The Potentiality of Natural Products and Herbal Medicine as Novel Medications for Parkinson’s Disease: A Promising Therapeutic Approach
Abstract
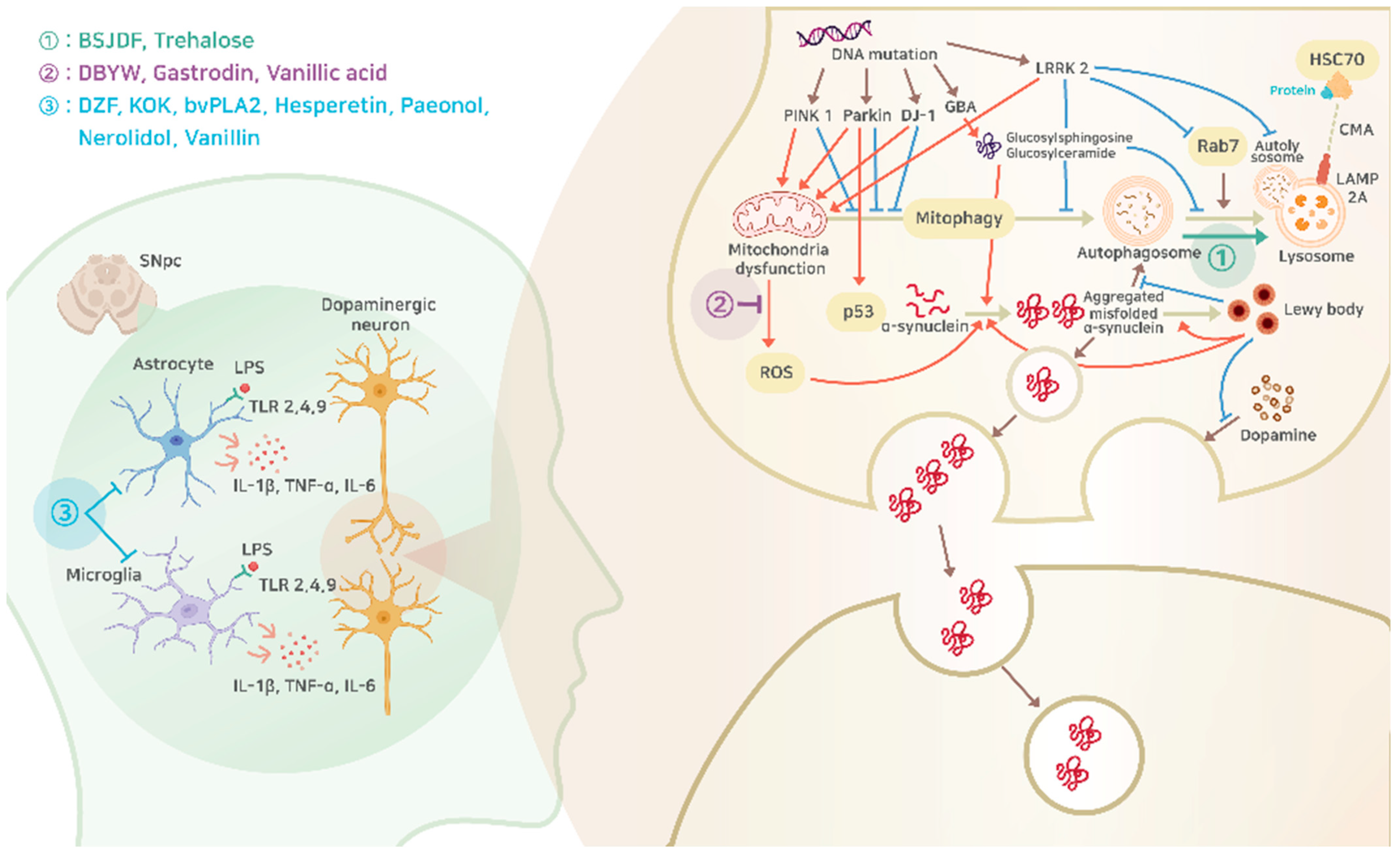
:1. Introduction
2. Pathophysiology of Parkinson’s Disease
2.1. The Main Cause of Parkinson’s Disease
2.2. Mitochondrial Dysfunction
2.3. Lysosomal Disorders
2.3.1. Autophagy–Lysosomal Pathway
2.3.2. Glucocerebrosidase (GCase)
2.3.3. LRRK2
2.4. Inflammation
2.4.1. Microglia
2.4.2. Astrocytes
2.4.3. TLRs
2.4.4. Pro-Inflammatory Cytokines and Parkinson’s Disease
3. Current Medications for Parkinson’s Disease
3.1. Levodopa
3.2. COMT Inhibitor
3.3. MAO-B Inhibitor
3.4. Dopamine Agonist
4. Evidence of Natural Products from Pre-Clinical Studies
4.1. Duzhong Fang
4.2. Kyung-Ok-Ko (KOK)
4.3. Da-Bu-Yin-Wan (DBYW)
4.4. Bee Venom Phospholipase A2 (BvPLa2)
4.5. Hesperetin
4.6. Paeonol
4.7. Gastrodin
4.8. Trehalose
4.9. Bu-Shen-Jie-Du-Fang (BSJDF)
4.10. Nerolidol (NRD)
4.11. Vanillic Acid (VA)
4.12. Vanillin
| Origin of Extraction | Mechanism | Cell or Animal Model | Inducer | Mode of Action and Target Signal | Site of Action (Figure 1) | Ref. |
|---|---|---|---|---|---|---|
| Duzhong Fang | Inflammation | C57bl/6 mice | MPTP | ↓ locomotor dysfunction, inflammation, Iba1, microglia reactivity state ↑ striatal dopamine content, dopaminergic neurons, TH | 3 | [128] |
| KOK | Inflammation | C57BL/6 mice | MPTP ML385 | ↓ neurological dysfunction and motor impairments, the loss of dopaminergic neurons and fibers, Iba1, the upregulation of inflammatory mediators (IL-6, TNF-α, COX-2, and iNOS), neurotoxicity (microglial activation and inflammatory response ↓), BBB disruption markers (PECAM-1 and GFAP), neurotoxicity and inflammation (phosphorylated forms of ERK, JNK, and p38 & IκB and NF-κB ↓), ROS, MAPKs and NF-κB signaling pathways ↑ Nrf2 signaling (decreases the expression levels of Keap1 (a repressor protein that binds to Nrf2), and increases the expression levels of Nrf2 transcription factor, Nrf2 targeting genes HO-1 and NQO-1) | 3 | [130] |
| DBYW | Mitochondrial dysfunction | Rat PC-12 cells | pDJ-1 transfection MPP+ | ↓ DJ-1, mitochondrial dysfunction ↑ mitochondrial mass, total ATP content, the Akt phosphorylation | 2 | [132] |
| BvPLA2 | Inflammation | Human A53T α-Syn Transgenic mice | A53T Transgenes | ↓ motor dysfunction, α-Syn, the activation and numbers of microglia, and the ratio of M1/M2 | 3 | [135] |
| Hesperetin | Inflammation | Wistar rats | 6-OHDA | ↓ astrogliosis (GFAP ↓), apoptosis (nigral DNA fragmentation ↓), the loss of SNC dopaminergic neurons ↑ striatal catalase activity and GSH content, Bcl2 | 3 | [136] |
| Paeonol | Inflammation | C57BL/6 mice | MPTP | ↓ motor dysfunction, oxidative stress (the activity levels of SOD, CAT, and GSH ↑), neuroinflammation(the number of Iba1-positive and IL-1β-positive cells ↓), ↑ TH-positive neurons, BDNF, dopaminergic neurons protection | 3 | [138] |
| Gastrodin | Mitochondrial dysfunction | Drosophila melanogaster | PINK1 gene mutant | ↓ the loss of dopaminergic neurons, the onset of Parkinson-like phenotypes ↑ lifespan, climbing ability, resistance to oxidative stress, enzyme activities of superoxide dismutase (SOD) and catalase (CAT), the expression of anti-oxidative genes | 2 | [140] |
| Trehalose | Lysosomal Disorders | Human A53T α-Syn Transgenic mice | A53T Transgenes | ↓ α-Synuclein-Induced Behavioral Impairment, α-Synuclein Accumulation ↑ DA Neuronal Survival, protection against the reduction of TH protein expression, autophagosome formation, LC3-II levels | 1 | [143] |
| BSJDF | Lysosomal Disorders | Pheochromocytoma12 (PC12) | MPP+ (MPTP) | improved cell survival in the PC12 cell PD model activated the autophagic process in PC12 cells. increased expression of Atg12 and LC3 proteins and upregulated Atg12 mRNA. | 1 | [146] |
| NRD | inflammation | Wistar rats | Rotenone | ↑ level of superoxide dismutase, catalase, and glutathione ↓ level of malondialdehyde inhibited the release of proinflammatory cytokines and inflammatory mediators prevented ROT-induced glial cell activation and the loss of dopaminergic neurons and nerve fibers attenuated rotenone-induced dopaminergic neurodegeneration. | 3 | [148] |
| Vanillic acid | Mitochondrial dysfunction | Sprague Dawley rats | Rotenone | ↓ Weight gain, Catalepsy, Rearing TBARS level (at 25 mg/kg and 50 mg/kg) SAG(superoxide anion generation) ↑ behaviour, CAT | 2 | [149] |
| Vanillin | Inflammation | Male Wistar rats | 6-OHDA | ↓ apomorphine-induced rotations, free radical release, expression of pro-inflammatory cytokines, lipid peroxidation ↑ striatal dopamine content, glutathione and superoxide dismutase enzyme protection of dopaminergic neurons | 3 | [151] |
5. Evidence of Natural Products from Clinical Trials
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, K.h. Korean Medicine Clinical Practice Guideline for Parkinson’s Disease; National Institute for Korean Medicine Development: Seoul, Republic of Korea, 2021.
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.M.; Xu, Z.; Tan, L.C.S. Epidemiology of Parkinson’s Disease—East Versus. West. Mov. Disord. Clin. Pract. 2018, 5, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Jerry, M.; Arcona, S.; McMorrow, D.; Schwartz, H.; Princic, N.; Sasane, R. Work Loss and Direct and Indirect Costs Associated with Parkinson’s Disease. Clin. Outcomes Res. 2023, 15, 309–319. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef]
- Kempster, P.A.; Williams, D.R.; Selikhova, M.; Holton, J.; Revesz, T.; Lees, A.J. Patterns of levodopa response in Parkinson’s disease: A clinico-pathological study. Brain 2007, 130, 2123–2128. [Google Scholar] [CrossRef]
- Bäckström, D.; Granåsen, G.; Domellöf, M.E.; Linder, J.; Jakobson Mo, S.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L. Early predictors of mortality in parkinsonism and Parkinson disease: A population-based study. Neurology 2018, 91, e2045–e2056. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Lim, J.S.; Lee, Y.K.; Sim, S.; Kim, M. Mortality and cause of death in South Korean patients with Parkinson’s disease: A longitudinal follow-up study using a national sample cohort. BMJ Open 2019, 9, e029776. [Google Scholar] [CrossRef]
- Rong, S.; Xu, G.; Liu, B.; Sun, Y.; Snetselaar, L.G.; Wallace, R.B.; Li, B.; Liao, J.; Bao, W. Trends in Mortality From Parkinson Disease in the United States, 1999–2019. Neurology 2021, 97, e1986–e1993. [Google Scholar] [CrossRef]
- Macleod, A.D.; Taylor, K.S.; Counsell, C.E. Mortality in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1615–1622. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef]
- Schaeffer, E.; Postuma, R.B.; Berg, D. Prodromal PD: A new nosological entity. Prog. Brain Res. 2020, 252, 331–356. [Google Scholar] [PubMed]
- Crouse, J.J.; Phillips, J.R.; Jahanshahi, M.; Moustafa, A.A. Postural instability and falls in Parkinson’s disease. Rev. Neurosci. 2016, 27, 549–555. [Google Scholar] [CrossRef]
- Palakurthi, B.; Burugupally, S.P. Postural Instability in Parkinson’s Disease: A Review. Brain Sci. 2019, 9, 239. [Google Scholar] [CrossRef]
- Schrag, A.; Ben-Shlomo, Y.; Quinn, N. How common are complications of Parkinson’s disease? J. Neurol. 2002, 249, 419–423. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Marino, B.L.B.; de Souza, L.R.; Sousa, K.P.A.; Ferreira, J.V.; Padilha, E.C.; da Silva, C.; Taft, C.A.; Hage-Melim, L.I.S. Parkinson’s Disease: A Review from Pathophysiology to Treatment. Mini Rev. Med. Chem. 2020, 20, 754–767. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Biundo, R.; Dobkin, R.; Goldman, J.; Lewis, S. Management of psychiatric and cognitive complications in Parkinson’s disease. BMJ 2022, 379, e068718. [Google Scholar] [CrossRef]
- Yang, J.Y.; Jin, C.; Lee, J.; Lee, H.G.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; et al. Patients with Parkinson Disease in a Traditional Korean Medicine Hospital: A Five-Year Audit. Evid. Based Complement. Altern. Med. 2021, 2021, 6842863. [Google Scholar] [CrossRef] [PubMed]
- Jun, P.; Zhao, H.; Kwon, O.; Jang, J.H. Efficacy of Traditional Herbal Medicine Treatment Based on Pattern Identification for Idiopathic Parkinson’s Disease: A Protocol for Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 4777849. [Google Scholar] [CrossRef]
- Bae, N.; Chung, S.; Kim, H.J.; Cha, J.W.; Oh, H.; Gu, M.Y.; Oh, M.S.; Yang, H.O. Neuroprotective effect of modified Chungsimyeolda-tang, a traditional Korean herbal formula, via autophagy induction in models of Parkinson’s disease. J. Ethnopharmacol. 2015, 159, 93–101. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Dagra, A.; Miller, D.R.; Lin, M.; Gopinath, A.; Shaerzadeh, F.; Harris, S.; Sorrentino, Z.A.; Støier, J.F.; Velasco, S.; Azar, J.; et al. α-Synuclein-induced dysregulation of neuronal activity contributes to murine dopamine neuron vulnerability. NPJ Park. Dis. 2021, 7, 76. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Breydo, L.; Wu, J.W.; Uversky, V.N. A-synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta 2012, 1822, 261–885. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Rideout, H.J.; Stefanis, L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011, 10, 1015–1025. [Google Scholar] [CrossRef]
- Cookson, M.R. Alpha-Synuclein and neuronal cell death. Mol. Neurodegener. 2009, 4, 9. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Olanow, C.W.; Perl, D.P.; DeMartino, G.N.; McNaught, K.S. Lewy-body formation is an aggresome-related process: A hypothesis. Lancet Neurol. 2004, 3, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Ricarte, D.; Ortiz, D.; Lee, S.-J. Models of multiple system atrophy. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Lee, H.-J.; Bae, E.-J.; Lee, S.-J. Extracellular α-synuclein—A novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014, 10, 92–98. [Google Scholar] [CrossRef] [PubMed]
- McCann, H.; Cartwright, H.; Halliday, G.M. Neuropathology of α-synuclein propagation and braak hypothesis. Mov. Disord. 2016, 31, 152–160. [Google Scholar] [CrossRef]
- Schulz-Schaeffer, W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef]
- Kefalopoulou, Z.; Politis, M.; Piccini, P.; Mencacci, N.; Bhatia, K.; Jahanshahi, M.; Widner, H.; Rehncrona, S.; Brundin, P.; Björklund, A.; et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: Two case reports. JAMA Neurol. 2014, 71, 83–87. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Burke, R.E.; Dauer, W.T.; Vonsattel, J.P. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann. Neurol. 2008, 64, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 2023, 18, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Santamaria, J.; Gaig, C.; Compta, Y. Chapter 14—Nonmotor Aspects of Parkinson’s Disease. In Blue Books of Neurology; Schapira, A.H.V., Lang, A.E.T., Fahn, S., Eds.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 229–251. [Google Scholar]
- Jellinger, K.A. Is Braak staging valid for all types of Parkinson’s disease? J. Neural. Transm. 2019, 126, 423–431. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Kang, S.S.; Liu, X.; Chen, G.; Zhang, Z.; Chandrasekharan, B.; Alam, A.M.; Neish, A.S.; Cao, X.; Ye, K. Initiation of Parkinson’s disease from gut to brain by δ-secretase. Cell Res. 2020, 30, 70–87. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 782082. [Google Scholar] [CrossRef]
- Deng, J.H.; Zhang, H.W.; Liu, X.L.; Deng, H.Z.; Lin, F. Morphological changes in Parkinson’s disease based on magnetic resonance imaging: A mini-review of subcortical structures segmentation and shape analysis. World J. Psychiatry 2022, 12, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Painous, C.; Pascual-Diaz, S.; Muñoz-Moreno, E.; Sánchez, V.; Pariente, J.C.; Prats-Galino, A.; Soto, M.; Fernández, M.; Pérez-Soriano, A.; Camara, A.; et al. Midbrain and pons MRI shape analysis and its clinical and CSF correlates in degenerative parkinsonisms: A pilot study. Eur. Radiol. 2023, 33, 4540–4551. [Google Scholar] [CrossRef]
- Kuzkina, A.; Rößle, J.; Seger, A.; Panzer, C.; Kohl, A.; Maltese, V.; Musacchio, T.; Blaschke, S.J.; Tamgüney, G.; Kaulitz, S.; et al. Combining skin and olfactory α-synuclein seed amplification assays (SAA)-towards biomarker-driven phenotyping in synucleinopathies. NPJ Park. Dis. 2023, 9, 79. [Google Scholar] [CrossRef]
- Kuzkina, A.; Bargar, C.; Schmitt, D.; Rößle, J.; Wang, W.; Schubert, A.L.; Tatsuoka, C.; Gunzler, S.A.; Zou, W.Q.; Volkmann, J.; et al. Diagnostic value of skin RT-QuIC in Parkinson’s disease: A two-laboratory study. NPJ Park. Dis. 2021, 7, 99. [Google Scholar] [CrossRef]
- Fricova, D.; Harsanyiova, J.; Trancikova, A.K. Alpha-Synuclein in the Gastrointestinal Tract as a Potential Biomarker for Early Detection of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8666. [Google Scholar] [CrossRef] [PubMed]
- Ruffmann, C.; Bengoa-Vergniory, N.; Poggiolini, I.; Ritchie, D.; Hu, M.T.; Alegre-Abarrategui, J.; Parkkinen, L. Detection of alpha-synuclein conformational variants from gastro-intestinal biopsy tissue as a potential biomarker for Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2018, 44, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lim, K.L.; Tan, E.K. Intestine-derived α-synuclein initiates and aggravates pathogenesis of Parkinson’s disease in Drosophila. Transl. Neurodegener. 2022, 11, 44. [Google Scholar] [CrossRef]
- Horsager, J.; Knudsen, K.; Sommerauer, M. Clinical and imaging evidence of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 164, 105626. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Kaur, R.; Mehan, S.; Singh, S. Understanding multifactorial architecture of Parkinson’s disease: Pathophysiology to management. Neurol. Sci. 2019, 40, 13–23. [Google Scholar] [CrossRef]
- Charan, R.A.; Johnson, B.N.; Zaganelli, S.; Nardozzi, J.D.; LaVoie, M.J. Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin. Cell Death Dis. 2014, 5, e1313. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J. Mitochondrial complex I deficiency and Parkinson disease. Nat. Rev. Neurosci. 2023, 24, 193. [Google Scholar] [CrossRef]
- Chen, C.; McDonald, D.; Blain, A.; Mossman, E.; Atkin, K.; Marusich, M.F.; Capaldi, R.; Bone, L.; Smith, A.; Filby, A.; et al. Parkinson’s disease neurons exhibit alterations in mitochondrial quality control proteins. NPJ Park. Dis. 2023, 9, 120. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- O’Hara, D.M.; Pawar, G.; Kalia, S.K.; Kalia, L.V. LRRK2 and α-Synuclein: Distinct or Synergistic Players in Parkinson’s Disease? Front. Neurosci. 2020, 14, 577. [Google Scholar] [CrossRef]
- Zanon, A.; Pramstaller, P.P.; Hicks, A.A.; Pichler, I. Environmental and Genetic Variables Influencing Mitochondrial Health and Parkinson’s Disease Penetrance. Park. Dis. 2018, 2018, 8684906. [Google Scholar] [CrossRef]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef]
- Park, J.S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Madsen, D.A.; Schmidt, S.I.; Blaabjerg, M.; Meyer, M. Interaction between Parkin and α-Synuclein in PARK2-Mediated Parkinson’s Disease. Cells 2021, 10, 283. [Google Scholar] [CrossRef]
- Matheoud, D.; Sugiura, A.; Bellemare-Pelletier, A.; Laplante, A.; Rondeau, C.; Chemali, M.; Fazel, A.; Bergeron, J.J.; Trudeau, L.E.; Burelle, Y.; et al. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell 2016, 166, 314–327. [Google Scholar] [CrossRef]
- Mencke, P.; Boussaad, I.; Romano, C.D.; Kitami, T.; Linster, C.L.; Krüger, R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells 2021, 10, 347. [Google Scholar] [CrossRef]
- Imberechts, D.; Kinnart, I.; Wauters, F.; Terbeek, J.; Manders, L.; Wierda, K.; Eggermont, K.; Madeiro, R.F.; Sue, C.; Verfaillie, C.; et al. DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy. Brain 2022, 145, 4368–4384. [Google Scholar] [CrossRef] [PubMed]
- Ammal Kaidery, N.; Thomas, B. Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem. Int. 2018, 117, 91–113. [Google Scholar] [CrossRef]
- Pan, T.; Kondo, S.; Le, W.; Jankovic, J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 2008, 131, 1969–1978. [Google Scholar] [CrossRef]
- Navarro-Romero, A.; Montpeyó, M.; Martinez-Vicente, M. The Emerging Role of the Lysosome in Parkinson’s Disease. Cells 2020, 9, 2399. [Google Scholar] [CrossRef]
- Abe, T.; Kuwahara, T. Targeting of Lysosomal Pathway Genes for Parkinson’s Disease Modification: Insights From Cellular and Animal Models. Front. Neurol. 2021, 12, 681369. [Google Scholar] [CrossRef]
- Lehtonen, Š.; Sonninen, T.M.; Wojciechowski, S.; Goldsteins, G.; Koistinaho, J. Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front. Neurosci. 2019, 13, 457. [Google Scholar] [CrossRef]
- Jackson, M.P.; Hewitt, E.W. Cellular proteostasis: Degradation of misfolded proteins by lysosomes. Essays Biochem. 2016, 60, 173–180. [Google Scholar]
- Senkevich, K.; Gan-Or, Z. Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Park. Relat. Disord. 2020, 73, 60–71. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Dion, P.A.; Rouleau, G.A. Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy 2015, 11, 1443–1457. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Lwin, A.; Orvisky, E.; Goker-Alpan, O.; LaMarca, M.E.; Sidransky, E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 2004, 81, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016, 5, e12813. [Google Scholar] [CrossRef]
- Orenstein, S.J.; Kuo, S.H.; Tasset, I.; Arias, E.; Koga, H.; Fernandez-Carasa, I.; Cortes, E.; Honig, L.S.; Dauer, W.; Consiglio, A.; et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 2013, 16, 394–406. [Google Scholar] [CrossRef]
- Gómez-Suaga, P.; Fdez, E.; Ramírez, M.B.; Hilfiker, S. A Link between Autophagy and the Pathophysiology of LRRK2 in Parkinson’s Disease. Park. Dis. 2012, 2012, 324521. [Google Scholar] [CrossRef]
- Teixeira, M.; Sheta, R.; Idi, W.; Oueslati, A. Alpha-Synuclein and the Endolysosomal System in Parkinson’s Disease: Guilty by Association. Biomolecules 2021, 11, 1333. [Google Scholar] [CrossRef]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Qian, L.; Flood, P.M. Microglial cells and Parkinson’s disease. Immunol. Res. 2008, 41, 155–164. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals 2014, 7, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Talpalar, A.E.; Ben-Yaakov, K.; Schwartz, M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol. Cell. Neurosci. 2005, 29, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Spittau, B.; Krieglstein, K. TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J. Neuroinflammation 2012, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Isik, S.; Kiyak, B.Y.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Kianmehr, M.; Anaeigoudari, A. Effects of Medicinal Plants and Flavonoids on Parkinson’s Disease: A Review on Basic and Clinical Evidences. Adv. Pharm. Bull 2021, 11, 224–232. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Yu, L.; Cui, R.; Zhang, Y.; Ding, H.; Yan, G. Shaoyao-Gancao Decoction Promoted Microglia M2 Polarization via the IL-13-Mediated JAK2/STAT6 Pathway to Alleviate Cerebral Ischemia-Reperfusion Injury. Mediat. Inflamm. 2022, 2022, 1707122. [Google Scholar] [CrossRef]
- Wang, T.; Yin, Y.; Jiang, X.; Ruan, Y.; Xu, J.; Hu, X.; Li, T.; Chu, L.; Li, L. Exploring the mechanism of luteolin by regulating microglia polarization based on network pharmacology and in vitro experiments. Sci. Rep. 2023, 13, 13767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Xue, R.; Fan, S.Y.; Fan, Q.Y.; An, L.; Li, J.; Zhu, L.; Ran, Y.H.; Zhang, L.M.; Zhong, B.H.; et al. Ammoxetine attenuates diabetic neuropathic pain through inhibiting microglial activation and neuroinflammation in the spinal cord. J. Neuroinflammation 2018, 15, 176. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflammation 2022, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Yang, C.; Mo, Y.; Xu, E.; Wen, H.; Wei, R.; Li, S.; Zheng, J.; Li, W.; Le, B.; Chen, Y.; et al. Astragaloside IV ameliorates motor deficits and dopaminergic neuron degeneration via inhibiting neuroinflammation and oxidative stress in a Parkinson’s disease mouse model. Int. Immunopharmacol. 2019, 75, 105651. [Google Scholar] [CrossRef]
- Maciel-Barón, L.; Moreno-Blas, D.; Morales-Rosales, S.L.; González-Puertos, V.Y.; López-Díazguerrero, N.E.; Torres, C.; Castro-Obregón, S.; Königsberg, M. Cellular Senescence, Neurological Function, and Redox State. Antioxid. Redox Signal. 2018, 28, 1704–1723. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Spinelli, C.C.; Martucciello, S.; Nori, S.L.; Capunzo, M.; Puca, A.A.; Ciaglia, E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018, 103, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.L.; Xie, X.H.; Ding, J.H.; Du, R.H.; Hu, G. Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. J. Neuroinflammation 2020, 17, 105. [Google Scholar] [CrossRef]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo. Nat. Commun. 2021, 12, 5382. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Gundersen, V. Parkinson’s Disease: Can Targeting Inflammation Be an Effective Neuroprotective Strategy? Front. Neurosci. 2020, 14, 580311. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Inflammatory process in Parkinson’s disease: Role for cytokines. Curr. Pharm. Des. 2005, 11, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Koprich, J.B.; Reske-Nielsen, C.; Mithal, P.; Isacson, O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflammation 2008, 5, 8. [Google Scholar] [CrossRef]
- de Bie, R.M.A.; Clarke, C.E.; Espay, A.J.; Fox, S.H.; Lang, A.E. Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet Neurol. 2020, 19, 452–461. [Google Scholar] [CrossRef]
- Ntetsika, T.; Papathoma, P.E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17. [Google Scholar] [CrossRef]
- Carrarini, C.; Russo, M.; Dono, F.; Di Pietro, M.; Rispoli, M.G.; Di Stefano, V.; Ferri, L.; Barbone, F.; Vitale, M.; Thomas, A.; et al. A Stage-Based Approach to Therapy in Parkinson’s Disease. Biomolecules 2019, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, J.H.; Kim, B.; Yang, G.; Kim, J.U. Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 8827. [Google Scholar] [CrossRef]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D. Parkinson’s disease treatment: Past, present, and future. J. Neural. Transm. 2020, 127, 785–791. [Google Scholar] [CrossRef]
- Kaakkola, S. Problems with the present inhibitors and a relevance of new and improved COMT inhibitors in Parkinson’s disease. Int. Rev. Neurobiol. 2010, 95, 207–225. [Google Scholar]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Malek, N.M.; Grosset, D.G. Investigational agents in the treatment of Parkinson’s disease: Focus on safinamide. J. Exp. Pharmacol. 2012, 4, 85–90. [Google Scholar] [CrossRef]
- Jost, W.H. A critical appraisal of MAO-B inhibitors in the treatment of Parkinson’s disease. J. Neural. Transm. 2022, 129, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A. Side effects of a dopamine agonist therapy for Parkinson’s disease: A mini-review of clinical pharmacology. Yale J. Biol. Med. 2016, 89, 37–47. [Google Scholar] [PubMed]
- Fan, S.; Yin, Q.; Li, D.; Ma, J.; Li, L.; Chai, S.; Guo, H.; Yang, Z. Anti-neuroinflammatory effects of Eucommia ulmoides Oliv. In a Parkinson’s mouse model through the regulation of p38/JNK-Fosl2 gene expression. J. Ethnopharmacol. 2020, 260, 113016. [Google Scholar]
- Li, L.; Fan, S.; Zhang, W.; Li, D.; Yang, Z.; Zhuang, P.; Han, J.; Guo, H.; Zhang, Y. Duzhong Fang Attenuates the POMC-Derived Neuroinflammation in Parkinsonian Mice. J. Inflamm. Res. 2021, 14, 3261–3276. [Google Scholar] [CrossRef]
- Jang, M.; Lee, M.J.; Lee, J.M.; Bae, C.S.; Kim, S.H.; Ryu, J.H.; Cho, I.H. Oriental medicine Kyung-Ok-Ko prevents and alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats. PLoS ONE 2014, 9, e87623. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, M.; Lee, J.I.; Chung, W.S.; Cho, I.H. Neuroprotective Effects of a Traditional Multi-Herbal Medicine Kyung-Ok-Ko in an Animal Model of Parkinson’s Disease: Inhibition of MAPKs and NF-κB Pathways and Activation of Keap1-Nrf2 Pathway. Front. Pharmacol. 2018, 9, 1444. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.M.; He, X.; Wang, Y.Y.; Gao, Y.S.; Wu, H.X.; Xu, H.; Gong, X.G.; Guo, Z.Y. Da-Bu-Yin-Wan and Qian-Zheng-San, two traditional Chinese herbal formulas, up-regulate the expression of mitochondrial subunit NADH dehydrogenase 1 synergistically in the mice model of Parkinson’s disease. J. Ethnopharmacol. 2013, 146, 363–371. [Google Scholar] [CrossRef]
- Zhang, Y.; XGong, G.; Sun, H.M.; Guo, Z.Y.; Hu, J.H.; Wang, Y.Y.; Feng, W.D.; Li, L.; Li, P.; Wang, Z.Z.; et al. Da-Bu-Yin-Wan Improves the Ameliorative Effect of DJ-1 on Mitochondrial Dysfunction Through Augmenting the Akt Phosphorylation in a Cellular Model of Parkinson’s Disease. Front. Pharmacol. 2018, 9, 1206. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef]
- Nakashima, S.; Kitamoto, K.; Arioka, M. The catalytic activity, but not receptor binding, of sPLA2s plays a critical role for neurite outgrowth induction in PC12 cells. Brain Res. 2004, 1015, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chung, H.S.; Lee, C.; Song, J.H.; Shim, I.; Kim, Y.S.; Bae, H. Bee venom phospholipase A2 ameliorates motor dysfunction and modulates microglia activation in Parkinson’s disease alpha-synuclein transgenic mice. Exp. Mol. Med 2016, 48, e244. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Khalili, M.; Baluchnejadmojarad, T.; Roghani, M. Protective Effect of Oral Hesperetin Against Unilateral Striatal 6-Hydroxydopamine Damage in the Rat. Neurochem. Res. 2016, 41, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, D.; Luo, N.; Yang, C.; Zhu, Y. Four active monomers from Moutan Cortex exert inhibitory effects against oxidative stress by activating Nrf2/Keap1 signaling pathway. Korean J. Physiol. Pharmacol. 2020, 24, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Y.H.; Liu, H.; Qu, H.D. Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced Parkinson’s disease in mice. Mol. Med. Rep. 2016, 14, 2397–2404. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Yang, S.; Li, Y.; Lin, X.; Xiu, M.; Li, X.; Liu, Y. Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson’s disease. Food Funct. 2021, 12, 7816–7824. [Google Scholar] [CrossRef]
- Pupyshev, A.B.; Klyushnik, T.P.; Akopyan, A.A.; Singh, S.K.; Tikhonova, M.A. Disaccharide trehalose in experimental therapies for neurodegenerative disorders: Molecular targets and translational potential. Pharmacol. Res. 2022, 183, 106373. [Google Scholar] [CrossRef]
- Bastin, A.R.; Nazari-Robati, M.; Sadeghi, H.; Doustimotlagh, A.H.; Sadeghi, A. Trehalose and N-Acetyl Cysteine Alleviate Inflammatory Cytokine Production and Oxidative Stress in LPS-Stimulated Human Peripheral Blood Mononuclear Cells. Immunol. Investig. 2022, 51, 963–979. [Google Scholar] [CrossRef]
- He, Q.; Koprich, J.B.; Wang, Y.; Yu, W.B.; Xiao, B.G.; Brotchie, J.M.; Wang, J. Treatment with Trehalose Prevents Behavioral and Neurochemical Deficits Produced in an AAV α-Synuclein Rat Model of Parkinson’s Disease. Mol. Neurobiol. 2016, 53, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.; Mok, S.W.; Wu, A.G.; Lam, C.W.; Yu, M.X.; Wong, V.K. New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy. Molecules 2016, 21, 359. [Google Scholar] [CrossRef]
- Chang, W.H.; Chen, C.H.; Lu, F.J. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002, 68, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, X.; Qiu, S.; Chen, W.; Li, W.; Zhang, H.; Wang, T.; Wang, X.; Wu, X. Chinese Herbal Complex ‘Bu Shen Jie Du Fang’ (BSJDF) Modulated Autophagy in an MPP(+)-Induced Cell Model of Parkinson’s Disease. Evid. Based Complement Altern. Med. 2019, 2019, 8920813. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Neto, J.D.; de Almeida, A.A.; da Silva Oliveira, J.; Santos, P.S.D.; de Sousa, D.P.; de Freitas, R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U.; et al. A review of the pharmacological characteristics of vanillic acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Lu, C.; Qu, S.; Zhong, Z.; Luo, H.; Lei, S.S.; Zhong, H.J.; Su, H.; Wang, Y.; Chong, C.M. The effects of bioactive components from the rhizome of gastrodia elata blume (Tianma) on the characteristics of Parkinson’s disease. Front. Pharmacol. 2022, 13, 963327. [Google Scholar] [CrossRef]
- Abuthawabeh, R.; Abuirmeileh, A.N.; Alzoubi, K.H. The beneficial effect of vanillin on 6-hydroxydopamine rat model of Parkinson’s disease. Restor. Neurol. Neurosci. 2020, 38, 369–373. [Google Scholar] [CrossRef]
- Petramfar, P.; Hajari, F.; Yousefi, G.; Azadi, S.; Hamedi, A. Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson’s disease, A randomized double blinded clinical trial. J. Ethnopharmacol. 2020, 247, 112226. [Google Scholar] [CrossRef]
- Zargaran, A.; Zarshenas, M.M.; Mehdizadeh, A.; Mohagheghzadeh, A. Management of tremor in medieval Persia. J. Hist Neurosci. 2013, 22, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Sheela, M.L.; Ramakrishna, M.K.; Salimath, B.P. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int. Immunopharmacol. 2006, 6, 494–498. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Parle, M.; Kulkarni, S.K. Memory enhancing activity of Glycyrrhiza glabra in mice. J. Ethnopharmacol. 2004, 91, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Xue, C.C.; Zhou, Z.W.; Li, C.G.; Du, Y.M.; Liang, J.; Zhou, S.F. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci. 2008, 82, 68–78. [Google Scholar] [CrossRef]
- Chahra, C.; Anis, H.; Bissene, D.; Mejda, S.; Jihène, M.; Salma, N.; Manel, N.; Sana, B.A.; Hedi, K.; Maha, B.F. The effect of Origanum majorana tea on motor and non-motor symptoms in patients with idiopathic Parkinson’s disease: A randomized controlled pilot study. Park. Relat. Disord. 2021, 91, 23–27. [Google Scholar] [CrossRef]
- Gu, S.C.; Ye, Q.; Wang, C.D.; Zhao, S.R.; Zhou, J.; Gao, C.; Zhang, Y.; Liu, Z.G.; Yuan, C.X. Pingchan Granule for Motor Symptoms and Non-Motor Symptoms of Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Pharmacol. 2022, 13, 739194. [Google Scholar]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Schol. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Radhakrishnan, D.M.; Goyal, V. Parkinson’s disease: A review. Neurol. India 2018, 66, S26–S35. [Google Scholar]
- Mullin, S.; Schapira, A. α-Synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol. Neurobiol. 2013, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Mazzulli, J.R.; Zunke, F.; Isacson, O.; Studer, L.; Krainc, D. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. USA 2016, 113, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- La Vitola, P.; Balducci, C.; Baroni, M.; Artioli, L.; Santamaria, G.; Castiglioni, M.; Cerovic, M.; Colombo, L.; Caldinelli, L.; Pollegioni, L.; et al. Peripheral inflammation exacerbates α-synuclein toxicity and neuropathology in Parkinson’s models. Neuropathol. Appl. Neurobiol. 2021, 47, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, Y.-J.; Lee, J.-U.; Yang, G.-S.; Yang, G.; Kim, S.-W.; Lee, J.-H.; Kim, J.-U. The Potentiality of Natural Products and Herbal Medicine as Novel Medications for Parkinson’s Disease: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 1071. https://doi.org/10.3390/ijms25021071
So Y-J, Lee J-U, Yang G-S, Yang G, Kim S-W, Lee J-H, Kim J-U. The Potentiality of Natural Products and Herbal Medicine as Novel Medications for Parkinson’s Disease: A Promising Therapeutic Approach. International Journal of Molecular Sciences. 2024; 25(2):1071. https://doi.org/10.3390/ijms25021071
Chicago/Turabian StyleSo, Yu-Jin, Jae-Ung Lee, Ga-Seung Yang, Gabsik Yang, Sung-Wook Kim, Jun-Ho Lee, and Jong-Uk Kim. 2024. "The Potentiality of Natural Products and Herbal Medicine as Novel Medications for Parkinson’s Disease: A Promising Therapeutic Approach" International Journal of Molecular Sciences 25, no. 2: 1071. https://doi.org/10.3390/ijms25021071
APA StyleSo, Y.-J., Lee, J.-U., Yang, G.-S., Yang, G., Kim, S.-W., Lee, J.-H., & Kim, J.-U. (2024). The Potentiality of Natural Products and Herbal Medicine as Novel Medications for Parkinson’s Disease: A Promising Therapeutic Approach. International Journal of Molecular Sciences, 25(2), 1071. https://doi.org/10.3390/ijms25021071





