. [Google Scholar] [CrossRef]
- Hirsch, R.; Dent, C.; Pfriem, H.; Allen, J.; Beekman, R.H., 3rd; Ma, Q.; Dastrala, S.; Bennett, M.; Mitsnefes, M.; Devarajan, P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr. Nephrol. 2007, 22, 2089–2095. [Google Scholar] [CrossRef]
- Sun, R.; Guo, Q.; Wang, J.; Zou, Y.; Chen, Z.; Wang, J.; Zhang, Y. Central venous pressure and acute kidney injury in critically ill patients with multiple comorbidities: A large retrospective cohort study. BMC Nephrol. 2022, 23, 83. [Google Scholar] [CrossRef]
- Nikolsky, E.; Mehran, R.; Lasic, Z.; Mintz, G.S.; Lansky, A.J.; Na, Y.; Pocock, S.; Negoita, M.; Moussa, I.; Stone, G.W.; et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005, 67, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Gong, X. Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention. Toxics 2024, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. SGLT2 Inhibition and Tubular Sodium Handling. J. Am. Soc. Nephrol. 2024, 35, 131–133. [Google Scholar] [CrossRef]
- Gullaksen, S.; Vernstrom, L.; Sorensen, S.S.; Ringgaard, S.; Laustsen, C.; Funck, K.L.; Poulsen, P.L.; Laugesen, E. Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: A randomised trial. Diabetologia 2023, 66, 813–825. [Google Scholar] [CrossRef]
- Heyman, S.N.; Armaly, Z.; Hamo-Giladi, D.B.; Abassi, Z. Novel perspectives regarding the physiologic mechanisms by which gliflozins induce reticulocytosis and erythrocytosis. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E621–E623. [Google Scholar] [CrossRef]
- Paliege, A.; Rosenberger, C.; Bondke, A.; Sciesielski, L.; Shina, A.; Heyman, S.N.; Flippin, L.A.; Arend, M.; Klaus, S.J.; Bachmann, S. Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010, 77, 312–318. [Google Scholar] [CrossRef]
- Darawshi, S.; Yaseen, H.; Gorelik, Y.; Faor, C.; Szalat, A.; Abassi, Z.; Heyman, S.N.; Khamaisi, M. Biomarker evidence for distal tubular damage but cortical sparing in hospitalized diabetic patients with acute kidney injury (AKI) while on SGLT2 inhibitors. Ren. Fail. 2020, 42, 836–844. [Google Scholar] [CrossRef]
- Cabuk, G.; Hazir, K.E. Do Sodium-Glucose Cotransporter 2 Inhibitors Decrease the Risk of Contrast-Associated Acute Kidney Injury in Patients with Type II Diabetes Mellitus? Anatol. J. Cardiol. 2024, 28, 222–228. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Cesaro, A.; Gallinoro, E.; Gragnano, F.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; Sansonetti, A.; et al. Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in diabetic patients with acute myocardial infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT registry. Diabetes Res. Clin. Pract. 2023, 202, 110766. [Google Scholar] [CrossRef]
- Bernardini, F.; Nusca, A.; Giannone, S.; Mangiacapra, F.; Melfi, R.; Ricottini, E.; Ussia, G.P.; Grigioni, F. 548 Role of new antidiabetic drugs in the prevention of contrast-induced nephropathy in diabetic patients undergoing percutaneous coronary intervention. Eur. Heart J. Suppl. 2022, 24, suac121.499. [Google Scholar] [CrossRef]
- Basutkar, R.S.; Cutinha, R.M.; Sathish, V.; Shahil, A.; Saneen Ck, N. Impact of SGLT2 Inhibitors on Renal Function in Type 2 Diabetic Patients with Coronary Artery Disease Undergoing Percutaneous Intervention: A Systematic Review and Meta-Analysis. Curr. Diabetes Rev. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, Y.; Bloch-Isenberg, N.; Heyman, S.N.; Khamaisi, M. Renal Functional Recovery Confounding the Assessment of Contrast Nephropathy: Propensity Score Analysis. Am. J. Nephrol. 2021, 52, 76–83. [Google Scholar] [CrossRef]
- Gorelik, Y.; Abassi, Z.; Bloch-Isenberg, N.; Khamaisi, M.; Heyman, S.N. Changing serum creatinine in the detection of acute renal failure and recovery following radiocontrast studies among acutely ill inpatients: Reviewing insights regarding renal functional reserve gained by large-data analysis. Pract. Lab. Med. 2022, 30, e00276. [Google Scholar] [CrossRef]
- Newhouse, J.H.; Kho, D.; Rao, Q.A.; Starren, J. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am. J. Roentgenol. 2008, 191, 376–382. [Google Scholar] [CrossRef]
- Abassi, Z.; Rosen, S.; Lamothe, S.; Heyman, S.N. Why Have Detection, Understanding and Management of Kidney Hypoxic Injury Lagged Behind those for the Heart? J. Clin. Med. 2019, 8, 267. [Google Scholar] [CrossRef]
- Lupu, L.; Rozenfeld, K.L.; Zahler, D.; Morgan, S.; Merdler, I.; Shtark, M.; Goldiner, I.; Banai, S.; Shacham, Y. Detection of Renal Injury Following Primary Coronary Intervention among ST-Segment Elevation Myocardial Infarction Patients: Doubling the Incidence Using Neutrophil Gelatinase-Associated Lipocalin as a Renal Biomarker. J. Clin. Med. 2021, 10, 2120. [Google Scholar] [CrossRef]
- Gorelik, Y.; Bloch-Isenberg, N.; Yaseen, H.; Heyman, S.N.; Khamaisi, M. Acute Kidney Injury After Radiocontrast-Enhanced Computerized Tomography in Hospitalized Patients With Advanced Renal Failure: A Propensity-Score-Matching Analysis. Invest. Radiol. 2020, 55, 677–687. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef]
- Kellum, J.A.; Ronco, C.; Bellomo, R. Conceptual advances and evolving terminology in acute kidney disease. Nat. Rev. Nephrol. 2021, 17, 493–502. [Google Scholar] [CrossRef]
- Tsutsui, H. Recent advances in the pharmacological therapy of chronic heart failure: Evidence and guidelines. Pharmacol. Ther. 2022, 238, 108185. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Heyman, S.N.; Evans, R.G.; Rosen, S.; Rosenberger, C. Cellular adaptive changes in AKI: Mitigating renal hypoxic injury. Nephrol. Dial. Transplant. 2012, 27, 1721–1728. [Google Scholar] [CrossRef]
- Rosenberger, C.; Rosen, S.; Shina, A.; Frei, U.; Eckardt, K.U.; Flippin, L.A.; Arend, M.; Klaus, S.J.; Heyman, S.N. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol. Dial. Transplant. 2008, 23, 3472–3478. [Google Scholar] [CrossRef]
- Locatelli, F.; Minutolo, R.; De Nicola, L.; Del Vecchio, L. Evolving Strategies in the Treatment of Anaemia in Chronic Kidney Disease: The HIF-Prolyl Hydroxylase Inhibitors. Drugs 2022, 82, 1565–1589. [Google Scholar] [CrossRef]
- Wanner, C.; Nangaku, M.; Kraus, B.J.; Zinman, B.; Mattheus, M.; Hantel, S.; Schumacher, M.; Ohneberg, K.; Schmoor, C.; Inzucchi, S.E. How do SGLT2 inhibitors protect the kidney? A mediation analysis of the EMPA-REG OUTCOME trial. Nephrol. Dial. Transplant. 2024, 39, 1504–1513. [Google Scholar] [CrossRef]
- Kwon, O.; Myong, J.P.; Lee, Y.; Choi, Y.J.; Yi, J.E.; Seo, S.M.; Jang, S.W.; Kim, P.J.; Lee, J.M. Sodium-Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction in Patients With Type 2 Diabetes: A Population-Based Investigation. J. Am. Heart Assoc. 2023, 12, e027824. [Google Scholar] [CrossRef]
- Chen, J.Y.; Pan, H.C.; Shiao, C.C.; Chuang, M.H.; See, C.Y.; Yeh, T.H.; Yang, Y.; Chu, W.K.; Wu, V.C. Impact of SGLT2 inhibitors on patient outcomes: A network meta-analysis. Cardiovasc. Diabetol. 2023, 22, 290. [Google Scholar] [CrossRef]
- Chen, X.; Hocher, C.F.; Shen, L.; Kramer, B.K.; Hocher, B. Reno- and cardioprotective molecular mechanisms of SGLT2 inhibitors beyond glycemic control: From bedside to bench. Am. J. Physiol. Cell Physiol. 2023, 325, C661–C681. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits IL-1beta-Mediated Inflammatory Response in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Foller, M.; Feger, M. The impact of SGLT2 inhibitors on alphaKlotho in renal MDCK and HK-2 cells. Front. Endocrinol. 2023, 14, 1069715. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Wu, D.; Li, X.; Guo, X.; Shi, S.; Lin, K. Biomarkers for the early prediction of contrast-induced nephropathy after percutaneous coronary intervention in adults: A systematic review and meta-analysis. Angiology 2022, 73, 207–217. [Google Scholar] [CrossRef]
- Seibert, F.S.; Heringhaus, A.; Pagonas, N.; Rudolf, H.; Rohn, B.; Bauer, F.; Timmesfeld, N.; Trappe, H.J.; Babel, N.; Westhoff, T.H. Biomarkers in the prediction of contrast media induced nephropathy—The BITCOIN study. PLoS ONE 2020, 15, e0234921. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosenberger, C.; Rosen, S.; Khamaisi, M. Why Is Diabetes Mellitus a Risk Factor for Contrast-Induced Nephropathy? Biomed. Res. Int. 2013, 2013, 123589. [Google Scholar] [CrossRef]
- Seeliger, E.; Flemming, B.; Wronski, T.; Ladwig, M.; Arakelyan, K.; Godes, M.; Mockel, M.; Persson, P.B. Viscosity of contrast media perturbs renal hemodynamics. J. Am. Soc. Nephrol. 2007, 18, 2912–2920. [Google Scholar] [CrossRef]
- Zang, J.; Liang, J.; Zhang, X.; Sang, D.; Duan, X.; Wang, Z.; Wei, W.; Wu, G. Short term sodium glucose transport protein 2 inhibitors are associated with post contrast acute kidney injury in patients with diabetes. Sci. Rep. 2024, 14, 22937. [Google Scholar] [CrossRef]
| Study | No. of Patients | Study Population | Contrast Volume (mL) | Baseline eGFR | CAN Definition | Statistical Methods | AKI Outcomes in Patients Given SGLT2i |
|---|---|---|---|---|---|---|---|
| Kültürsay et al. [10] | 130 of 295 on SGLT2is empagliflozin or dapagliflozin exposure for at least 6 months | Retrospective single-center study. Patients undergoing PCI for STEMI | Mean 291 and 265 mL in SGLT2i and control groups, respectively | ≥30 mL/min/1.73 m2 | A rise in sCr of ≥0.3 mg/dL above baseline within 48 h of contrast media exposure or an increase of at least 1.5 times the baseline value within 7 days | Doubly robust inverse probability weighted logistic regression analysis | AKI: lower occurrence, OR 0.86; 95% CI [0.76–0.98]; (p = 0.028) |
| Paolisso et al. [9] | 111 of 646 on SGLT2i chronic SGLT2-I therapy (started at least 3 months before hospitalization) | Retrospective single-center study. Patients with AMI (STEMI and non-STEMI) | Median 180 mL [IQR 140–240] | ≥30 mL/min/1.73 m2 | Not clearly defined | Multivariable Cox regression model | AKI: lower occurrence, 5.4% vs. 13.1% (p = 0.022) |
| Özkan and Gürdoğan [12] | 104 of 312 on SGLT2is, duration and timing of SGLT2 inhibitors were not indicated | Retrospective single-center study. Patients undergoing angiography with or without PCI | Mean 170 mL | ≥30 mL/min/1.73 m2 | sCr rise > 0.5 mg/dL or >25% above baseline within 48 h, or >1.5 times above baseline within 7 days or a urinary output of less than 0.5 mL/kg/h for at least 6 h | Multivariate binary logistic regression analysis | AKI: lower occurrence, OR 0.41, 95% CI [0.142–0.966]; p = 0.004 |
| Hua et al. [8] | 242 on SGLT2is matched with 242 non-users, canagliflozin, empagliflozin or dapagliflozin for at least 6 months till the date of PCI | Retrospective single-center study. Patients undergoing angiography with or without PCI | Mean 141 and 149 mL in the SGLT2i and control groups, respectively | serum creatinine ≤ 2.5 ng/dL | sCr rise ≥ 0.5 mg/dL or >1.25 times above baseline within 72 h | Propensity score matching followed by McNemar’s test | AKI: lower occurrence, OR 0.37; 95% CI [0.19–0.67]; (p < 0.01) |
| Meregildo-Rodriguez et al. [14] | 512 of 2572 on SGLT2is, the mean time of SGLT2 I therapy duration was 7.3 ± 3 months | Meta analysis of 4 observational studies following coronary angiography with or without PCI | Not reported | Not reported | Absolute increase in sCr by 0.3 to 0.5 mg/dL or 25 to 50% relative increase within 48–72 h following coronary intervention | Meta analysis | AKI: lower occurrence, RR 0.37; 95% CI [0.24–0.58] |
| Feitosa MPM et al. [11] | 22 patients on iSGLT-2 and 20 controls. The SGLT2i empagliflozin 25 mg daily was initiated at least 15 days before PCI and maintained until the end of the follow-up period | Prospective single-center open-label, randomized study of patients undergoing elective PCI | 144 ± 66 mL in the SGLT2i users vs. 176 ± 54 mL in non-users | 62.1 ± 22.5 mL/min in SGLT2i users and 68.2 ± 17.7 mL/min in non-users | A 25% increase in baseline creatinine or an absolute increase of 0.5 mg/dL between 48 and 72 h after contrast administration | Chi-square test | There was no difference in the primary endpoint of the study |
| Çabuk and Hazır [48] | 133 patients with DM on SGLT-2i matched with 212 non-users, patients who were using an SGLT2 inhibitor (empagliflozin or dapagliflozin) for at least 6 months | Cross-sectional and single-center study. Patients underwent CAG and/or PCI | 160.42 (±70.31) mL in the SGLT2i users vs. 158.72 (±81.24) mL in non-users | 71.44 mL/min (57.04–86.22) vs. 66.08 mL/min (52.23–84.16) | Increase in serum creatinine of ≥0.5 mg/dL or an absolute increase of ≥25% from baseline 72 h after CM exposure | Wilcoxon signed-rank test | CA-AKI incidence was significantly lower in patients using SGLT2 inhibitors (9.0%) compared with non-users (26.4%, p < 0.001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyman, S.N.; Aronson, D.; Abassi, Z. SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes. Int. J. Mol. Sci. 2024, 25, 10759. https://doi.org/10.3390/ijms251910759
Heyman SN, Aronson D, Abassi Z. SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes. International Journal of Molecular Sciences. 2024; 25(19):10759. https://doi.org/10.3390/ijms251910759
Chicago/Turabian StyleHeyman, Samuel N., Doron Aronson, and Zaid Abassi. 2024. "SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes" International Journal of Molecular Sciences 25, no. 19: 10759. https://doi.org/10.3390/ijms251910759
APA StyleHeyman, S. N., Aronson, D., & Abassi, Z. (2024). SGLT2 Inhibitors and the Risk of Contrast-Associated Nephropathy Following Angiographic Intervention: Contradictory Concepts and Clinical Outcomes. International Journal of Molecular Sciences, 25(19), 10759. https://doi.org/10.3390/ijms251910759





