Group VA Aromatic Thiosemicarbazone Complexes: Synthesis, Characterization, Biological Activity, and Topological Studies
Abstract
:1. Introduction
2. Results and Discussion
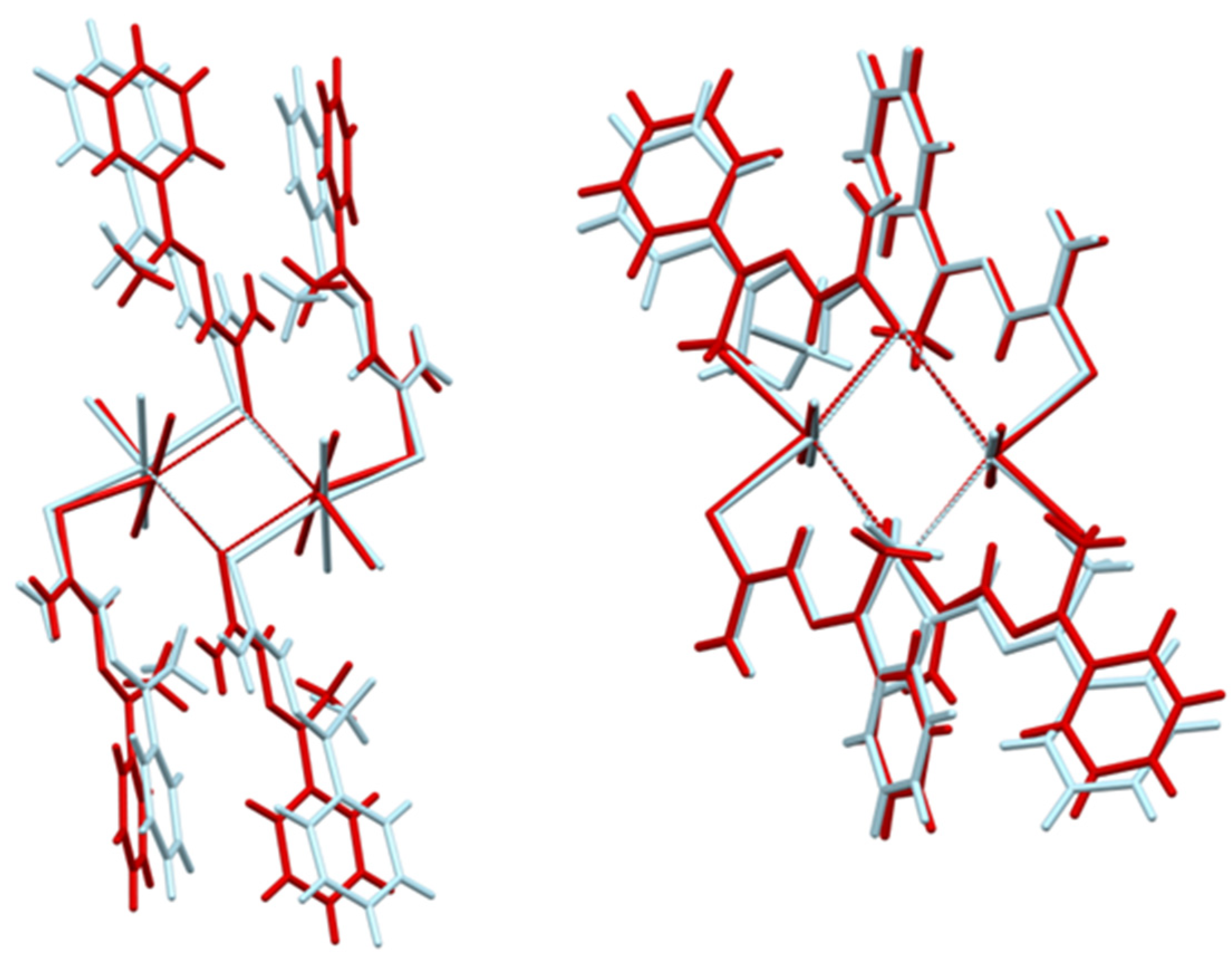
2.1. Structural Analysis
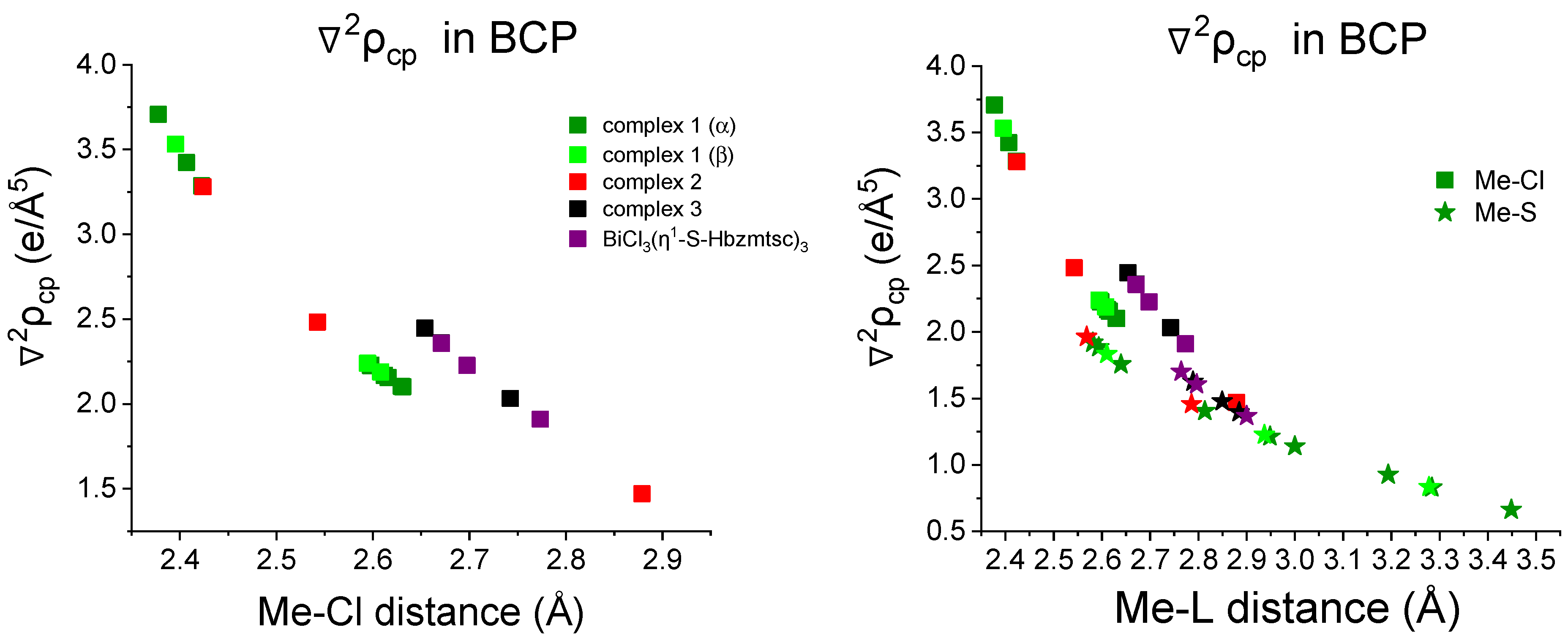
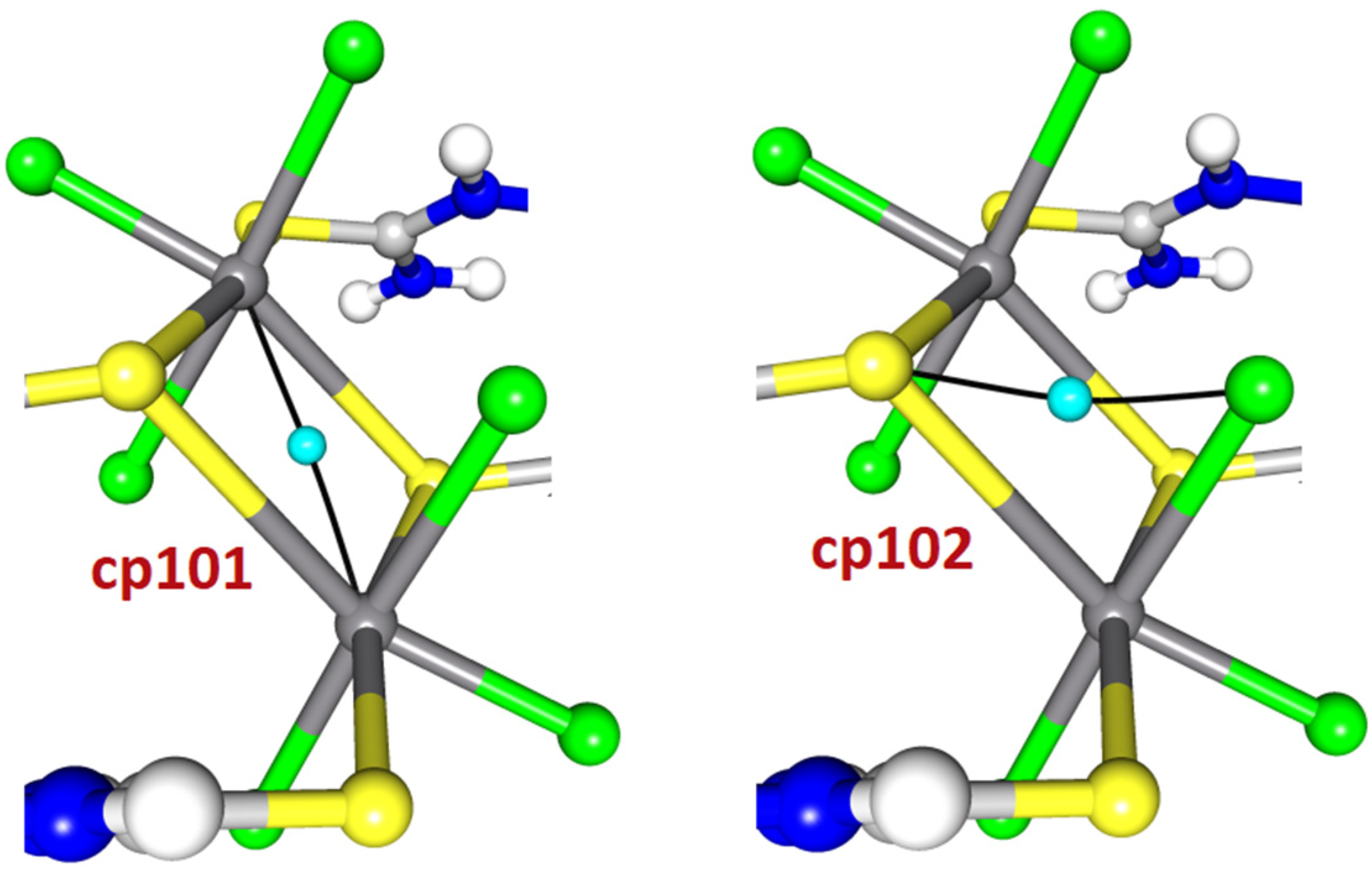
2.2. Topological Analysis
2.3. Spectroscopy
2.4. Thermal Analysis
2.5. Stability of Antimony (III) and Bismuth (III) Complexes in Solution
2.6. Biological Results
2.6.1. Antiproliferative Study
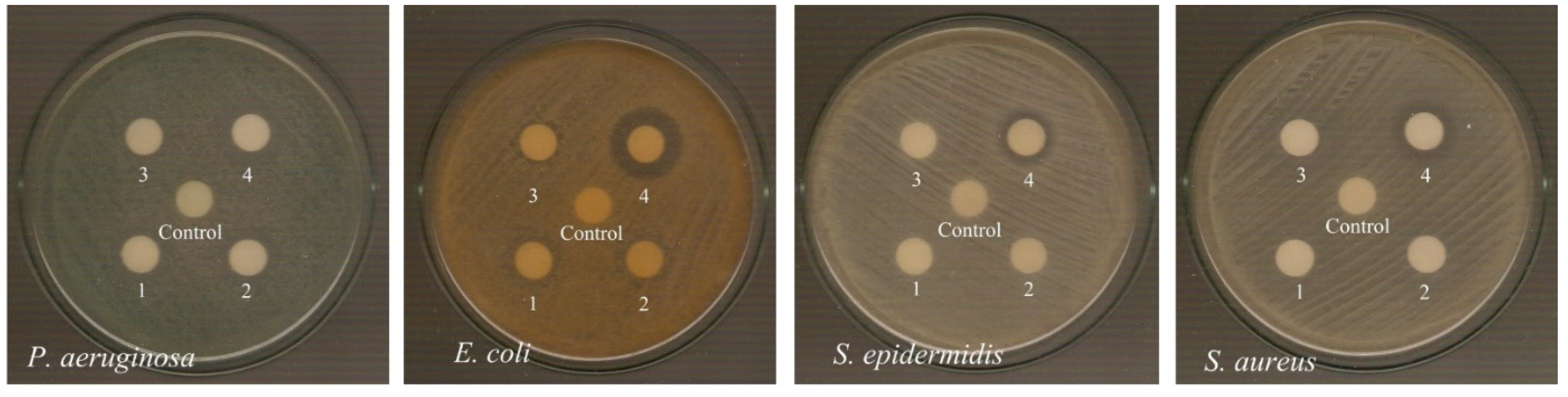
2.6.2. Antimicrobial Study
3. Materials and Methods
3.1. Materials and Instruments
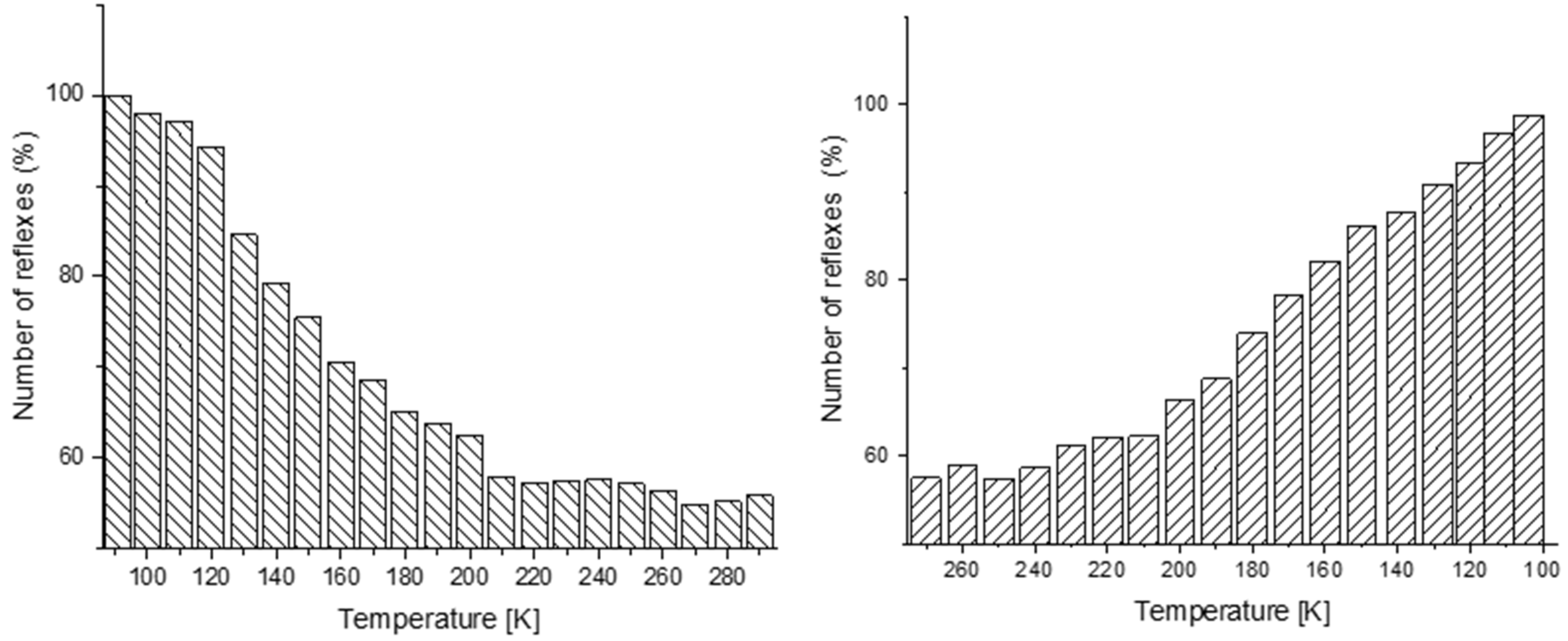
3.2. Single Crystal X-ray Measurement and Refinement
3.3. Powder Crystal X-ray Measurement
3.4. Preparation of Complexes
3.4.1. Preparation of {[SbCl3(µ2-S-Hacptsc)(η1-S-Hacptsc)], 2/3H2O,1/3CH2Cl2 (1)
3.4.2. Preparation of {[SbCl3(κ2-S, N-Hacpmtsc)(η1-S-Hacpmtsc)].CH2Cl2} (2)
3.4.3. Preparation of {[BiCl3(η1-S-Hbzmtsc)3]·C2H5OH} (3)
3.5. Antiproliferative Study
3.6. Determination of the Inhibition Zone (IZ) through Agar Disk-Diffusion Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCallum, I. Antimony in Medicinal History: An Account of the Medical Uses of Antimony and Its Compounds since Early Times to the Present; Pentland Press: Edinburgh, UK; Cambridge, UK, 1999. [Google Scholar]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef] [PubMed]
- European Commission: Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs. Study on the Critical Raw Materials for the EU 2023—Final Report; Grohol, M., Veeh, C., Eds.; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 9 September 2024).
- Rosário, J.d.S.; Moreira, F.H.; Rosa, L.H.F.; Guerra, W.; Silva-Caldeira, P.P. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules 2023, 28, 5921. [Google Scholar] [CrossRef]
- Keogan, D.M.; Griffith, D.M. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Bialek, B.; Hansel, R. Medical Use of Bismuth: The Two Sides of the Coin. J. Clinic. Toxicol. 2011, 3. [Google Scholar]
- Hadjikakou, S.; Ozturk, I.; Banti, C.; Kourkoumelis, N.; Hadjiliadis, N. Recent advances on antimony (III/V) compounds with potential activity against tumor cells. J. Inorg. Biochem. 2015, 153, 293–305. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis(thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef] [PubMed]
- Palamarciuc, O.; Milunović, M.N.M.; Sîrbu, A.; Stratulat, E.; Pui, A.; Gligorijevic, N.; Radulovic, S.; Kožíšek, J.; Darvasiová, D.; Rapta, P.; et al. Investigation of the cytotoxic potential of methyl imidazole-derived thiosemicarbazones and their copper(ii) complexes with dichloroacetate as a co-ligand. New J. Chem. 2018, 43, 1340–1357. [Google Scholar] [CrossRef]
- da Silva, A.P.; Martini, M.V.; de Oliveira, C.M.; Cunha, S.; de Carvalho, J.E.; Ruiz, A.L.; da Silva, C.C. Antitumor activity of (−)-α-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti–Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Aygun, O.; Grześkiewicz, A.M.; Banti, C.N.; Hadjikakou, S.K.; Kubicki, M.; Ozturk, I.I. Monomeric octahedral bismuth (III) benzaldehyde-N1-alkyl thiosemicarbazones: Synthesis, characterization and biological properties. Polyhedron 2022, 215, 115683. [Google Scholar] [CrossRef]
- Ozturk, I.; Banti, C.; Hadjikakou, S.; Panagiotou, N.; Tasiopoulos, A. Bismuth (III) halide complexes of aromatic thiosemicarbazones: Synthesis, structural characterization and biological evaluation. Polyhedron 2021, 208, 115388. [Google Scholar] [CrossRef]
- Grześkiewicz, A.M.; Dutkiewicz, G.; Aygun, O.; Ozturk, I.I.; Kubicki, M. Solid-to-solid polymorphic phase transitions in two isostructural Bi (III) complexes with 1-phenylethyl-N-ethylthiosemicarbazide and halogens. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990; ISBN 9780198558651. [Google Scholar]
- Ozturk, I.I.; Sirinkaya, E.T.; Cakmak, M.; Gürgan, M.; Ceyhan, D.; Panagiotou, N.; Tasiopoulos, A.J. Structural and biological features of bismuth (III) halide complexes with heterocyclic thioamides. J. Mol. Struct. 2020, 1227, 129730. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Macchi, P.; Proserpio, D.M.; Sironi, A. Experimental Electron Density in a Transition Metal Dimer: Metal–Metal and Metal–Ligand Bonds. J. Am. Chem. Soc. 1998, 120, 13429–13435. [Google Scholar] [CrossRef]
- Bianchi, R.; Gervasio, G.; Marabello, D. Experimental Electron Density Analysis of Mn2(CO)10: Metal–Metal and Metal–Ligand Bond Characterization. Inorg. Chem. 2000, 39, 2360–2366. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Haribabu, J.; Arulkumar, R.; Mahendiran, D.; Jeyalakshmi, K.; Swaminathan, S.; Venuvanalingam, P.; Bhuvanesh, N.; Santibanez, J.F.; Karvembu, R. Effect of coordination mode of thiosemicarbazone on the biological activities of its Ru(II)-benzene complexes: Biomolecular interactions and anticancer activity via ROS-mediated mitochondrial apoptosis. Inorganica Chim. Acta 2024, 565. [Google Scholar] [CrossRef]
- Lobana, T.S.; Khanna, S.; Sharma, R.; Hundal, G.; Sultana, R.; Chaudhary, M.; Butcher, R.J.; Castineiras, A. Versatility of Thiosemicarbazones in the Construction of Monomers, Dimers and Hydrogen-Bonded Networks of Silver(I) Complexes. Cryst. Growth Des. 2008, 8, 1203–1212. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Yarar, S.; Gürgan, M.; Ceyhan, D.; Panagiotou, N.; Tasiopoulos, A.J.; Demirkesen, S.; Aral, C. Novel binuclear antimony(III) halide complexes of 5-methoxy-2-mercaptobenzimidazole: Synthesis, structural characterization, and biological studies. J. Coord. Chem. 2020, 73, 485–505. [Google Scholar] [CrossRef]
- Turk, K.; Grześkiewicz, A.M.; Banti, C.N.; Hadjikakou, S.K.; Kubicki, M.; Ozturk, I.I. Synthesis, characterization, and biological properties of mono-, di- and poly-nuclear bismuth(III) halide complexes containing thiophene-2-carbaldehyde thiosemicarbazones. J. Inorg. Biochem. 2022, 237, 111987. [Google Scholar] [CrossRef] [PubMed]
- Urgut, O.; Ozturk, I.; Banti, C.; Kourkoumelis, N.; Manoli, M.; Tasiopoulos, A.; Hadjikakou, S. Addition of tetraethylthiuram disulfide to antimony(III) iodide; synthesis, characterization and biological activity. Inorganica Chim. Acta 2016, 443, 141–150. [Google Scholar] [CrossRef]
- Han, A.; Ozturk, I.; Banti, C.; Kourkoumelis, N.; Manoli, M.; Tasiopoulos, A.; Owczarzak, A.; Kubicki, M.; Hadjikakou, S. Antimony(III) halide compounds of thioureas: Structures and biological activity. Polyhedron 2014, 79, 151–160. [Google Scholar] [CrossRef]
- Ozturk, I.; Filimonova, S.; Hadjikakou, S.K.; Kourkoumelis, N.; Dokorou, V.; Manos, M.J.; Tasiopoulos, A.J.; Barsan, M.M.; Butler, I.S.; Milaeva, E.R.; et al. Structural Motifs and Biological Studies of New Antimony(III) Iodide Complexes with Thiones. Inorg. Chem. 2009, 49, 488–501. [Google Scholar] [CrossRef]
- Ozturk, I.; Yarar, S.; Banti, C.; Kourkoumelis, N.; Chrysouli, M.; Manoli, M.; Tasiopoulos, A.; Hadjikakou, S. QSAR studies on antimony(III) halide complexes with N-substituted thiourea derivatives. Polyhedron 2017, 123, 152–161. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Turk, K.; Grześkiewicz, A.M.; Kubicki, M.; Banti, C.N.; Hadjikakou, S.K. Heteroleptic six-coordinate bismuth(iii) complexes with 2-acetylthiophene thiosemicarbazones: Synthesis, characterization, and biological properties. New J. Chem. 2023, 47, 12779–12789. [Google Scholar] [CrossRef]
- Arda, M.; Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Manoli, M.; Tasiopoulos, A.J.; Hadjikakou, S.K. Novel bismuth compounds: Synthesis, characterization and biological activity against human adenocarcinoma cells. RSC Adv. 2016, 6, 29026–29044. [Google Scholar] [CrossRef]
- Yarar, S.; Ozturk, I.; Banti, C.; Panagiotou, N.; Papatriantafyllopoulou, C.; Manoli, M.; Manos, M.; Tasiopoulos, A.; Hadjikakou, S. Synthesis, characterization and cytotoxic properties of bismuth(III) chloride complexes with heterocyclic thioamides. Inorg. Chim. Acta 2018, 471, 23–33. [Google Scholar] [CrossRef]
- Ozturk, I.; Banti, C.; Hadjikakou, S.; Panagiotou, N.; Tasiopoulos, A. Structural architectures and biological properties of main group bismuth(III) iodide complexes with heterocyclic thioamides. Inorg. Chim. Acta 2019, 497, 119094. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- CrysAlisPRO. Oxford Diffraction; Agilent Technologies UK Ltd.: Oxfordshire, UK, 2022. [Google Scholar]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGXsuite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Jelsch, C.; Guillot, B.; Lagoutte, A.; Lecomte, C. Advances in Proteins and Small Molecules. Charge Density Refinement Methods using software MoPro. J. Appl. Cryst. 2005, 38, 38–54. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Knížek, K. Kalvados—Software for Crystal Structure and Powder Diffraction. Available online: https://www.fzu.cz/~knizek/kalvados/obr.html (accessed on 9 September 2024).
- Banti, C.N.; Papatriantafyllopoulou, C.; Manoli, M.; Tasiopoulos, A.J.; Hadjikakou, S.K. Nimesulide Silver Metallodrugs, Containing the Mitochondriotropic, Triaryl Derivatives of Pnictogen; Anticancer Activity against Human Breast Cancer Cells. Inorg. Chem. 2016, 55, 8681–8696. [Google Scholar] [CrossRef]
- Milionis, I.; Banti, C.N.; Sainis, I.; Raptopoulou, C.P.; Psycharis, V.; Kourkoumelis, N.; Hadjikakou, S.K. Silver ciprofloxacin (CIPAG): A successful combination of chemically modified antibiotic in inorganic–organic hybrid. JBIC J. Biol. Inorg. Chem. 2018, 23, 705–723. [Google Scholar] [CrossRef]
- Chakansin, C.; Yostaworaku, J.; Warin, C.; Kulthong, K.L.; Boonrungsiman, S. Resazurin rapid screening for antibacterial activities of organic andinorganic nanoparticles: Potential, limitations and precautions. Anal. Biochem. 2022, 637, 114449. [Google Scholar] [CrossRef] [PubMed]
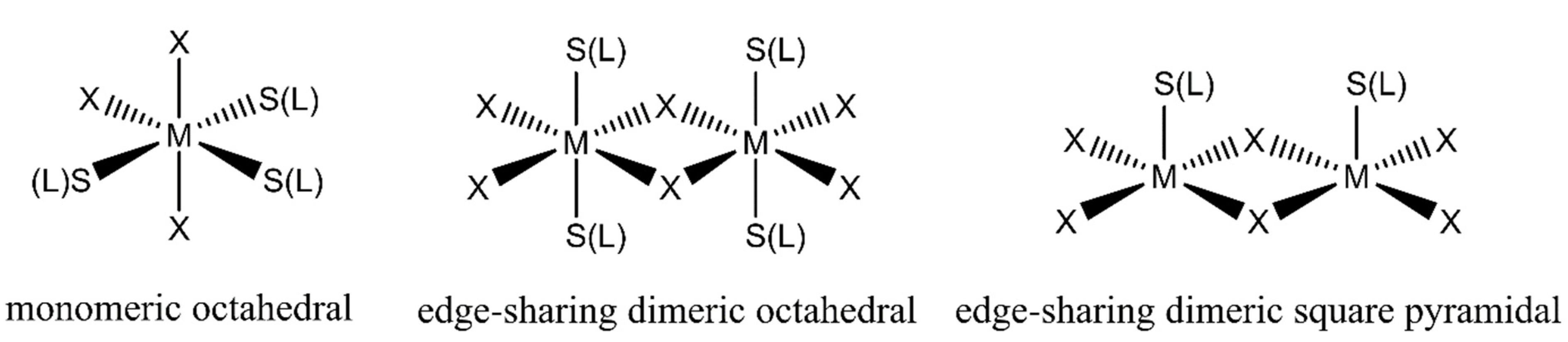


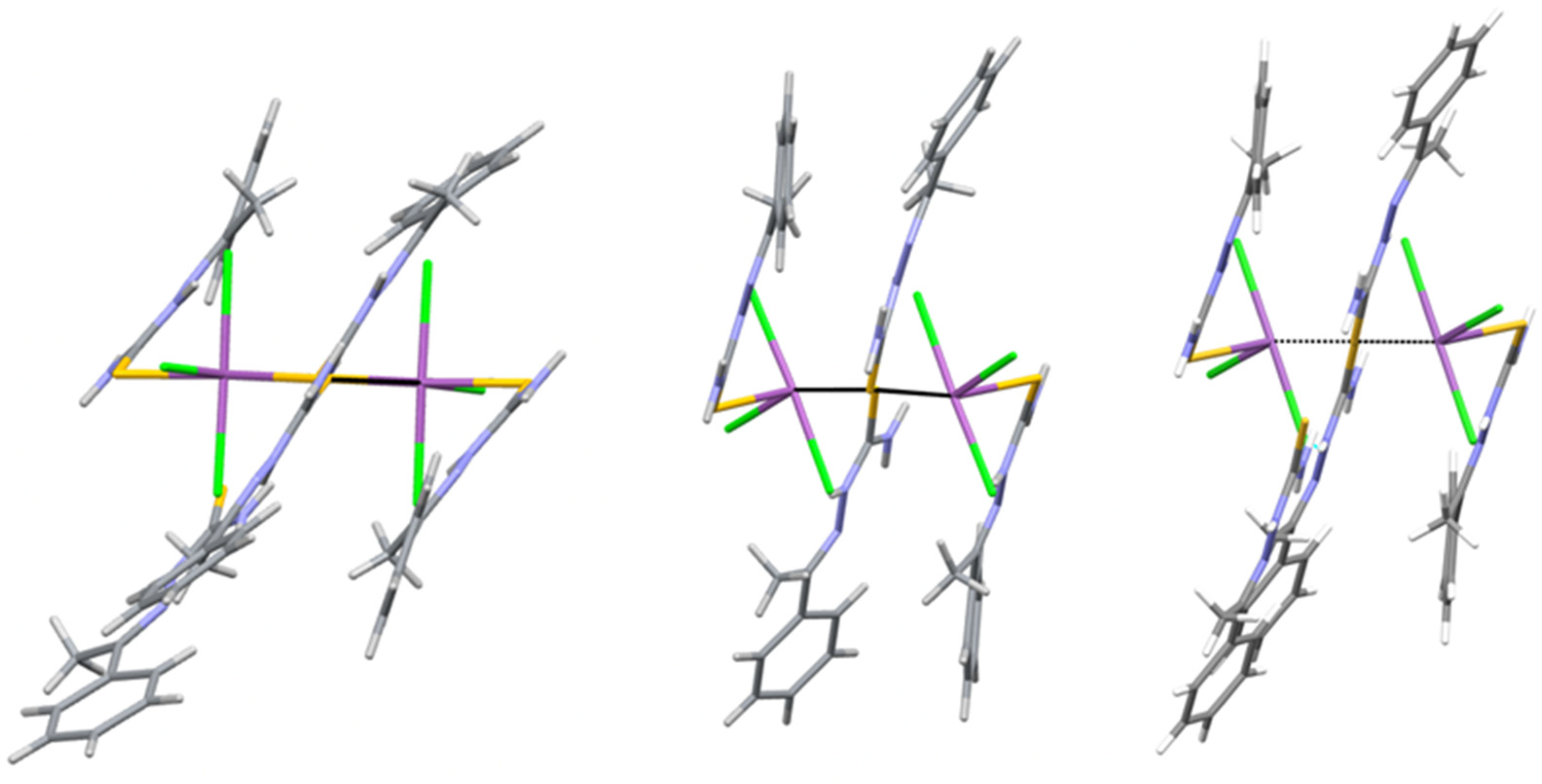
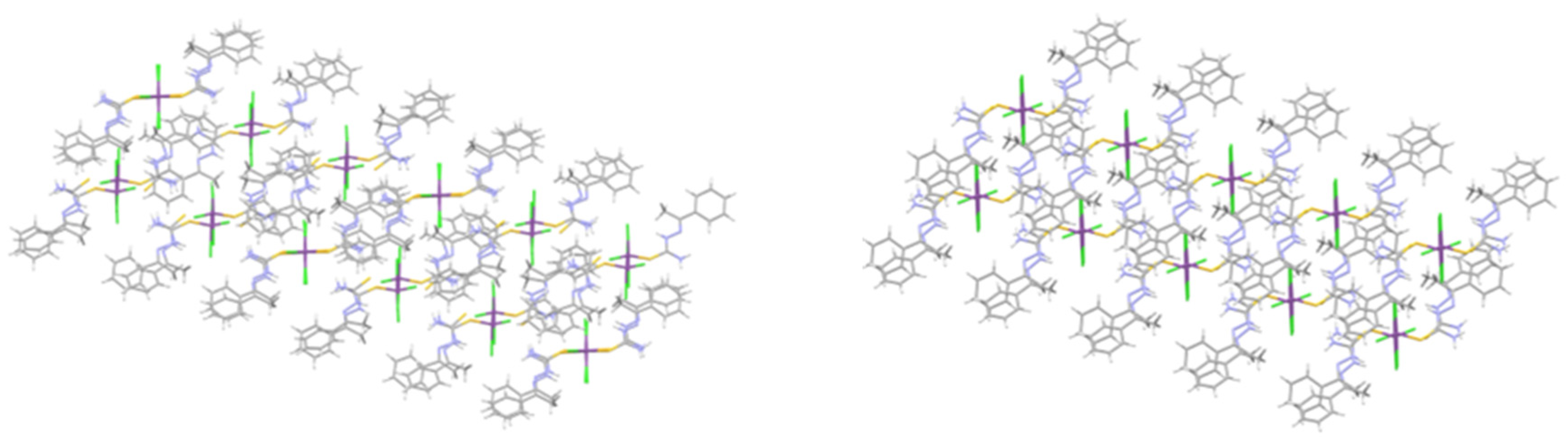
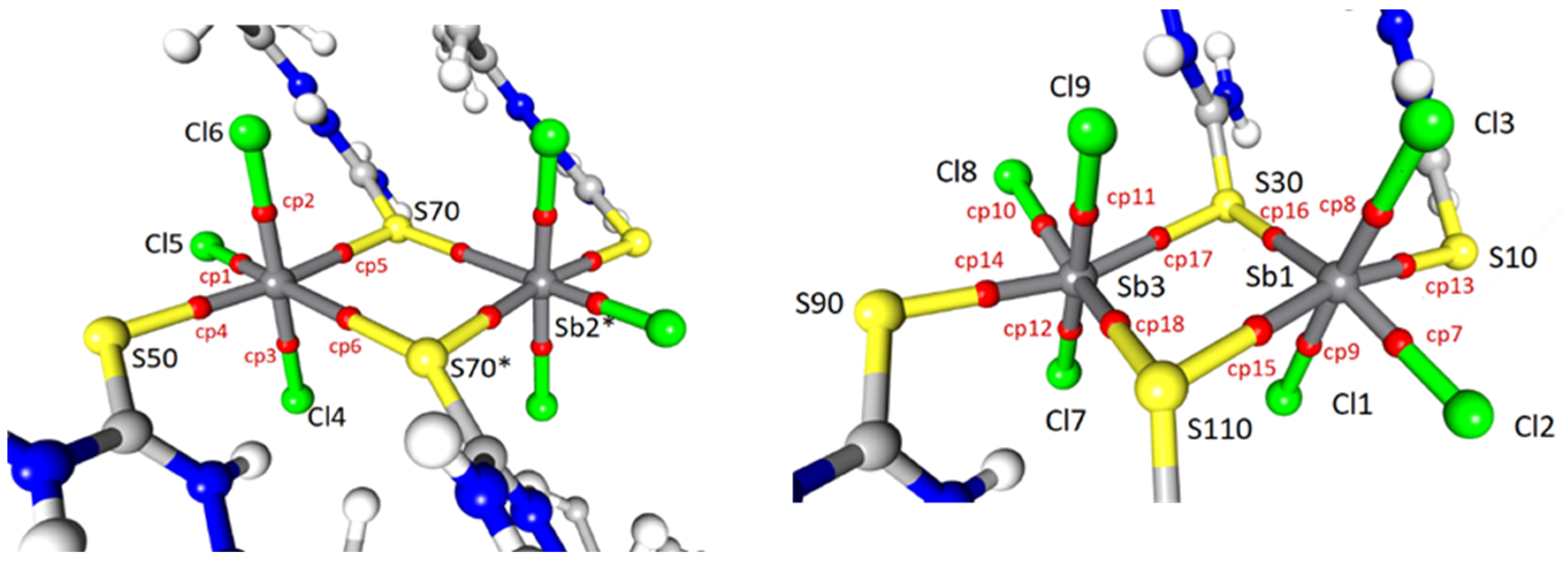
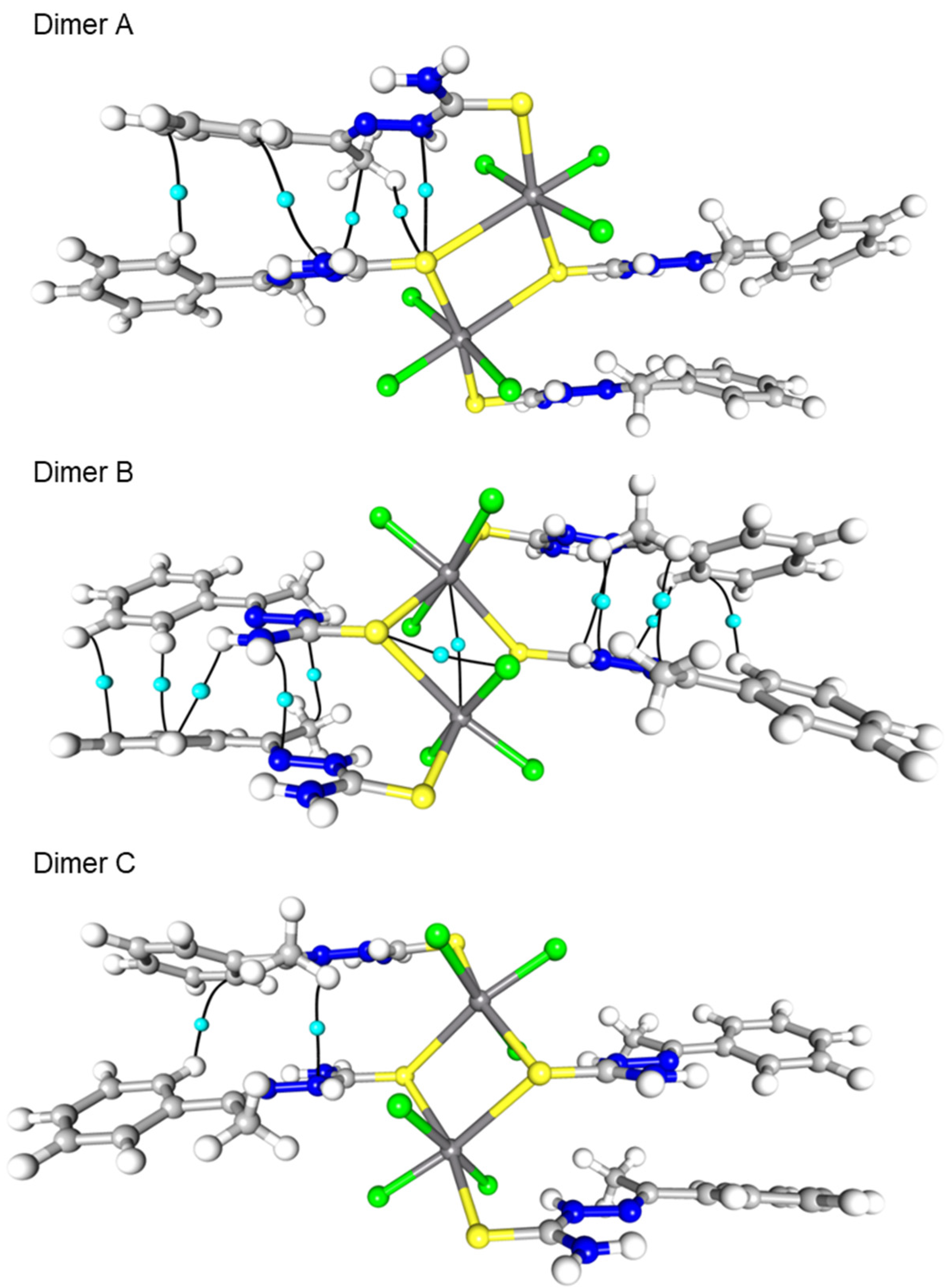
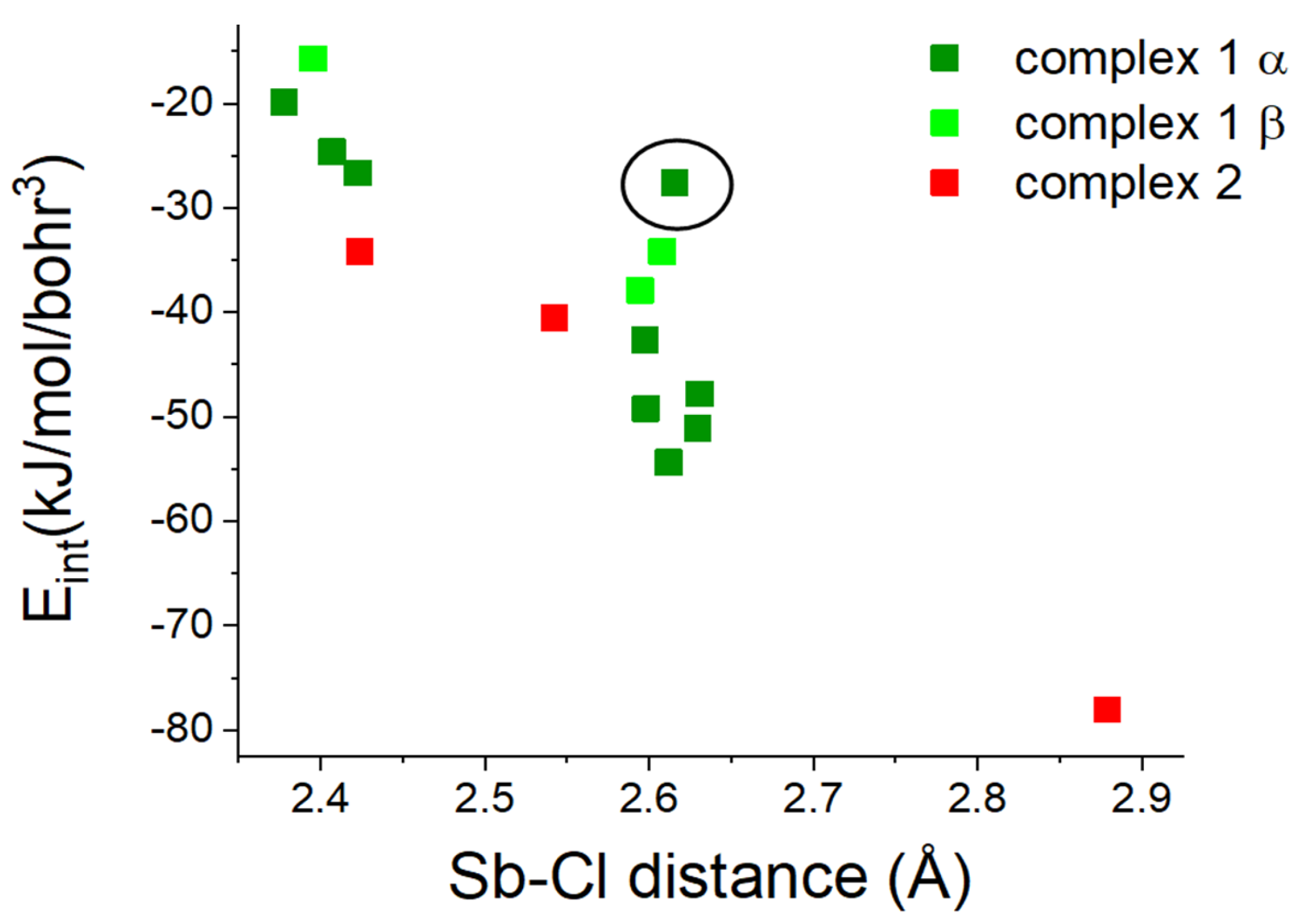

| Atom1 | Atom2 | Cp no | Gcp | Vcp | R1,2 | ρcp | ∇2ρcp I | Hcp | Gcp/ρcp | Vcp/Gcp | Eint |
|---|---|---|---|---|---|---|---|---|---|---|---|
| dimerA | |||||||||||
| Sb2 | S50 | cp4 | 86.95 | −126.07 | 2.64 | 0.35 | 1.756 | −39.12 | 0.094 | −1.45 | −63.035 |
| Sb2 | S70 | cp5 | 60.66 | −83.07 | 2.81 | 0.27 | 1.404 | −22.41 | 0.086 | −1.37 | −41.535 |
| Sb2 | S70 * | cp6 | 18.65 | −19.26 | 3.45 | 0.10 | 0.662 | −0.61 | 0.072 | −1.03 | −9.630 |
| dimer B | |||||||||||
| Sb1 | S10 | cp13 | 98.70 | −145.12 | 2.58 | 0.39 | 1.92 | −46.42 | 0.098 | −1.47 | −72.560 |
| Sb3 | S90 | cp14 | 96.21 | −141.07 | 2.59 | 0.38 | 1.89 | −44.86 | 0.097 | −1.47 | −70.535 |
| Sb1 | S110 | cp15 | 42.50 | −53.85 | 3.00 | 0.20 | 1.14 | −11.35 | 0.080 | −1.27 | −26.925 |
| Sb1 | S30 | cp16 | 30.05 | −34.87 | 3.19 | 0.15 | 0.93 | −4.82 | 0.076 | −1.16 | −17.435 |
| Sb3 | S30 | cp17 | 46.91 | −60.78 | 2.95 | 0.22 | 1.21 | −13.87 | 0.082 | −1.30 | −30.390 |
| Sb3 | S110 | cp18 | 25.70 | −28.59 | 3.28 | 0.13 | 0.84 | −2.89 | 0.075 | −1.11 | −14.295 |
| dimer C | |||||||||||
| Sb1 | S10 | cp22 | 92.58 | −135.22 | 2.61 | 0.37 | 1.83 | −42.64 | 0.10 | −1.46 | −67.61 |
| Sb2 | S30 | cp23 | 25.67 | −28.65 | 3.28 | 0.13 | 0.83 | −2.98 | 0.07 | −1.12 | −14.33 |
| Sb3 | S30 * | cp24 | 47.87 | −62.34 | 2.94 | 0.22 | 1.23 | −14.47 | 0.08 | −1.30 | −31.17 |
| dimer A | |||||||||||
| Sb2 | Cl5 | cp1 | 173.42 | −245.87 | 2.38 | 0.53 | 3.71 | −72.45 | 0.13 | −1.42 | −122.94 |
| Sb2 | Cl6 | cp2 | 98.91 | −137.14 | 2.60 | 0.37 | 2.23 | −38.23 | 0.10 | −1.39 | −68.57 |
| Sb2 | Cl4 | cp3 | 91.70 | −126.18 | 2.63 | 0.35 | 2.10 | −34.48 | 0.11 | −1.38 | −63.09 |
| dimer B | |||||||||||
| Sb1 | Cl2 | cp7 | 153.58 | −217.58 | 2.42 | 0.49 | 3.29 | −64.00 | 0.121 | −1.42 | −108.79 |
| Sb1 | Cl3 | cp8 | 95.64 | −132.20 | 2.61 | 0.36 | 2.17 | −36.56 | 0.102 | −1.38 | −66.10 |
| Sb1 | Cl1 | cp9 | 95.05 | −131.28 | 2.62 | 0.36 | 2.16 | −36.23 | 0.102 | −1.38 | −65.64 |
| Sb3 | Cl8 | cp10 | 160.17 | −227.01 | 2.41 | 0.50 | 3.43 | −66.84 | 0.122 | −1.42 | −113.51 |
| Sb3 | Cl9 | cp11 | 92.12 | −126.80 | 2.63 | 0.35 | 2.11 | −34.68 | 0.101 | −1.38 | −63.40 |
| Sb3 | Cl7 | cp12 | 98.93 | −137.18 | 2.60 | 0.37 | 2.23 | −38.25 | 0.103 | −1.39 | −68.59 |
| dimer C | |||||||||||
| Sb1 | Cl1 | cp 19 | 96.68 | −133.76 | 2.61 | 0.36 | 2.19 | −37.08 | 0.10 | −1.38 | −66.88 |
| Sb1 | Cl2 | cp20 | 165.18 | −234.16 | 2.40 | 0.51 | 3.53 | −68.98 | 0.12 | −1.42 | −117.08 |
| Sb1 | Cl3 | cp21 | 99.63 | −138.25 | 2.59 | 0.37 | 2.24 | −38.62 | 0.10 | −1.39 | −69.13 |
| Atom1 | Atom2 | Cp no | Gcp | Vcp | R1,2 | ρcp | ∇2ρcp | Hcp | Gcp/ρcp | Eint | Symm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sb1 | Sb3 | cp101 | 12.21 | −12.40 | 3.92 | 0.075 | 0.44 | −0.19 | 0.062 | −6.20 | x, y, z |
| Cl9 | S110 | cp102 | 22.44 | −20.14 | 3.29 | 0.093 | 0.91 | 2.30 | 0.092 | −10.07 | x, y, z |
| Compounds | IC50 (μΜ) | Ref. |
|---|---|---|
| Hacptsc | >30 | * |
| Hacpmtsc | >30 | * |
| Hbzmtsc | >30 | * |
| Complex 1 | 20.6 ± 1.8 | * |
| Complex 2 | 27.7 ± 3.4 | * |
| Complex 3 | >30 | * |
| [SbCl3(DMTU)3] | 9.0 ± 0.3 | [29] |
| [SbBr3(TMTU)3] | 12.7 ± 2.5 | [27] |
| [SbI2(µ2-I)(EtMBZIM)2]2.3H2O | 3.2 ± 1.7 | [28] |
| [SbCl3(µ2-S)(MtMBZIM)2]2 | 4.72 ± 1.34 | [24] |
| {[SbBr(Me2DTC)2]n} | 0.046 ± 0.004 | [26] |
| ([BiCl3(MBZT)2].H2O) | >30 | [32] |
| [BiI3(tHPMT)3] | >30 | [33] |
| [BiCl3(Hattsc)3] | 4.5 ± 0.4 | [30] |
| {[BiI2(µ2-I)(Hattsc)2]2} | 5.6 ± 0.3 | [30] |
| {[BiI(µ2-I)(Et2DTC)2]2}n | 0.05 ± 0.006 | [31] |
| Inhibition Zone (mm) | Ref. | ||||
|---|---|---|---|---|---|
| P. aeruginosa | E. coli | S. epidermidis | S. aureus | ||
| DMSO | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | [15] |
| Hacptsc | 9.0 ± 0.0 | 9.3 ± 0.7 | 9.0 ± 0.0 | 9.0 ± 0.0 | [15] |
| Hacpmtsc | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.0 ± 0.0 | [15] |
| Hbzmtsc | 9.0 ± 0.0 | 9.2 ± 0.3 | 9.0 ± 0.0 | 9.0 ± 0.0 | [14] |
| 1 | 12.0 ± 0.0 | 11.7 ± 0.7 | 9.0 ± 0.0 | 9.0 ± 0.0 | * |
| 2 | 13.3 ± 0.5 | 10.8 ± 1.5 | 9.0 ± 0.0 | 9.0 ± 0.0 | * |
| 3 | 12.5 ± 1.0 | 10.7 ± 1.3 | 9.0 ± 0.0 | 9.0 ± 0.0 | * |
| {[BiCl3(Htetsc)3].CH3OH} | 15.0 ± 1.1 | 15.5 ± 1.0 | 14.0 ± 0.0 | 15.0 ± 0.0 | [34] |
| {BiBr3(Hbztsc)3} | 10.7 ± 0.9 | 12.8 ± 1.2 | 9.0 ± 0.0 | 9.6 ± 0.9 | [15] |
| {BiBr3(Hbzmtsc)3} | 11.0 ± 1.3 | 12.7 ± 1.4 | 9.0 ± 0.0 | 10.1 ± 1.1 | [14] |
| 1 (α) | 1 (β) | 2 | 3 | |
|---|---|---|---|---|
| Chemical formula | C54H66Cl9N18S6Sb3 ·CH2Cl2·2H20 | C54H66Cl9N18S6Sb3 ·CH2Cl2·2H20 | C20H26Cl3N6S2Sb·CH2Cl2 | C27H33BiCl3N9S3· C2H6O |
| Formula weight | 1314.22 | 1314.22 | 727.63 | 941.20 |
| Crystal system, | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | ||||
| Temperature (K) | 100.00(10) | 293(2) | 138.00(50) | 100.00(10) |
| a, b, c (Å) | 14.1625(5), 16.2995(7), 18.7401(7) | 8.4190(2), 10.9693(3), 14.5152 (4) | 9.1243(3), 9.6716(4), 10.1365(3) | 9.1909(5), 9.3371(5), 12.2582(5) |
| α, β, γ (°) | 66.292(4), 82.772(3), 75.475(3) | 84.163(2), 87.856(2), 83.201 (2) | 98.337(3), 105.052(3), 117.973(4) | 107.110(4), 94.469(4), 108.663(5) |
| V (Å3) | 3832.8(3) | 1323.72 (6) | 724.61(5) | 935.17(9) |
| Z | 2 | 2 | 1 | 1 |
| μ (mm−1) | 1.65 | 1.53 | 13.33 | 5.13 |
| No. of measured, independent and observed [I > 2s(I)] reflections | 29377, 14804, 12546 | 29518, 5645, 5061 | 11970, 5496, 5475 | 12760, 7454, 7449 |
| Rint | 0.0220 | 0.040 | 0.0463 | 0.0238 |
| R[F2> 2s(F2)], wR(F2), S | 0.030, 0.072, 1.09 | 0.031, 0.079, 1.07 | 0.038, 0.104, 1.05 | 0.025, 0.058, 1.05 |
| No. of reflections | 14804 | 5645 | 5496 | 7454 |
| No. of parameters | 844 | 273 | 321 | 421 |
| Δρmax, Δρmin (e·Å−3) | 1.653–0.956 | 0.59−0.85 | 1.203–1.039 | 1.445–1.168 |
| CCDC number | 2310421 | 2376681 | 2310419 | 2310420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, I.I.; Sumer, E.I.; Dutkiewicz, G.; Banti, C.N.; Hadjikakou, S.K.; Grześkiewicz, A.M.; Kubicki, M. Group VA Aromatic Thiosemicarbazone Complexes: Synthesis, Characterization, Biological Activity, and Topological Studies. Int. J. Mol. Sci. 2024, 25, 10794. https://doi.org/10.3390/ijms251910794
Ozturk II, Sumer EI, Dutkiewicz G, Banti CN, Hadjikakou SK, Grześkiewicz AM, Kubicki M. Group VA Aromatic Thiosemicarbazone Complexes: Synthesis, Characterization, Biological Activity, and Topological Studies. International Journal of Molecular Sciences. 2024; 25(19):10794. https://doi.org/10.3390/ijms251910794
Chicago/Turabian StyleOzturk, Ibrahim I., Emine I. Sumer, Grzegorz Dutkiewicz, Christina N. Banti, Sotiris K. Hadjikakou, Anita M. Grześkiewicz, and Maciej Kubicki. 2024. "Group VA Aromatic Thiosemicarbazone Complexes: Synthesis, Characterization, Biological Activity, and Topological Studies" International Journal of Molecular Sciences 25, no. 19: 10794. https://doi.org/10.3390/ijms251910794









