Membrane-Bound Redox Enzyme Cytochrome bd-I Promotes Carbon Monoxide-Resistant Escherichia coli Growth and Respiration
Abstract
1. Introduction
2. Results
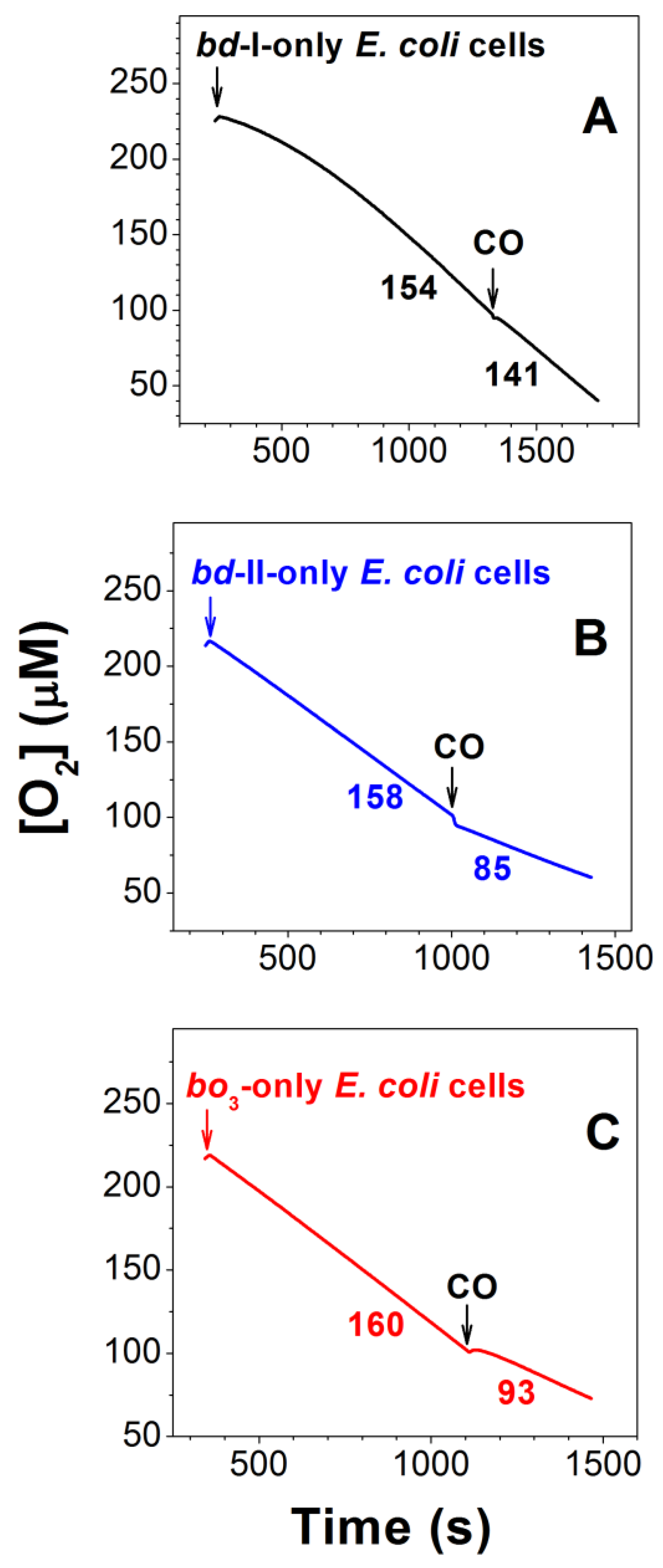
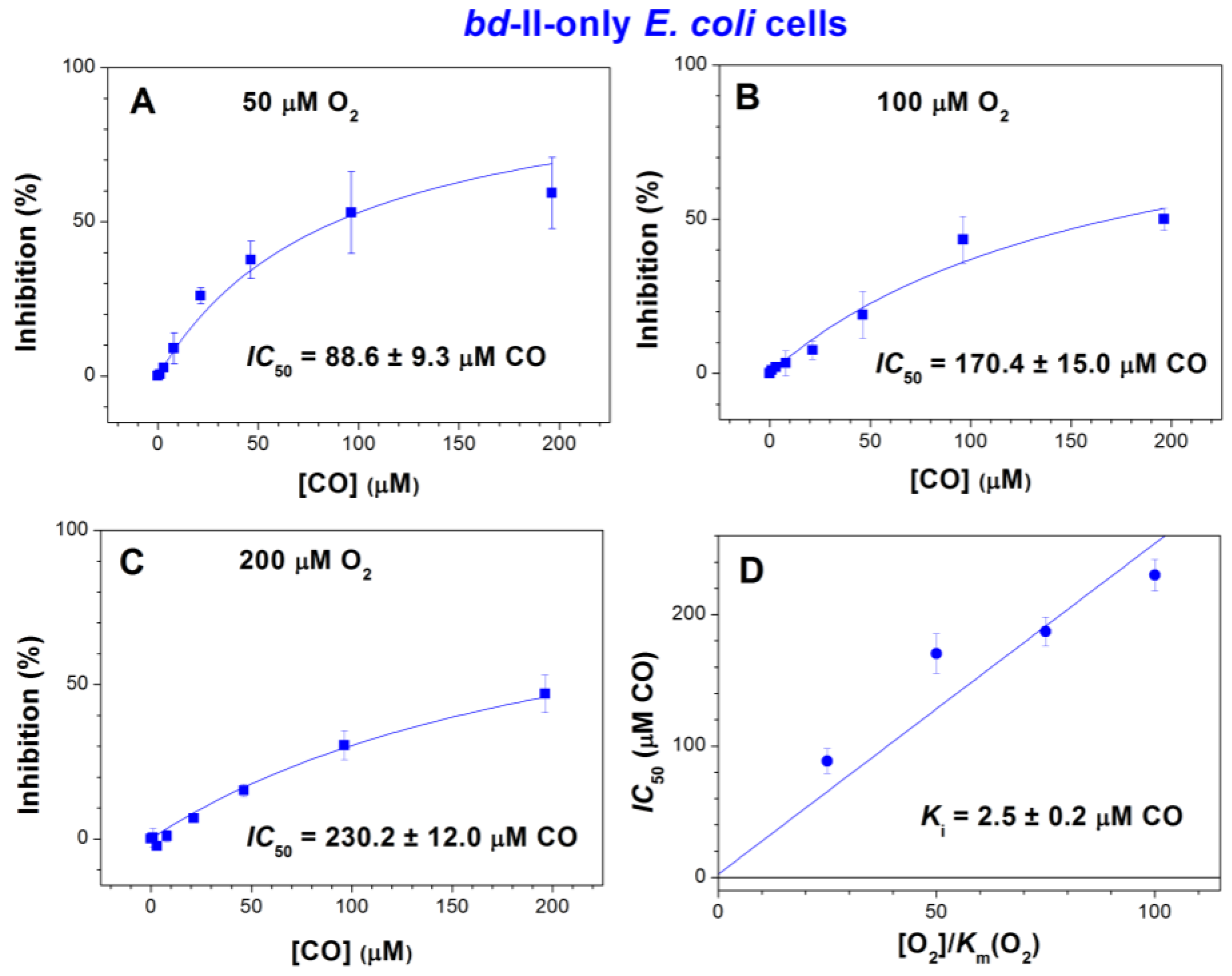
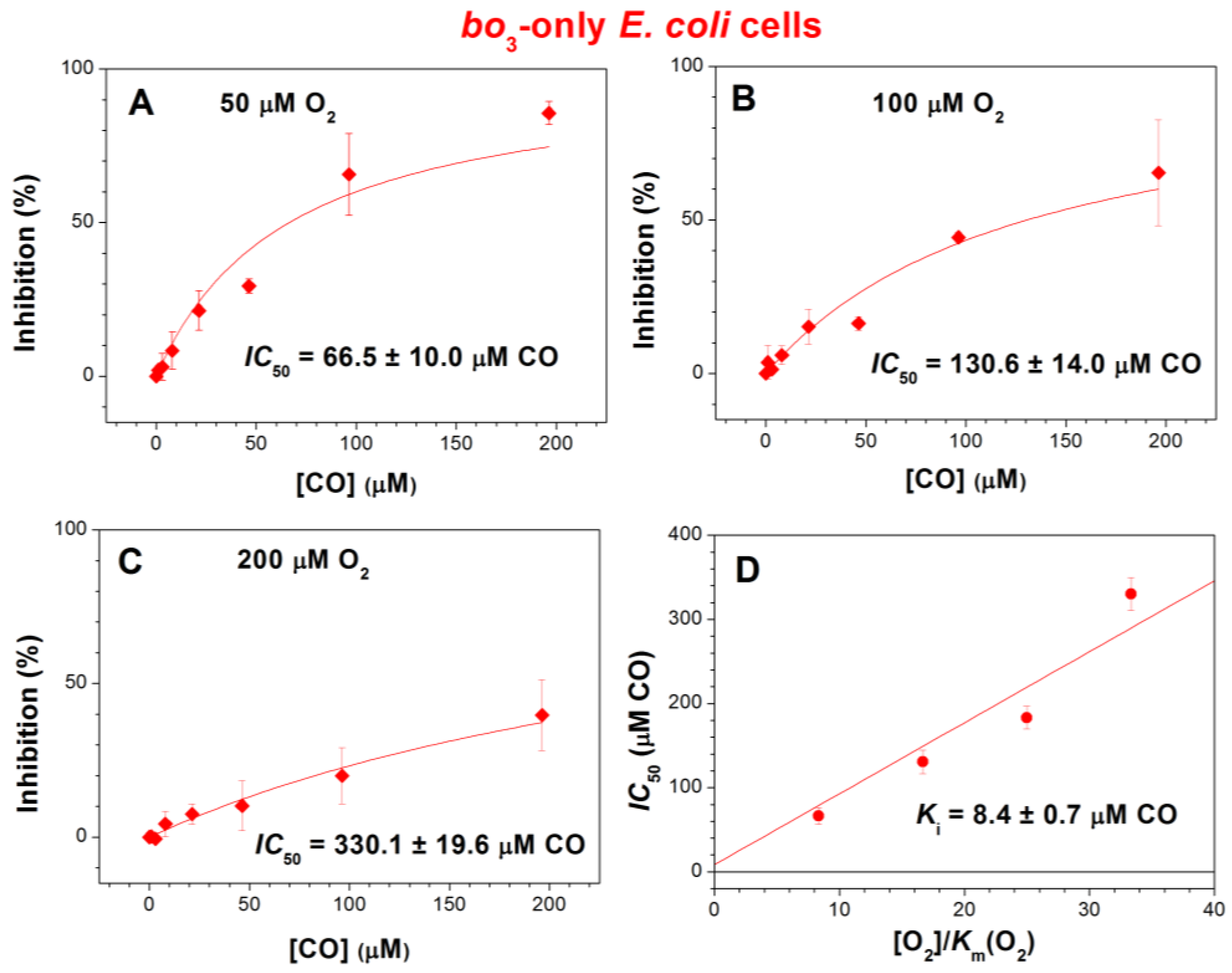
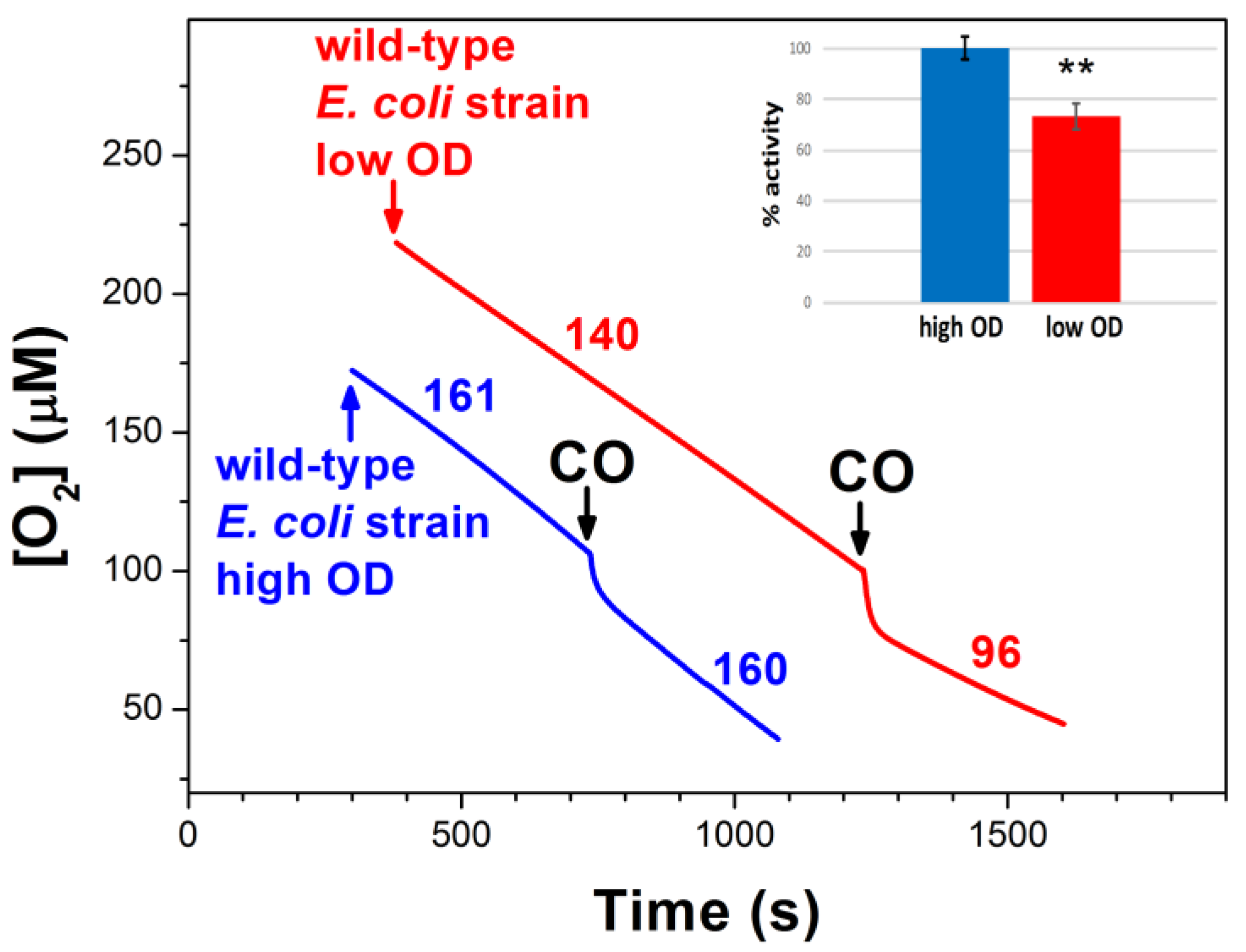
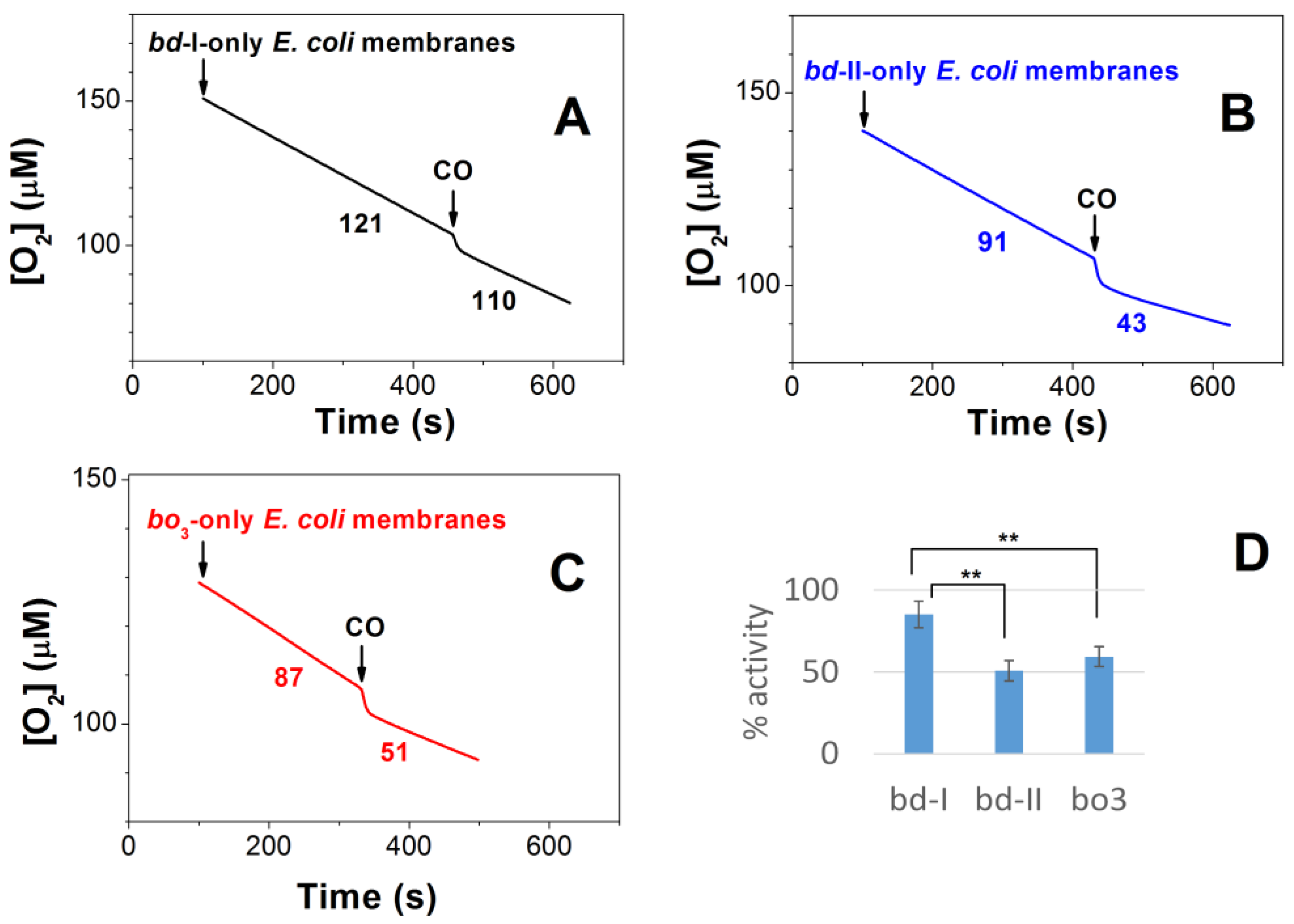
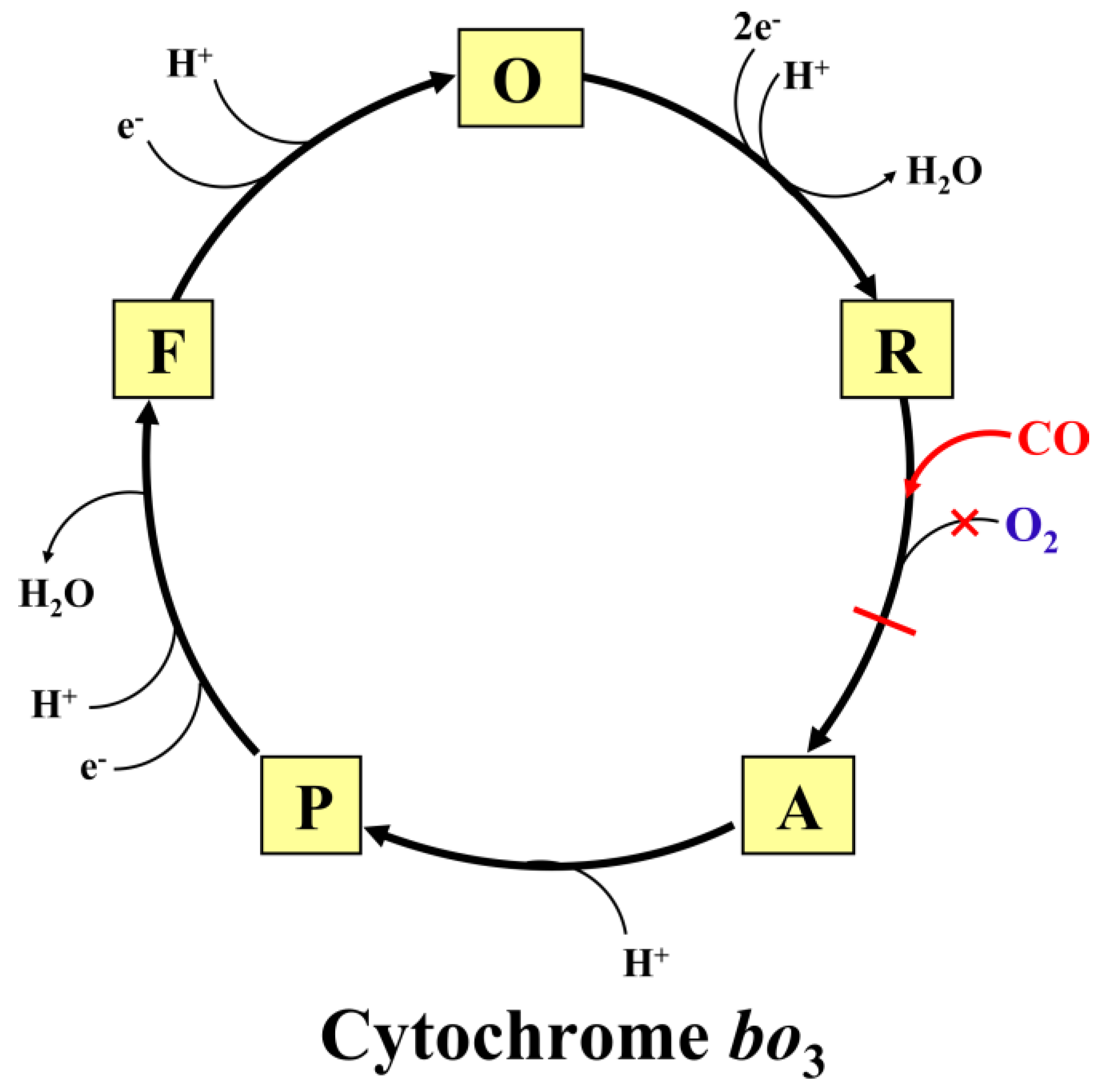
2.1. Effect of CO on E. coli Aerobic Respiration
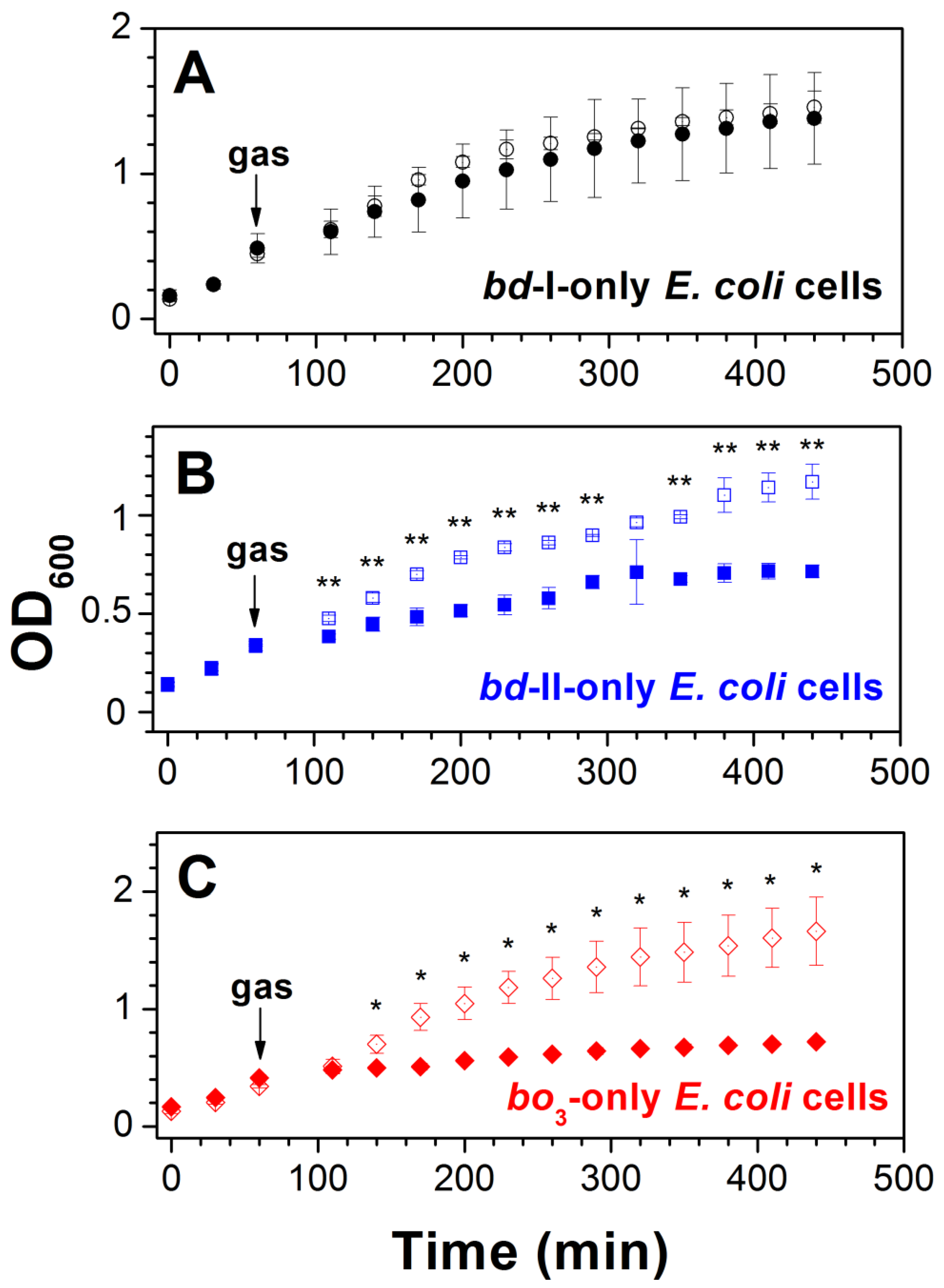
2.2. Effect of CO on E. coli Cell Growth
3. Discussion
4. Materials and Methods
4.1. Materials, E. coli Mutant Strains and Growth Conditions
4.2. Investigation of the Effect of CO on Respiration of Wild-Type E. coli Cells
4.3. Isolation of Membranes from E. coli Respiratory Mutants
4.4. Oxygraphic Measurements
4.5. Spectroscopic Measurements
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingi, T.; Ronnett, G.V. Direct demonstration of a physiological role for carbon monoxide in olfactory receptor neurons. J. Neurosci. 1995, 15, 8214–8222. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Schultz, G.; Koesling, D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. Embo J. 1996, 15, 6863–6868. [Google Scholar] [CrossRef] [PubMed]
- Siow, R.C.; Sato, H.; Mann, G.E. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: Anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc. Res. 1999, 41, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Boehning, D.; Snyder, S.H. Circadian rhythms. Carbon monoxide and clocks. Science 2002, 298, 2339–2340. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Sethi, J.; Choi, A.M. Carbon monoxide-dependent signaling. Crit. Care Med. 2002, 30, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell Biochem. 2002, 234-235, 249–263. [Google Scholar] [CrossRef]
- Bilban, M.; Haschemi, A.; Wegiel, B.; Chin, B.Y.; Wagner, O.; Otterbein, L.E. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J. Mol. Med. (Berl.) 2008, 86, 267–279. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 2008, 45, 562–569. [Google Scholar] [CrossRef]
- Olson, K.R.; Donald, J.A. Nervous control of circulation-the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009, 111, 244–256. [Google Scholar] [CrossRef]
- Wang, X.M.; Kim, H.P.; Nakahira, K.; Ryter, S.W.; Choi, A.M. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 2009, 182, 3809–3818. [Google Scholar] [CrossRef]
- Farrugia, G.; Szurszewski, J.H. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology 2014, 147, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Maki, T.; Mandeville, E.T.; Koh, S.H.; Hayakawa, K.; Arai, K.; Kim, Y.M.; Whalen, M.J.; Xing, C.; Wang, X.; et al. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat. Med. 2016, 22, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Wood, H. Traumatic brain injury: Carbon monoxide-a potential therapy for traumatic brain injury? Nat. Rev. Neurol. 2016, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, Y.; Kim, S.K.; Park, S.U.; Park, J.; Chen, Y.; Kim, J.; Ryu, J.; Cho, G.J.; Surh, Y.J.; et al. Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK-eIF2alpha-ATF4 pathway. Free Radic. Biol. Med. 2017, 110, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Klemz, R.; Reischl, S.; Wallach, T.; Witte, N.; Jurchott, K.; Klemz, S.; Lang, V.; Lorenzen, S.; Knauer, M.; Heidenreich, S.; et al. Reciprocal regulation of carbon monoxide metabolism and the circadian clock. Nat. Struct. Mol. Biol. 2017, 24, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.L.; Hauser, C.J.; Ji, X.; Wang, B.; Camara, N.O.S.; Robson, S.C.; et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- Joe, Y.; Kim, S.; Kim, H.J.; Park, J.; Chen, Y.; Park, H.J.; Jekal, S.J.; Ryter, S.W.; Kim, U.H.; Chung, H.T. FGF21 induced by carbon monoxide mediates metabolic homeostasis via the PERK/ATF4 pathway. Faseb J. 2018, 32, 2630–2643. [Google Scholar] [CrossRef]
- Minegishi, S.; Sagami, I.; Negi, S.; Kano, K.; Kitagishi, H. Circadian clock disruption by selective removal of endogenous carbon monoxide. Sci. Rep. 2018, 8, 11996. [Google Scholar] [CrossRef]
- Chen, Y.; Joe, Y.; Park, J.; Song, H.C.; Kim, U.H.; Chung, H.T. Carbon monoxide induces the assembly of stress granule through the integrated stress response. Biochem. Biophys. Res. Commun. 2019, 512, 289–294. [Google Scholar] [CrossRef]
- Rahman, F.U.; Park, D.R.; Joe, Y.; Jang, K.Y.; Chung, H.T.; Kim, U.H. Critical Roles of Carbon Monoxide and Nitric Oxide in Ca(2+) Signaling for Insulin Secretion in Pancreatic Islets. Antioxid. Redox Signal. 2019, 30, 560–576. [Google Scholar] [CrossRef]
- Stucki, D.; Steinhausen, J.; Westhoff, P.; Krahl, H.; Brilhaus, D.; Massenberg, A.; Weber, A.P.M.; Reichert, A.S.; Brenneisen, P.; Stahl, W. Endogenous Carbon Monoxide Signaling Modulates Mitochondrial Function and Intracellular Glucose Utilization: Impact of the Heme Oxygenase Substrate Hemin. Antioxidants 2020, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zeng, J.S.; Sahasrabudhe, A.; Jin, K.; Fink, Y.; Manthiram, K.; Anikeeva, P. Electrochemical Modulation of Carbon Monoxide-Mediated Cell Signaling. Angew. Chem. Int. Ed. Engl. 2021, 60, 20325–20330. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon monoxide signaling: Examining its engagement with various molecular targets in the context of binding affinity, concentration, and biologic response. Pharmacol. Rev. 2022, 74, 823–873. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.P.; De La Cruz, L.K.; Lyles, K.V.; Wareham, L.K.; Gilbert, J.A.; Eichenbaum, Z.; Magierowski, M.; Poole, R.K.; Wollborn, J.; Wang, B. Role of carbon monoxide in host-gut microbiome communication. Chem. Rev. 2020, 120, 13273–13311. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.R.; Weaver, B.R.; Roberts, M.G.; Burstyn, J.N. Carbon monoxide-sensing transcription factors: Regulators of microbial carbon monoxide oxidation pathway gene expression. J. Bacteriol. 2023, 205, e0033222. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.F.; Parente, M.R.; Justino, M.C.; Oleastro, M.; Nobre, L.S.; Saraiva, L.M. The bactericidal activity of carbon monoxide-releasing molecules against Helicobacter pylori. PLoS ONE 2013, 8, e83157. [Google Scholar] [CrossRef]
- Desmard, M.; Davidge, K.S.; Bouvet, O.; Morin, D.; Roux, D.; Foresti, R.; Ricard, J.D.; Denamur, E.; Poole, R.K.; Montravers, P.; et al. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. Faseb J. 2009, 23, 1023–1031. [Google Scholar] [CrossRef]
- Davidge, K.S.; Sanguinetti, G.; Yee, C.H.; Cox, A.G.; McLeod, C.W.; Monk, C.E.; Mann, B.E.; Motterlini, R.; Poole, R.K. Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J. Biol. Chem. 2009, 284, 4516–4524. [Google Scholar] [CrossRef]
- Wareham, L.K.; McLean, S.; Begg, R.; Rana, N.; Ali, S.; Kendall, J.J.; Sanguinetti, G.; Mann, B.E.; Poole, R.K. The broad-spectrum antimicrobial potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a water-soluble CO-releasing molecule (CORM-401): Intracellular accumulation, transcriptomic and statistical analyses, and membrane polarization. Antioxid. Redox. Signal. 2018, 28, 1286–1308. [Google Scholar] [CrossRef]
- Southam, H.M.; Smith, T.W.; Lyon, R.L.; Liao, C.; Trevitt, C.R.; Middlemiss, L.A.; Cox, F.L.; Chapman, J.A.; El-Khamisy, S.F.; Hippler, M.; et al. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018, 18, 114–123. [Google Scholar] [CrossRef]
- Sousa, F.L.; Alves, R.J.; Ribeiro, M.A.; Pereira-Leal, J.B.; Teixeira, M.; Pereira, M.M. The superfamily of heme-copper oxygen reductases: Types and evolutionary considerations. Biochim. Biophys. Acta 2012, 1817, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Gennis, R.B.; Hemp, J. Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA’ in Archaea. ISME J. 2021, 15, 3534–3548. [Google Scholar] [CrossRef] [PubMed]
- Siletsky, S.A. Investigation of the Mechanism of Membrane Potential Generation by Heme-Copper Respiratory Oxidases in a Real Time Mode. Biochemistry 2023, 88, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Safari, C.; Ghosh, S.; Andersson, R.; Johannesson, J.; Bath, P.; Uwangue, O.; Dahl, P.; Zoric, D.; Sandelin, E.; Vallejos, A.; et al. Time-resolved serial crystallography to track the dynamics of carbon monoxide in the active site of cytochrome c oxidase. Sci. Adv. 2023, 9, eadh4179. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.; Dimogkioka, A.R.; Rapaport, D.; Ojemyr, L.N.; Brzezinski, P. Structure and function of the S. pombe III-IV-cyt c supercomplex. Proc. Natl. Acad. Sci. USA 2023, 120, e2307697120. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui-Hassani, B.; Blaby-Haas, C.E.; Verissimo, A.; Daldal, F. The Escherichia coli MFS-type transporter genes yhjE, ydiM, and yfcJ are required to produce an active bo3 quinol oxidase. PLoS ONE 2023, 18, e0293015. [Google Scholar] [CrossRef]
- Shimada, A.; Etoh, Y.; Kitoh-Fujisawa, R.; Sasaki, A.; Shinzawa-Itoh, K.; Hiromoto, T.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. X-ray structures of catalytic intermediates of cytochrome c oxidase provide insights into its O2 activation and unidirectional proton-pump mechanisms. J. Biol. Chem. 2020, 295, 5818–5833. [Google Scholar] [CrossRef]
- Shimada, A.; Hara, F.; Shinzawa-Itoh, K.; Kanehisa, N.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. Critical roles of the CuB site in efficient proton pumping as revealed by crystal structures of mammalian cytochrome c oxidase catalytic intermediates. J. Biol. Chem. 2021, 100967. [Google Scholar] [CrossRef]
- Shimada, A.; Baba, J.; Nagao, S.; Shinzawa-Itoh, K.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. Crystallographic cyanide-probing for cytochrome c oxidase reveals structural bases suggesting that a putative proton transfer H-pathway pumps protons. J. Biol. Chem. 2023, 299, 105277. [Google Scholar] [CrossRef]
- Panda, S.; Phan, H.; Karlin, K.D. Heme-copper and Heme O2-derived synthetic (bioinorganic) chemistry toward an understanding of cytochrome c oxidase dioxygen chemistry. J. Inorg. Biochem. 2023, 249, 112367. [Google Scholar] [CrossRef]
- Ishigami, I.; Sierra, R.G.; Su, Z.; Peck, A.; Wang, C.; Poitevin, F.; Lisova, S.; Hayes, B.; Moss, F.R., 3rd; Boutet, S.; et al. Structural insights into functional properties of the oxidized form of cytochrome c oxidase. Nat. Commun. 2023, 14, 5752. [Google Scholar] [CrossRef] [PubMed]
- Verameyenka, K.G.; Naumouskaya, V.A.; Maximova, N.P. Cytochrome c oxidase is one of the key enzymes providing the ability to produce phenazines in Pseudomonas chlororaphis subsp. aurantiaca. World J. Microbiol. Biotechnol. 2023, 39, 279. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Karimullina, E.; Emde, T.; Otwinowski, Z.; Borek, D.; Savchenko, A. Monomer and dimer structures of cytochrome bo3 ubiquinol oxidase from Escherichia coli. Protein Sci. 2023, e4616. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, L.; Vallese, F.; Ding, Z.; Choi, S.K.; Hong, S.; Luo, Y.; Liu, B.; Chan, C.K.; Tajkhorshid, E.; et al. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Jose, A.; Schaefer, A.W.; Roveda, A.C., Jr.; Transue, W.J.; Choi, S.K.; Ding, Z.; Gennis, R.B.; Solomon, E.I. The three-spin intermediate at the O-O cleavage and proton-pumping junction in heme-Cu oxidases. Science 2021, 373, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Asseri, A.H.; Godoy-Hernandez, A.; Goojani, H.G.; Lill, H.; Sakamoto, J.; McMillan, D.G.G.; Bald, D. Cardiolipin enhances the enzymatic activity of cytochrome bd and cytochrome bo3 solubilized in dodecyl-maltoside. Sci. Rep. 2021, 11, 8006. [Google Scholar] [CrossRef]
- Kaila, V.R.I.; Wikstrom, M. Architecture of bacterial respiratory chains. Nat. Rev. Microbiol. 2021, 19, 319–330. [Google Scholar] [CrossRef]
- Wikstrom, M.; Springett, R. Thermodynamic efficiency, reversibility, and degree of coupling in energy conservation by the mitochondrial respiratory chain. Commun. Biol. 2020, 3, 451. [Google Scholar] [CrossRef]
- Di Trani, J.M.; Gheorghita, A.A.; Turner, M.; Brzezinski, P.; Adelroth, P.; Vahidi, S.; Howell, P.L.; Rubinstein, J.L. Structure of the bc1-cbb3 respiratory supercomplex from Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2023, 120, e2307093120. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, T.; Yang, J.; Hao, S.; Sun, Z.; Peng, Y. Anammox Coupled with Photocatalyst for Enhanced Nitrogen Removal and the Activated Aerobic Respiration of Anammox Bacteria Based on cbb3-Type Cytochrome c Oxidase. Environ. Sci. Technol. 2023, 57, 17910–17919. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Yin, Z.; Chen, M.; Song, J.; Li, P.; Yang, L. New insights into the proton pumping mechanism of ba3 cytochrome c oxidase: The functions of key residues and water. Phys. Chem. Chem. Phys. 2023, 25, 25105–25115. [Google Scholar] [CrossRef] [PubMed]
- Noodleman, L.; Gotz, A.W.; Han Du, W.G.; Hunsicker-Wang, L. Reaction pathways, proton transfer, and proton pumping in ba3 class cytochrome c oxidase: Perspectives from DFT quantum chemistry and molecular dynamics. Front. Chem. 2023, 11, 1186022. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, M.; Pecorilla, C.; Sharma, V. The mitochondrial respiratory chain. Enzymes 2023, 54, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, M.; Gennis, R.B.; Rich, P.R. Structures of the intermediates in the catalytic cycle of mitochondrial cytochrome c oxidase. Biochim. Biophys. Acta. Bioenerg. 2023, 1864, 148933. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.K.; Cook, G.M. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 2000, 43, 165–224. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.; Teixeira, M. Supramolecular organization of bacterial aerobic respiratory chains: From cells and back. Biochim. Biophys. Acta 2016, 1857, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Melin, F.; Sabuncu, S.; Choi, S.K.; Leprince, A.; Gennis, R.B.; Hellwig, P. Role of the tightly bound quinone for the oxygen reaction of cytochrome bo3 oxidase from Escherichia coli. FEBS Lett. 2018, 592, 3380–3387. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Impact of hydrogen sulfide on mitochondrial and bacterial bioenergetics. Int. J. Mol. Sci. 2021, 22, 12688. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffre, A.; Poole, R.K. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function and utility as drug targets. Antioxid. Redox Signal. 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Rolfe, M.D.; Ter Beek, A.; Graham, A.I.; Trotter, E.W.; Asif, H.M.; Sanguinetti, G.; de Mattos, J.T.; Poole, R.K.; Green, J. Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J. Biol. Chem. 2011, 286, 10147–10154. [Google Scholar] [CrossRef]
- Alexeeva, S.; Hellingwerf, K.; Teixeira de Mattos, M.J. Quantitative assessment of oxygen availability: Perceived aerobiosis and its effect on flux distribution in the respiratory chain of Escherichia coli. J. Bacteriol. 2002, 184, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Trotter, E.W.; Rolfe, M.D.; Hounslow, A.M.; Craven, C.J.; Williamson, M.P.; Sanguinetti, G.; Poole, R.K.; Green, J. Reprogramming of Escherichia coli K-12 metabolism during the initial phase of transition from an anaerobic to a micro-aerobic environment. PLoS ONE 2011, 6, e25501. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Riistama, S.; Larsson, G.; Jasaitis, A.; Svensson-Ek, M.; Laakkonen, L.; Puustinen, A.; Iwata, S.; Wikstrom, M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat. Struct. Biol. 2000, 7, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Hahn, A.; Mills, D.J.; Radloff, M.; Eisinger, M.L.; Nikolaev, A.; Meier-Credo, J.; Melin, F.; Miyoshi, H.; Gennis, R.B.; et al. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science 2019, 366, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Thesseling, A.; Rasmussen, T.; Burschel, S.; Wohlwend, D.; Kagi, J.; Muller, R.; Bottcher, B.; Friedrich, T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 2019, 10, 5138. [Google Scholar] [CrossRef] [PubMed]
- Grauel, A.; Kagi, J.; Rasmussen, T.; Makarchuk, I.; Oppermann, S.; Moumbock, A.F.A.; Wohlwend, D.; Muller, R.; Melin, F.; Gunther, S.; et al. Structure of Escherichia coli cytochrome bd-II type oxidase with bound aurachin D. Nat. Commun. 2021, 12, 6498. [Google Scholar] [CrossRef] [PubMed]
- Grund, T.N.; Radloff, M.; Wu, D.; Goojani, H.G.; Witte, L.F.; Josting, W.; Buschmann, S.; Muller, H.; Elamri, I.; Welsch, S.; et al. Mechanistic and structural diversity between cytochrome bd isoforms of Escherichia coli. Proc. Natl. Acad. Sci. USA 2021, 118, e2114013118. [Google Scholar] [CrossRef]
- Samukaite Bubniene, U.; Zukauskas, S.; Ratautaite, V.; Vilkiene, M.; Mockeviciene, I.; Liustrovaite, V.; Drobysh, M.; Lisauskas, A.; Ramanavicius, S.; Ramanavicius, A. Assessment of cytochrome c and chlorophyll a as natural redox mediators for enzymatic biofuel cells powered by glucose. Energies 2022, 15, 6838. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffre, A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007, 355, 97–102. [Google Scholar] [CrossRef]
- Mason, M.G.; Shepherd, M.; Nicholls, P.; Dobbin, P.S.; Dodsworth, K.S.; Poole, R.K.; Cooper, C.E. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 2009, 5, 94–96. [Google Scholar] [CrossRef]
- Shepherd, M.; Achard, M.E.; Idris, A.; Totsika, M.; Phan, M.D.; Peters, K.M.; Sarkar, S.; Ribeiro, C.A.; Holyoake, L.V.; Ladakis, D.; et al. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci. Rep. 2016, 6, 35285. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Siletsky, S.A.; Sarti, P.; Giuffre, A. Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim. Biophys. Acta 2015, 1847, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Siletsky, S.A.; Borisov, V.B. In Escherichia coli ammonia inhibits cytochrome bo3 but activates cytochrome bd-I. Antioxidants 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Sarti, P.; Giuffre, A.; Forte, E.; Mastronicola, D.; Barone, M.C.; Brunori, M. Nitric oxide and cytochrome c oxidase: Mechanisms of inhibition and NO degradation. Biochem. Biophys. Res. Commun. 2000, 274, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffre, A. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 2006, 580, 4823–4826. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E. Bioenergetics and reactive nitrogen species in bacteria. Int. J. Mol. Sci. 2022, 23, 7321. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaco, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffre, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Davletshin, A.; Mastronicola, D.; Sarti, P.; Giuffre, A. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 2013, 587, 2214–2218. [Google Scholar] [CrossRef]
- Al-Attar, S.; Yu, Y.; Pinkse, M.; Hoeser, J.; Friedrich, T.; Bald, D.; de Vries, S. Cytochrome bd displays significant quinol peroxidase activity. Sci. Rep. 2016, 6, 27631. [Google Scholar] [CrossRef]
- Forte, E.; Nastasi, M.R.; Borisov, V.B. Preparations of terminal oxidase cytochrome bd-II isolated from Escherichia coli reveal significant hydrogen peroxide scavenging activity. Biochemistry 2022, 87, 720–730. [Google Scholar] [CrossRef]
- Korshunov, S.; Imlay, K.R.; Imlay, J.A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016, 101, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Harikishore, A.; Mathiyazakan, V.; Pethe, K.; Gruber, G. Novel targets and inhibitors of the Mycobacterium tuberculosis cytochrome bd oxidase to foster anti-tuberculosis drug discovery. Expert Opin. Drug Discov. 2023, 18, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Bayly, K.; Cordero, P.R.F.; Kropp, A.; Huang, C.; Schittenhelm, R.B.; Grinter, R.; Greening, C. Mycobacteria tolerate carbon monoxide by remodeling their respiratory chain. mSystems 2021, 6, e01292-20. [Google Scholar] [CrossRef] [PubMed]
- Scharn, C.R.; Collins, A.C.; Nair, V.R.; Stamm, C.E.; Marciano, D.K.; Graviss, E.A.; Shiloh, M.U. Heme oxygenase-1 regulates inflammation and mycobacterial survival in human macrophages during Mycobacterium tuberculosis infection. J. Immunol. 2016, 196, 4641–4649. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Larsen, R.; Gallo, D.; Chin, B.Y.; Harris, C.; Mannam, P.; Kaczmarek, E.; Lee, P.J.; Zuckerbraun, B.S.; Flavell, R.; et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Invest. 2014, 124, 4926–4940. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Begg, R.; Jesse, H.E.; Van Beilen, J.W.; Ali, S.; Svistunenko, D.; McLean, S.; Hellingwerf, K.J.; Sanguinetti, G.; Poole, R.K. Carbon monoxide gas is not inert, but global, in its consequences for bacterial gene expression, iron acquisition, and antibiotic resistance. Antioxid. Redox. Signal. 2016, 24, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Siletsky, S.A.; Petrosino, M.; Giuffre, A. In the respiratory chain of Escherichia coli cytochromes bd-I and bd-II are more sensitive to carbon monoxide inhibition than cytochrome bo3. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148088. [Google Scholar] [CrossRef]
- Nastasi, M.R.; Borisov, V.B.; Forte, E. The terminal oxidase cytochrome bd-I confers carbon monoxide resistance to Escherichia coli cells. J. Inorg. Biochem. 2023, 247, 112341. [Google Scholar] [CrossRef]
- Kalia, N.P.; Singh, S.; Hards, K.; Cheung, C.Y.; Sviriaeva, E.; Banaei-Esfahani, A.; Aebersold, R.; Berney, M.; Cook, G.M.; Pethe, K.M. Tuberculosis relies on trace oxygen to maintain energy homeostasis and survive in hypoxic environments. Cell Rep. 2023, 42, 112444. [Google Scholar] [CrossRef]
- Bekker, M.; de Vries, S.; Ter Beek, A.; Hellingwerf, K.J.; de Mattos, M.J. Respiration of Escherichia coli can be fully uncoupled via the nonelectrogenic terminal cytochrome bd-II oxidase. J. Bacteriol. 2009, 191, 5510–5517. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.; Arutyunyan, A.M.; Osborne, J.P.; Gennis, R.B.; Konstantinov, A.A. Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands. Biochemistry 1999, 38, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Puustinen, A.; Wikstrom, M. The heme groups of cytochrome o from Escherichia coli. Proc. Natl. Acad. Sci. USA 1991, 88, 6122–6126. [Google Scholar] [CrossRef] [PubMed]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging diversity in lipid-protein interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef] [PubMed]
- Cournia, Z.; Allen, T.W.; Andricioaei, I.; Antonny, B.; Baum, D.; Brannigan, G.; Buchete, N.V.; Deckman, J.T.; Delemotte, L.; Del Val, C.; et al. Membrane protein structure, function, and dynamics: A perspective from experiments and theory. J. Membr. Biol. 2015, 248, 611–640. [Google Scholar] [CrossRef]
- Borisov, V.B. Effect of membrane environment on ligand-binding properties of the terminal oxidase cytochrome bd-I from Escherichia coli. Biochemistry 2020, 85, 1603–1612. [Google Scholar] [CrossRef]
- Andreev, I.M.; Konstantinov, A.A. Reaction of oxidized cytochrome oxidase with cyanide. Effects of pH, cytochrome c and membrane environment. Bioorg. Khim. (in Russian) 1983, 9, 216–227. [Google Scholar]
- Tsai, A.L.; Berka, V.; Martin, E.; Olson, J.S. A "sliding scale rule" for selectivity among NO, CO, and O2 by heme protein sensors. Biochemistry 2012, 51, 172–186. [Google Scholar] [CrossRef]
- Bartlett, G.J.; Newberry, R.W.; VanVeller, B.; Raines, R.T.; Woolfson, D.N. Interplay of hydrogen bonds and n-->pi* interactions in proteins. J. Am. Chem. Soc. 2013, 135, 18682–18688. [Google Scholar] [CrossRef]
- Wickham-Smith, C.; Malys, N.; Winzer, K. Improving carbon monoxide tolerance of Cupriavidus necator H16 through adaptive laboratory evolution. Front. Bioeng. Biotechnol. 2023, 11, 1178536. [Google Scholar] [CrossRef]
- Proshlyakov, D.A.; Pressler, M.A.; DeMaso, C.; Leykam, J.F.; DeWitt, D.L.; Babcock, G.T. Oxygen activation and reduction in respiration: Involvement of redox-active tyrosine 244. Science 2000, 290, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Rossius, S.G.; Dijk, M.; de Vries, S. Oxoferryl-porphyrin radical catalytic intermediate in cytochrome bd oxidases protects cells from formation of reactive oxygen species. J. Biol. Chem. 2012, 287, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Belevich, I.; Borisov, V.B.; Verkhovsky, M.I. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J. Biol. Chem. 2007, 282, 28514–28519. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastasi, M.R.; Borisov, V.B.; Forte, E. Membrane-Bound Redox Enzyme Cytochrome bd-I Promotes Carbon Monoxide-Resistant Escherichia coli Growth and Respiration. Int. J. Mol. Sci. 2024, 25, 1277. https://doi.org/10.3390/ijms25021277
Nastasi MR, Borisov VB, Forte E. Membrane-Bound Redox Enzyme Cytochrome bd-I Promotes Carbon Monoxide-Resistant Escherichia coli Growth and Respiration. International Journal of Molecular Sciences. 2024; 25(2):1277. https://doi.org/10.3390/ijms25021277
Chicago/Turabian StyleNastasi, Martina R., Vitaliy B. Borisov, and Elena Forte. 2024. "Membrane-Bound Redox Enzyme Cytochrome bd-I Promotes Carbon Monoxide-Resistant Escherichia coli Growth and Respiration" International Journal of Molecular Sciences 25, no. 2: 1277. https://doi.org/10.3390/ijms25021277
APA StyleNastasi, M. R., Borisov, V. B., & Forte, E. (2024). Membrane-Bound Redox Enzyme Cytochrome bd-I Promotes Carbon Monoxide-Resistant Escherichia coli Growth and Respiration. International Journal of Molecular Sciences, 25(2), 1277. https://doi.org/10.3390/ijms25021277







