Lepidium peruvianum as a Source of Compounds with Anticancer and Cosmetic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fingerprinting of L. peruvianum Extracts by HPLC-ESI-Q-TOF-MS/MS
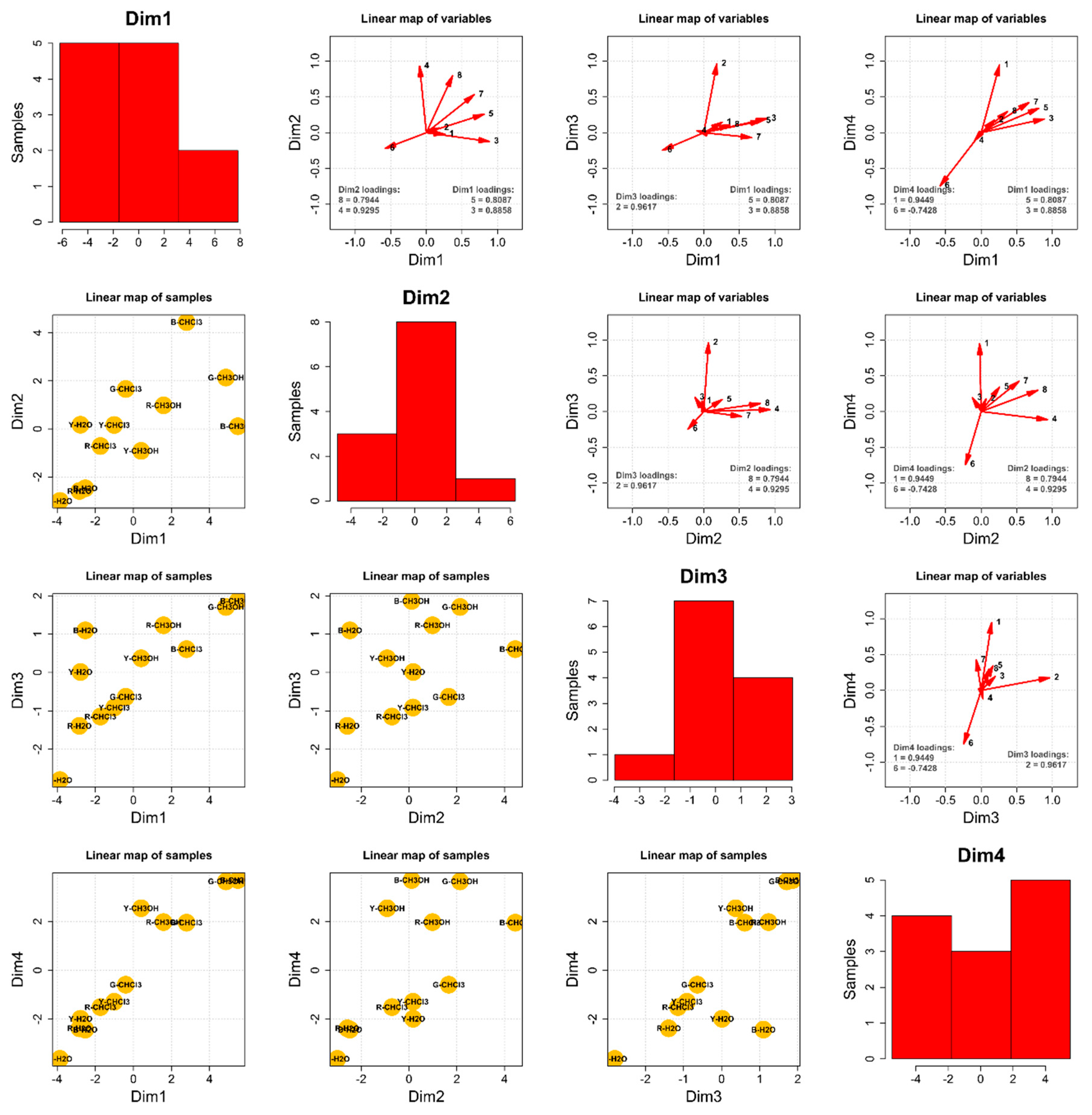
Principal Component Analysis—The Composition of the 12 Extracts
2.2. The Bioactivity Determination of the Tested Extracts
2.2.1. Comparative Analysis of the Antioxidant Activity of Maca Extracts
2.2.2. Tyrosinase Inhibitory Activity of Maca Extracts
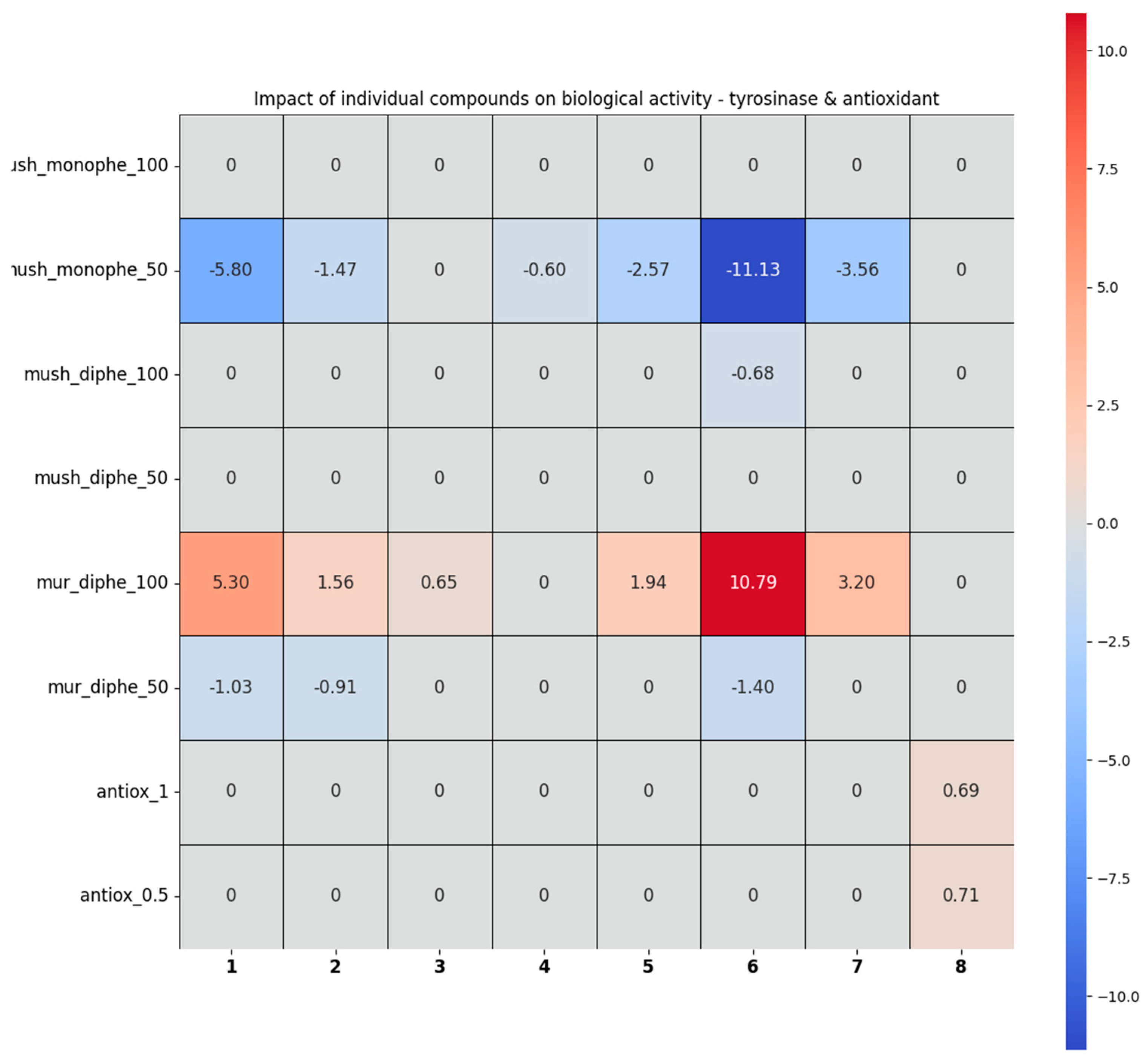
2.2.3. The Analysis of the Tyrosinase Inhibition and Antioxidant Activity Assays by the Chemometric Approach
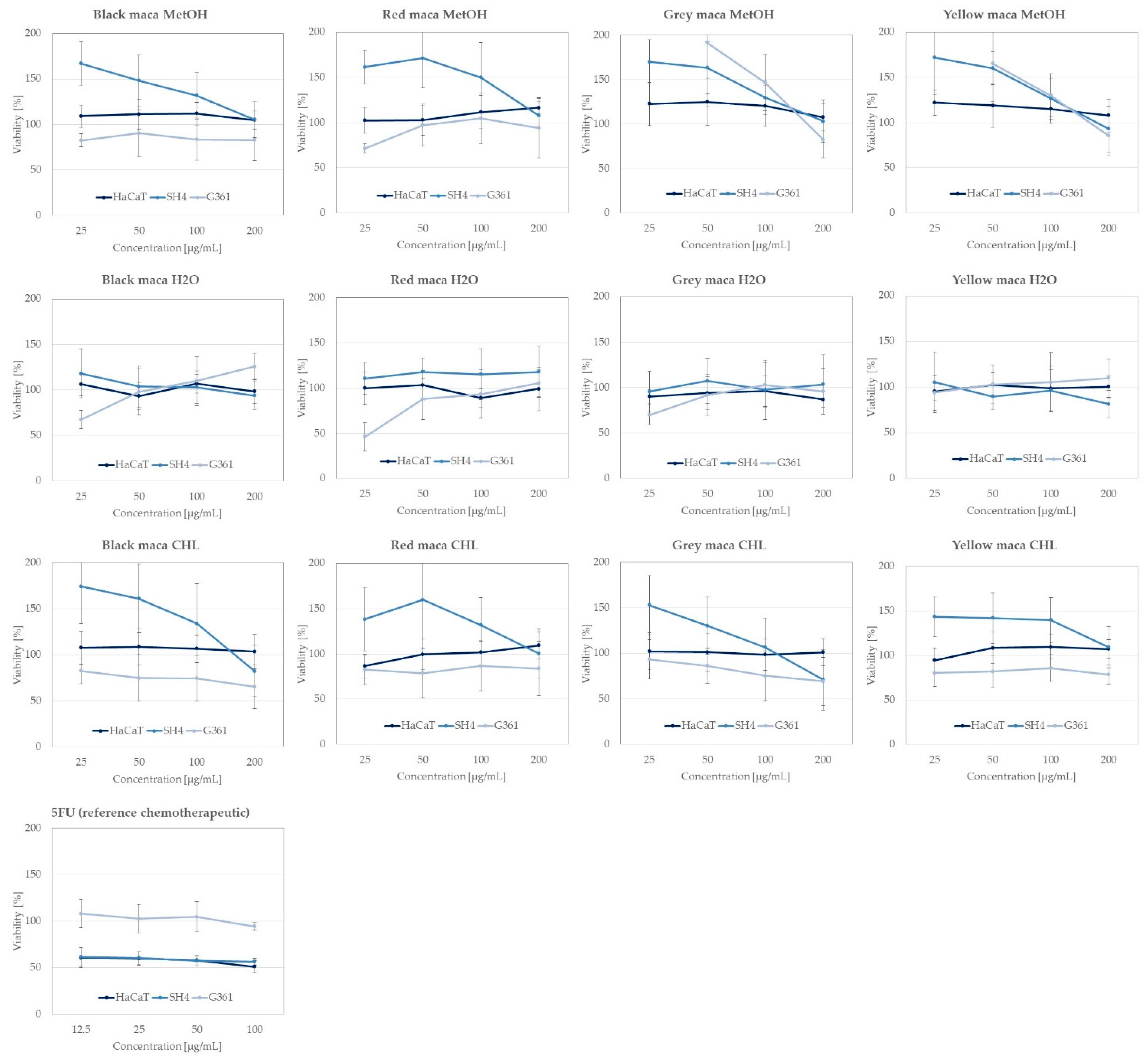
2.2.4. Anticancer Activity of Extracts towards Skin Cancer Lines
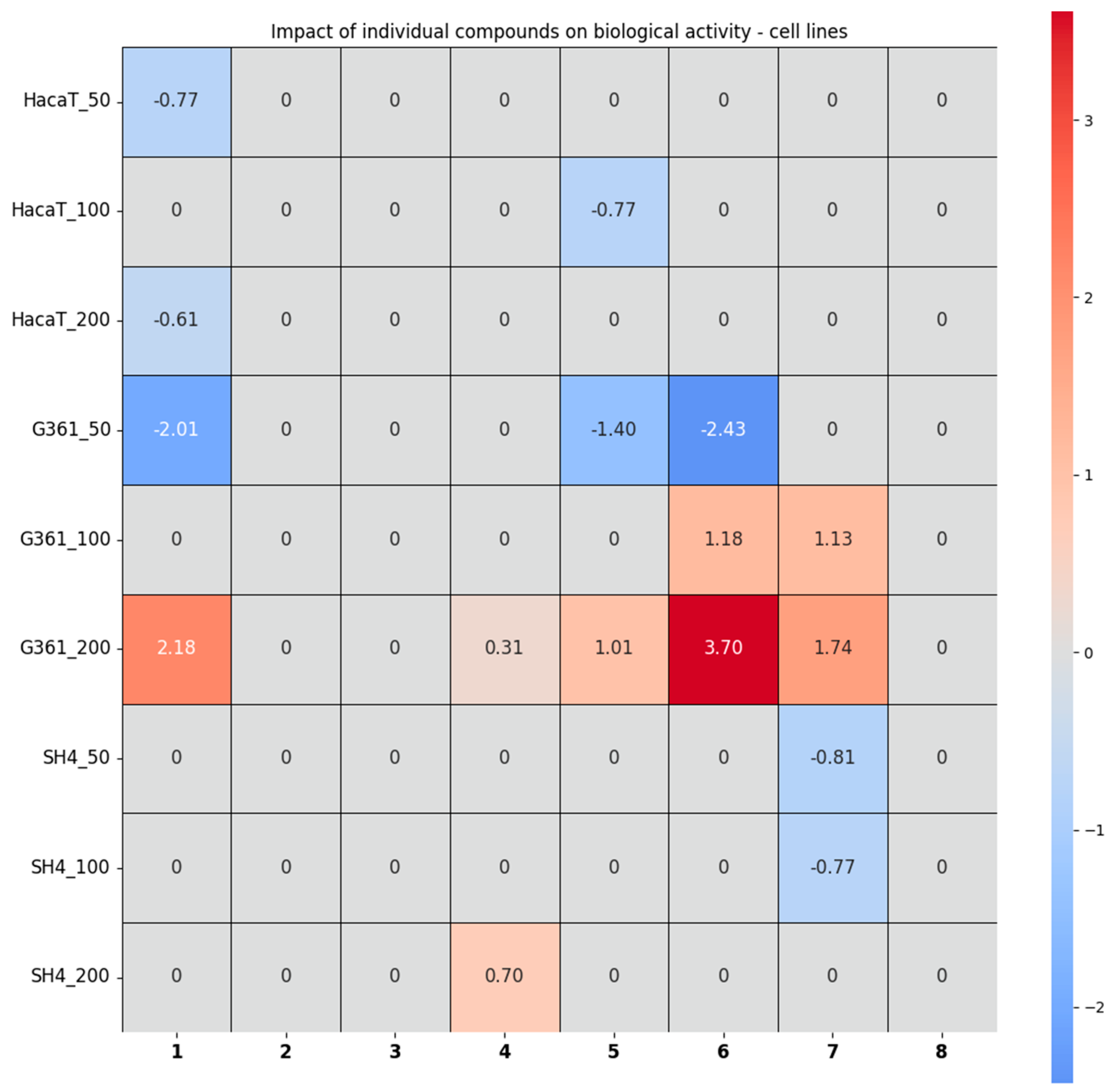
2.2.5. Chemometric Analysis of the Results Obtained from the Studies in Cells
3. Materials and Methods
3.1. The Reagents
3.2. The Extraction and Chromatographic Fingerprinting of L. peruvianum Extracts
3.3. Cell Lines
3.4. Cell Viability Assay
3.5. Tyrosinase Inhibition by the Tested Extracts
3.6. Antiradical Activity Determination of the Extracts
3.6.1. DPPH Scavenging Assay
3.6.2. Detection of Intracellular ROS Levels—H2DCFDA Assay
3.7. Statistical Analysis of Data
3.7.1. Principal Component Analysis (PCA)
3.7.2. Regression Analysis (RA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mush_monophe | Mushroom tyrosinase + L-tyrosine (monophenolase activity) |
| mush_diphe | Mushroom tyrosinase + L-DOPA (diphenolase activity) |
| mur_diphe | Murine tyrosinase + L-DOPA (diphenolase activity) |
| antiox | Antioxidant activity |
| HaCaT | Activity towards HaCaT cell lines |
| G361 | Activity towards G361 cell lines |
| SH4 | Activity towards SH4 cell lines |
| B-MetOH | Black maca methanol extract |
| B-CHL | Black maca chloroform extract |
| G-MetOH | Grey maca methanol extract |
| B-H2O | Black maca water extract |
| G-H2O | Grey maca water extract |
References
- Natural Cosmetics Market Size Analysis Report By Product (Skin Care, Hair Care, Fragrance, Color Cosmetics), By Distribution Channel (Supermarket/Hypermarket, Online), and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/natural-cosmetics-market (accessed on 10 June 2024).
- Sumit, K.; Vivek, S.; Sujata, S.; Ashish, B. Herbal Cosmetics: Used for Skin and Hair. Inven. Rapid Cosmeceuticals 2012, 2012, 1–7. [Google Scholar]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C.; Gonzales-Castañeda, C. Lepidium meyenii (Maca): A plant from the highlands of Peru—From tradition to science. Forsch Komplementmed. 2009, 16, 373–380. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2022, 11, 64. [Google Scholar] [CrossRef]
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 23, 48586. [Google Scholar] [CrossRef]
- Minich, D.M.; Ross, K.; Frame, J.; Fahoum, M.; Warner, W.; Meissner, H.O. Not All Maca Is Created Equal: A Review of Colors, Nutrition, Phytochemicals, and Clinical Uses. Nutrients 2024, 16, 530. [Google Scholar] [CrossRef]
- Sunan, W.; Fan, Z. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar]
- Esparza, E.; Hadzich, A.; Kofer, W.; Mithöfer, A.; Cosio, E.G. Bioactive maca (Lepidium meyenii) alkamides are a result of traditional Andean postharvest drying practices. Phytochemistry 2015, 116, 138–148. [Google Scholar] [CrossRef]
- Jin, W.; Chen, X.; Dai, P.; Yu, L. Lepidiline C, and D: Two new imidazole alkaloids from Lepidium meyenii Walpers (Brassicaceae) roots. Phytochem. Lett. 2016, 17, 158–161. [Google Scholar] [CrossRef]
- Chen, R.; Wei, J.; Gao, Y. A review of the study of active components and their pharmacology value in Lepidium meyenii (Maca). Phytother. Res. 2021, 35, 6706–6719. [Google Scholar] [CrossRef]
- Gonzales, C.; Rubio, J.; Gasco, M.; Nieto, J.; Yucra, S.; Gonzales, G.F. Effect of short-term and long-term treatments with three ecotypes of Lepidium meyenii (MACA) on spermatogenesis in rats. J. Ethnopharmacol. 2006, 103, 448–454. [Google Scholar] [CrossRef]
- Ohta, Y.; Yoshida, K.; Kamiya, S.; Kawate, N.; Takahashi, M.; Inaba, T.; Hatoya, S.; Morii, H.; Takahashi, K.; Ito, M.; et al. Feeding hydroalcoholic extract powder of Lepidium meyenii (maca) increases serum testosterone concentration and enhances steroidogenic ability of Leydig cells in male rats. Andrologia 2016, 48, 347–354. [Google Scholar] [CrossRef]
- Meissner, H.O.; Kapczynski, W.; Mscisz, A.; Lutomski, J. Use of gelatinized maca (Lepidium peruvianum) in early postmenopausal women. Int. J. Biomed. Sci. 2005, 1, 33–45. [Google Scholar] [CrossRef]
- Meissner, H.O.; Mscisz, A.; Reich-Bilinska, H. Hormone-Balancing Effect of Pre-Gelatinized Organic Maca (Lepidium peruvianum Chacon): (III) Clinical responses of early-postmenopausal women to Maca in a double-blind, randomized, Placebo-controlled, crossover configuration, outpatient study. Int. J. Biomed. Sci. 2006, 2, 375–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Ao, M.; Jin, W. Effect of ethanol extract of Lepidium meyenii Walp. On osteoporosis in ovariectomized rat. J. Ethnopharmacol. 2006, 105, 274–279. [Google Scholar] [CrossRef]
- Liu, H.; Jin, W.; Fu, C.; Dai, P.; Yu, Y.; Huo, Q.; Yu, L. Discovering anti-osteoporosis constituents of maca (Lepidium meyenii) by combined virtual screening and activity verification. Food Res. Int. 2015, 77, 215–222. [Google Scholar] [CrossRef]
- Stojanovska, L.; Law, C.; Lai, B.; Chung, T.; Nelson, K.; Day, S.; Apostolopoulos, V.; Haines, C. Maca reduces blood pressure and depression, in a pilot study in postmenopausal women. Climacteric 2015, 18, 69–78. [Google Scholar] [CrossRef]
- Guo, S.S.; Gao, X.F.; Gu, Y.R.; Wan, Z.X.; Lu, A.M.; Qin, Z.H.; Luo, L. Preservation of Cognitive Function by Lepidium meyenii (Maca) Is Associated with Improvement of Mitochondrial Activity and Upregulation of Autophagy-Related Proteins in Middle-Aged Mouse Cortex. Evidence-based Complement. Altern. Med. 2016, 2016, 4394261. [Google Scholar] [CrossRef]
- Ai, Z.; Cheng, A.-F.; Yu, Y.-T.; Yu, L.-J.; Jin, W. Antidepressant-Like Behavioral, Anatomical, and Biochemical Effects of Petroleum Ether Extract from Maca (Lepidium meyenii) in Mice Exposed to Chronic Unpredictable Mild Stress. J. Med. Food 2014, 17, 535–542. [Google Scholar] [CrossRef]
- del Valle Mendoza, J.; Pumarola, T.; Gonzales, L.A.; del Valle, L.J. Antiviral activity of maca (Lepidium meyenii) against human influenza virus. Asian Pac. J. Trop. Med. 2014, 7, 415–420. [Google Scholar] [CrossRef]
- Rodríguez-Huamán, Á.; Casimiro-Gonzales, S.; Chávez-Pérez, J.A.; Gonzales-Arimborgo, C.; Cisneros-Fernández, R.; Aguilar-Mendoza, L.Á.; Gonzales, G.F. Antioxidant and neuroprotector effect of Lepidium meyenii (maca) methanol leaf extract against 6-hydroxy dopamine (6-OHDA)-induced toxicity in PC12 cells. Toxicol. Mech. Methods 2017, 27, 279–285. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Bai, L.; Pan, M.H. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef]
- Ulloa del Carpio, N.; Alvarado-Corella, D.; Quiñones-Laveriano, D.M.; Araya-Sibaja, A.; Vega-Baudrit, J.; Monagas-Juan, M.; Navarro-Hoyos, M.; Villar-López, M. Exploring the chemical and pharmacological variability of Lepidium meyenii: A comprehensive review of the effects of maca. Front. Pharmacol. 2024, 15, 1360422. [Google Scholar] [CrossRef]
- Nuñez, D.; Olavegoya, P.; Gonzales, G.F.; Gonzales-Castañeda, C. Red Maca (Lepidium meyenii), a Plant from the Peruvian Highlands, Promotes Skin Wound Healing at Sea Level and at High Altitude in Adult Male Mice. High Alt. Med. Biol. 2017, 18, 372–383. [Google Scholar] [CrossRef]
- Gonzales-Castañeda, C.; Gonzales, G.F. Hypocotyls of Lepidium meyenii (maca), a plant of the Peruvian highlands, prevent ultraviolet A-, B-, and C-induced skin damage in rats. Photodermatol. Photoimmunol. Photomed. 2008, 24, 24–31. [Google Scholar] [CrossRef]
- Elmets, C.A.; Singh, D.; Tubesing, K. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Lee, K.J.; Dabrowski, K.; Sandoval, M. Activity-guided fractionation of phytochemicals of maca meal, their antioxidant activities and effects on growth, feed utilization, and survival in rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture 2005, 244, 293–301. [Google Scholar] [CrossRef]
- Xie, H.; Chun, F.K.; Rutz, J. Sulforaphane Impact on Reactive Oxygen Species (ROS) in Bladder Carcinoma. Int. J. Mol. Sci. 2021, 31, 5938. [Google Scholar] [CrossRef]
- Li, A.; Duan, S.; Dang, Y. Origin identification of Chinese Maca using electronic nose coupled with GC-MS. Sci. Rep. 2019, 21, 12216. [Google Scholar] [CrossRef]
- Buchynskyy, A.; Gillespie, J.R.; Herbst, Z.M.; Ranade, R.M.; Buckner, F.S.; Gelb, M.H. 1-Benzyl-3-aryl-2-thiohydantoin Derivatives as New Anti-Trypanosoma brucei Agents: SAR and in Vivo Efficacy. Med. Chem. Lett. 2017, 8, 886–891. [Google Scholar] [CrossRef]
- Poyraz, S.; Belveren, S.; Ülger, M.; Şahin, E.; Döndaş, H.A. Synthesis, characterization, crystal structure, and antituberculosis activity of some novel polysubstituted aminocarbothiol/thiohydantoin-pyrrolidine derivatives. Mon. Chem. 2017, 148, 2173–2182. [Google Scholar] [CrossRef]
- Zuo, M.; Xu, X.; Xie, Z.; Ge, R.; Zhang, Z.; Li, Z.; Bian, J. Design and synthesis of indoline thiohydantoin derivatives based on enzalutamide as antiproliferative agents against prostate cancer. J. Med. Chem. 2017, 125, 1002–1022. [Google Scholar] [CrossRef]
- Bassetto, M.; Ferla, S.; Pertusati, F.; Kandil, S.; Westwell, A.D.; Brancale, A.; Mcguigan, C. Design and synthesis of novel bicalutamide and enzalutamide derivatives as antiproliferative agents for the treatment of prostate cancer. Eur. J. Med. Chem. 2016, 118, 230–243. [Google Scholar] [CrossRef]
- Castellaneta, A.; Losito, I.; Cisternino, G.; Leoni, C.; Santamaria, P.; Calvano, C.D.; Bianco, G.; Cataldi, T.I. All Ion Fragmentation Analysis Enhances the Untargeted Profiling of Glucosinolates in Brassica Microgreens by Liquid Chromatography and High-Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom 2022, 33, 2108–2119. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Lelario, F.; Bufo, S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2374–2388. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC-ESI-Orbitrap MS coupled with UHPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 17, 44660. [Google Scholar] [CrossRef]
- Yabar, E.; Pedreschi, R.; Chirinos, R.; Campos, D. Glucosinolate content and myrosinase activity evolution in three maca (Lepidium meyenii Walp.) ecotypes during preharvest, harvest and postharvest drying. Food Chem. 2011, 127, 1576–1583. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, J.; Hao, L. Quality Evaluation of Lepidium meyenii (Maca) Based on HPLC and LC-MS Analysis of its Glucosinolates from Roots. Food Anal. Methods 2017, 10, 2143–2151. [Google Scholar] [CrossRef]
- Clément, C.; Diaz Grados, D.A.; Avula, B.; Khan, I.A.; Mayer, A.C.; Ponce Aguirre, D.D.; Manrique, I.; Kreuzer, M. Influence of colour type and previous cultivation on secondary metabolites in hypocotyls and leaves of maca (Lepidium meyenii Walpers). J. Sci. Food Agric. 2010, 90, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Uto-Kondo, H.; Naito, Y.; Ichikawa, M.; Nakata, R.; Hagiwara, A.; Kotani, K. Antioxidant activity, total polyphenol, anthocyanin and benzyl-glucosinolate contents in different phenotypes and portion of Japanese Maca (Lepidiummeyenii). Heliyon 2024, 10, e32778. [Google Scholar] [CrossRef]
- Meissner, H.O.; Mscisz, A.; Baraniak, M.; Piatkowska, E.; Pisulewski, P.; Mrozikiewicz, M.; Bobkiewicz-Kozlowska, T. Peruvian Maca (Lepidium peruvianum)—III: The Effects of Cultivation Altitude on Phytochemical and Genetic Differences in the Four Prime Maca Phenotypes. Int. J. Biomed. Sci. 2017, 13, 58–73. [Google Scholar] [CrossRef]
- Huerta Ojeda, Á.; Rodríguez Rojas, J.; Cuevas Guíñez, J.; Ciriza Velásquez, S.; Cancino-López, J.; Barahona-Fuentes, G.; Yeomans-Cabrera, M.-M.; Pavez, L.; Jorquera-Aguilera, C. The Effects of Maca (Lepidium meyenii Walp) on Cellular Oxidative Stress: A Systematic Review and Meta-Analysis. Antioxidants 2024, 13, 1046. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014, 860479, Erratum in: Oxid. Med. Cell. Longev. 2020, 2020, 1969760. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea Biebersteinii Afan. Extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef]
- Yang, J.; Cho, H.; Gil, M.; Kim, K.E. Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants 2023, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Blum, A.; Volkenandt, M. Hautkrebs [Skin cancer]. Dtsch. Med. Wochenschr. 2002, 127, 1679–1681. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, X.J. TGFβ Signaling in Photoaging and UV-Induced Skin Cancer. J. Investig. Dermatol. 2020, 141, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Flohil, S.; Vries, E.; Neumann, H.A.M.; Coebergh, J.; Nijsten, T. Incidence, Prevalence and Future Trends of Primary Basal Cell Carcinoma in the Netherlands. Acta Derm.-Venereol. 2011, 91, 24–30. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts and Figures. 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 8 January 2024).
- Bassan, P.; Bhushan, S.; Kaur, T.; Arora, R.; Arora, S.; Vig, A.P. Extraction, profiling and bioactivity analysis of volatile glucosinolates present in oil extract of Brassica juncea var. raya. Physiol. Mol. Biol. Plants 2018, 24, 399–409. [Google Scholar] [CrossRef]
- Blažević, I.; Đulović, A.; Maravić, A.; Čulić, V.Č.; Montaut, S.; Rollin, P. Antimicrobial and Cytotoxic Activities of Lepidium latifolium L. Hydrodistillate, Extract and Its Major Sulfur Volatile Allyl Isothiocyanate. Chem. Biodivers. 2019, 16, 31. [Google Scholar] [CrossRef]
- Jamuna, K.S.; Suma, M.S.; Ramesh, C.K.; Mahmood, R.; Nanjundaswamy, L. Studies on in vitro antiproliferate activities in cruciferous vegetables. J. Appl. Hortic. 2017, 19, 230–234. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.; Shao, X.; Jin, D.; Zheng, D.; Zhao, T.; Zhu, H.; Zhang, L.; et al. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 5, 542–553. [Google Scholar] [CrossRef]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yang, Y.; Gao, M.; Qu, Y.; Guo, X.; Liu, Y.; Cui, X.; Wang, C. Lepidium meyenii Walpers polysaccharide and its cationic derivative re-educate tumor-associated macrophages for synergistic tumor immunotherapy. Carbohydr. Polym. 2020, 250, 116904. [Google Scholar] [CrossRef]
- Szwejda-Grzybowska, J. Antykancerogenne składniki warzyw kapustnych i ich znaczenie w profilaktyce chorób nowotworowych. Bromat. Chem. Toksykol. 2011, 4, 1039–1046. [Google Scholar]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Matejic, J.S.; Dzamic, A.M.; Mihajilov-Krstev, T.; Randelovic, V.N.; Krivosej, Z.D.; Marin, P.D. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. Taxa. Cent. Eur. J. Biol. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R.; RStudio Team: Vienna, Austria, 2019. [Google Scholar]
- Borchers, H.W. Pracma: Practical Numerical Math Functions 2022, Version 2.3.8 from R-Forge; R Package Vignette: Madison, WI, USA, 2022. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 4 October 2024).
- Friendly, M.; Fox, J.; Chalmers, P. Matlib: Matrix Functions for Teaching and Learning Linear Algebra and Multivariate Statistics. 2021. Available online: https://cran.r-project.org/web/packages/matlib/matlib.pdf (accessed on 4 October 2024).





| No. | Ion (+/−) | Rt (min) | Molecular Formula | m/z Calculated | m/z Experimental | Delta (mmu) | DBE | MS/MS Fragments | Proposed Compound |
|---|---|---|---|---|---|---|---|---|---|
| 1 | [M-H]− | 9.3 | C14H19NO9S2 | 408.0471 | 408.0452 | −10.15 | 6 | 408.0346; 274.9853; 240.9993; 211.9993; 195.0387; 166.0321 | Glucotropaeolin |
| 2 | [M-H]− | 12.1 | C15H21NO10S2 | 438.0534 | 438.0550 | −3.62 | 6 | 274.9855; 259.0156; 243.0192; 195.0305; 145.0588; | Glucolimnanthin |
| 3 | [M-H]− | 13.0 | C17H22N2O19S2 | 477.0643 | 477.0658 | −3.12 | 8 | - | 4-Methoxyglucobrassicin |
| 4 | [M-H]− | 15.8 | C22H36O10N2S5 | 551.1733 | 551.1781 | 0.16 | 5.5 | 389.1287; 370.1942; 341.0926; 317.0991; 309.4013; 283.0377; 261.9778; 235.8406; 216.3231; 193.0498; 134.0416; | 4-Methoxyindolyl-3-hexylhydroxy-glucosinolate |
| 5 | [M-H]− | 16.5 | C15H29O10NS2 | 446.1154 | 446.1178 | −4.0 | 2 | - | Indolil-5-methylglucosinolate |
| 6 | [M-H]− | 17.17 | C12H24O7N2S3 | 371.0946 | 371.0987 | −0.09 | 11.5 | 249.0673; 231.0436; 193.0621; 175.0191 | Pent-4-enylglucosinolate (Glucobrassicanapin) |
| 7 | [M-H]− | 17.6 | C17H21O10NS2 | 450.0528 | 450.0548 | −0.04 | 8.5 | 437.5571; 417.1699; 386.6801 356.1078; 259.9393 | Glucoalyssin |
| 8 | [M-H]− | 26.9 | C25H20O8 | 447.1085 | 447.1082 | 0.76 | 16 | - | A derivative of ferulic acid |
| 9 | [M-H]− | 5.47 | C14H19NO10S2 | 424.0378 | 424.0392 | −3.39 | 6 | 408.5279; 335.1320; 274.9969; 285.5466; 228.0066; 195.3503 | Hydroxybenzyl-glucosinolate |
| 10 | [M-H]− | 10.5 | C15H21NO10S2 | 438.0534 | 438.00553 | −4.3 | 6 | 421.1190; 336.0780; 274.9955; 259.0090; | Methoxybenzylglucosinolate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, D.; Gaweł-Bęben, K.; Kukula-Koch, W.; Strzępek-Gomółka, M.; Wawruszak, A.; Woźniak, S.; Chrzanowska, M.; Czech, K.; Borzyszkowska-Bukowska, J.; Głowniak, K.; et al. Lepidium peruvianum as a Source of Compounds with Anticancer and Cosmetic Applications. Int. J. Mol. Sci. 2024, 25, 10816. https://doi.org/10.3390/ijms251910816
Kasprzak D, Gaweł-Bęben K, Kukula-Koch W, Strzępek-Gomółka M, Wawruszak A, Woźniak S, Chrzanowska M, Czech K, Borzyszkowska-Bukowska J, Głowniak K, et al. Lepidium peruvianum as a Source of Compounds with Anticancer and Cosmetic Applications. International Journal of Molecular Sciences. 2024; 25(19):10816. https://doi.org/10.3390/ijms251910816
Chicago/Turabian StyleKasprzak, Dorota, Katarzyna Gaweł-Bęben, Wirginia Kukula-Koch, Marcelina Strzępek-Gomółka, Anna Wawruszak, Sylwia Woźniak, Marcelina Chrzanowska, Karolina Czech, Julia Borzyszkowska-Bukowska, Kazimierz Głowniak, and et al. 2024. "Lepidium peruvianum as a Source of Compounds with Anticancer and Cosmetic Applications" International Journal of Molecular Sciences 25, no. 19: 10816. https://doi.org/10.3390/ijms251910816
APA StyleKasprzak, D., Gaweł-Bęben, K., Kukula-Koch, W., Strzępek-Gomółka, M., Wawruszak, A., Woźniak, S., Chrzanowska, M., Czech, K., Borzyszkowska-Bukowska, J., Głowniak, K., Matosiuk, D., Orihuela-Campos, R. C., Jodłowska-Jędrych, B., Laskowski, T., & Meissner, H. O. (2024). Lepidium peruvianum as a Source of Compounds with Anticancer and Cosmetic Applications. International Journal of Molecular Sciences, 25(19), 10816. https://doi.org/10.3390/ijms251910816








