Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion
Abstract
:1. Introduction
2. Results
2.1. 1,1-Diphenyl-2-picrylhydrazyl Radical-Scavenging Assay
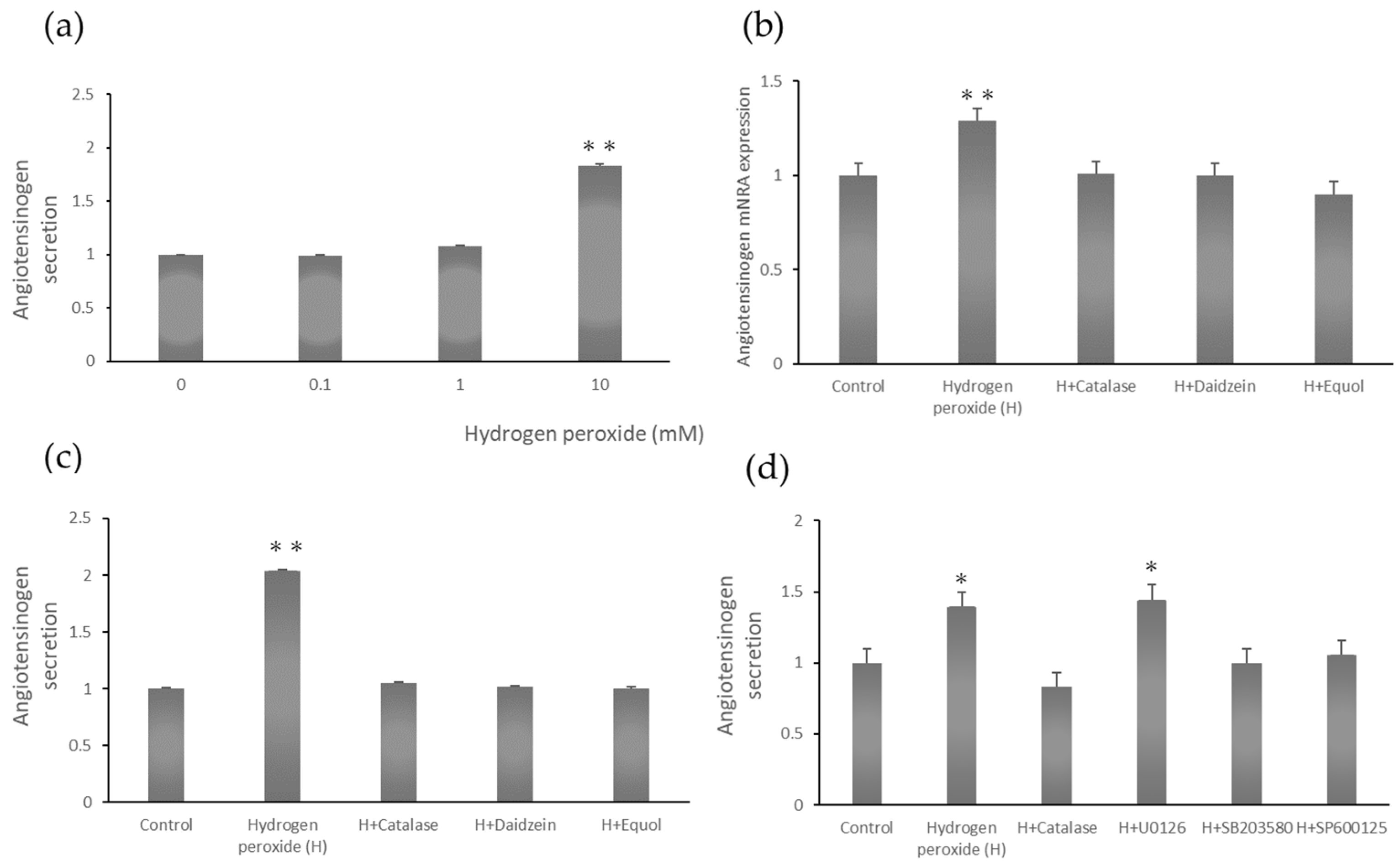
2.2. Dose-Dependent Angiotensinogen Secretion
2.3. Angiotensinogen mRNA Expression Stimulated by Hydrogen Peroxide
2.4. Angiotensinogen Protein Secretion Stimulated by Hydrogen Peroxide
2.5. Angiotensinogen Secretion Stimulated by Hydrogen Peroxide
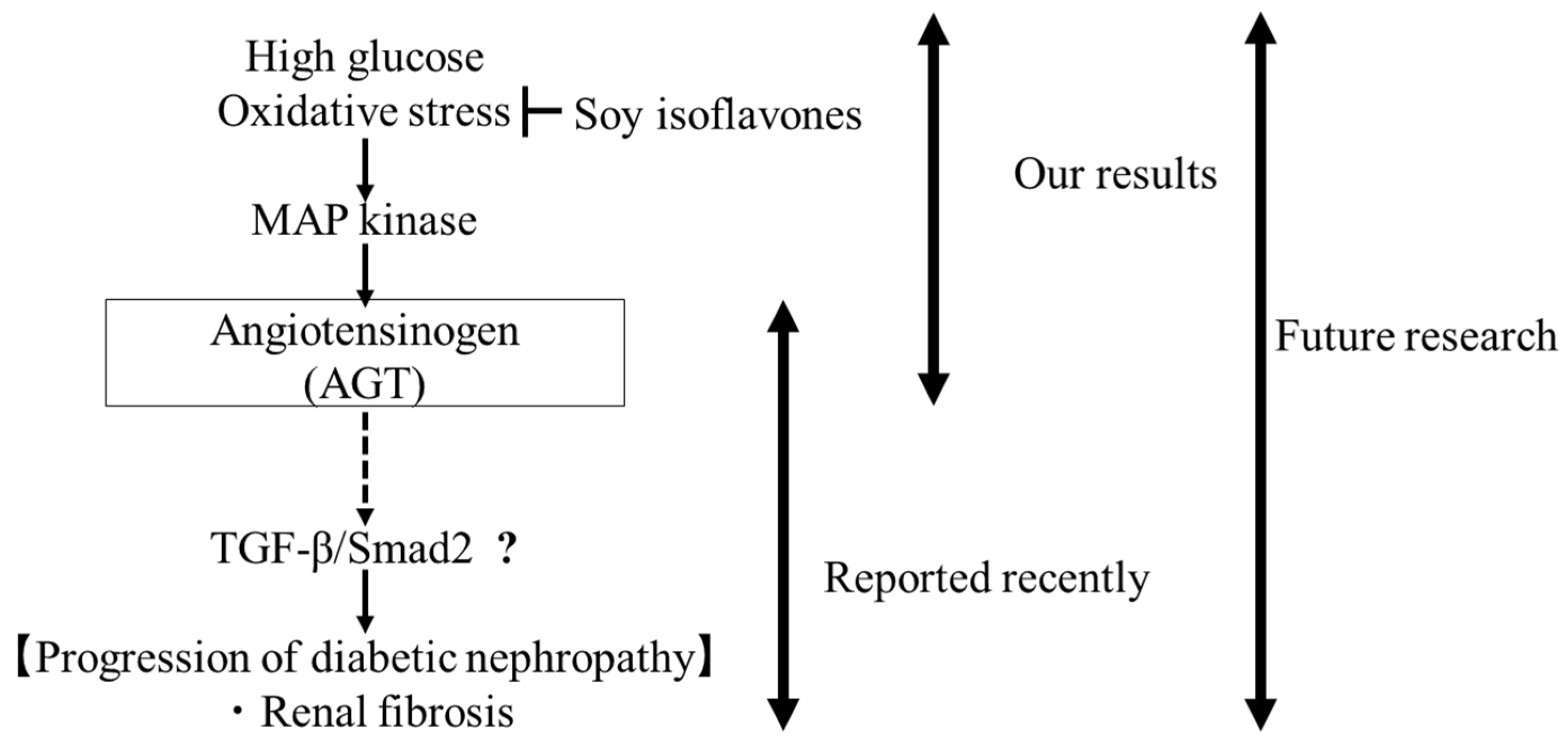
3. Discussion
4. Materials and Methods
4.1. 1,1-Diphenyl-2-picrylhydrazyl Assay
4.2. Cell Culture and Treatment
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGT | angiotensinogen |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GFR | glomerular filtration rate |
| RAS | renin–angiotensin system |
| ROS | reactive oxygen species |
| STZ | streptozotocin |
| Tg | transgenic |
| WT | wild-type |
References
- Powers, S.K.; Morton, A.B.; Hyatt, H.; Hinkley, M.J. The Renin-Angiotensin System and Skeletal Muscle. Exerc. Sport Sci. Rev. 2018, 46, 205–214. [Google Scholar] [CrossRef]
- Mo, C.; Ke, J.; Zhao, D.; Zhang, B. Role of the renin-angiotensin-aldosterone system in bone metabolism. J. Bone Miner. Metab. 2020, 38, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-Angiotensin System and Cardiovascular Functions. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e108–e116. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Sun, X.; Sun, J.; Jiang, Y.; Lin, F.; Kong, F.; Li, F.; Zhu, J.; Huan, L.; Zheng, B.; et al. Tissue Renin-Angiotensin System (tRAS) Induce Intervertebral Disc Degeneration by Activating Oxidative Stress and Inflammatory Reaction. Oxid. Med. Cell. Longev. 2021, 2021, 3225439. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.; Silva, J.A., Jr.; Ganten, D.; Bader, M. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension 2000, 35, 409–412. [Google Scholar] [CrossRef]
- Dell’Italia, L.J.; Meng, Q.C.; Balcells, E.; Wei, C.C.; Palmer, R.; Hageman, G.R.; Durand, J.; Hankes, G.H.; Oparil, S. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J. Clin. Investig. 1997, 100, 253–258. [Google Scholar] [CrossRef]
- Mazzocchi, G.; Malendowicz, L.K.; Markowska, A.; Albertin, G.; Nussdorfer, G.G. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1027–E1030. [Google Scholar] [CrossRef]
- Danser, A.H.; Admiraal, P.J.; Derkx, F.H.; Schalekamp, M.A. Angiotensin I-to-II conversion in the human renal vascular bed. J. Hypertens. 1998, 16, 2051–2056. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef]
- Carey, R.M.; Siragy, H.M. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol. Metab. 2003, 14, 274–281. [Google Scholar] [CrossRef]
- Navar, L.G.; Harrison-Bernard, L.M.; Nishiyama, A.; Kobori, H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002, 39, 316–322. [Google Scholar] [CrossRef]
- Kamiyama, M.; Iijima, K.; Okuzawa, R.; Kawata, R.; Kimura, A.; Shinohara, Y.; Shimada, A.; Yamanaka, M.; Youda, A.; Iwamoto, T. Augmented Intrarenal and Urinary Angiotensinogen in Diabetic Nephropathy: The Role of Isoflavones. Int. J. Mol. Sci. 2025, 26, 1443. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Kamiyama, M.; Harrison-Bernard, L.M.; Navar, L.G. Cardinal role of the intrarenal renin-angiotensin system in the pathogenesis of diabetic nephropathy. J. Investig. Med. 2013, 61, 256–264. [Google Scholar] [CrossRef]
- Kamiyama, M.; Urushihara, M.; Morikawa, T.; Konishi, Y.; Imanishi, M.; Nishiyama, A.; Kobori, H. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int. J. Mol. Sci. 2013, 14, 23045–23062. [Google Scholar] [CrossRef]
- Kamiyama, M.; Garner, M.K.; Farragut, K.M.; Sofue, T.; Hara, T.; Morikawa, T.; Konishi, Y.; Imanishi, M.; Nishiyama, A.; Kobori, H. Detailed localization of augmented angiotensinogen mRNA and protein in proximal tubule segments of diabetic kidneys in rats and humans. Int. J. Biol. Sci. 2014, 10, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Zsombok, A.; Kobori, H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J. Pharmacol. Sci. 2012, 119, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Varghese, S.M.; Gabhale, S.; Shah, A.; Shashank, C.; Thakkar, S. Estimation of the Various Urinary Biomarkers among the Non-Hypertensive Type 2 Diabetic Patients with Nephropathy. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S1), S286–S289. [Google Scholar] [CrossRef]
- Mahapatra, H.S.; Kulshreshtha, B.; Goyal, P.; Chitkara, A.; Kumari, A.; Arora, A.; Sekhar, V.; Gupta, Y.P. Comparative diagnostic utility of different urinary biomarkers during pre-albuminuric stages of non-hypertensive type 2 diabetic nephropathy. Indian J. Med. Res. 2022, 156, 46–55. [Google Scholar] [CrossRef]
- Dagdeviren Cakir, A.; Saygili, S.K.; Canpolat, N.; Konukoglu, D.; Turan, H.; Caliskan, S.; Sever, L.; Ercan, O.; Evliyaoglu, O. Elevated Urinary VEGF-A, Transferrin, and Angiotensinogen Levels in Normoalbuminuric Children and Adolescents with Type 1 Diabetes: Can They Be Early Markers of Diabetic Kidney Disease? Horm. Res. Paediatr. 2021, 94, 426–432. [Google Scholar] [CrossRef]
- Kasama, T.; Sun, M.; Kaji, N.; Akiyama, S.; Yuzawa, Y.; Tokeshi, M.; Matsuo, S.; Baba, Y. Microchip Immunoassays for Monitoring Renal Function: Rapid, Low-Cost, and Highly Sensitive Quantification of Urinary Biomarkers of Diabetic Nephropathy. Micromachines 2021, 12, 1353. [Google Scholar] [CrossRef]
- Satirapoj, B.; Pooluea, P.; Nata, N.; Supasyndh, O. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: A prospective cohort study. J. Diabetes Its Complicat. 2019, 33, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Davisson, R.L.; Hardy, D.O.; Zhu, L.J.; Merrill, D.C.; Catterall, J.F.; Sigmund, C.D. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J. Biol. Chem. 1997, 272, 28142–28148. [Google Scholar] [CrossRef]
- Kimura, S.; Mullins, J.J.; Bunnemann, B.; Metzger, R.; Hilgenfeldt, U.; Zimmermann, F.; Jacob, H.; Fuxe, K.; Ganten, D.; Kaling, M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992, 11, 821–827. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, E.R.; Chitescu, C.L.; Borda, D.; Lupoae, M.; Gird, C.E.; Geana, E.I.; Blaga, G.V.; Boscencu, R. Comparison of the Polyphenolic Profile of Medicago sativa L. and Trifolium pratense L. Sprouts in Different Germination Stages Using the UHPLC-Q Exactive Hybrid Quadrupole Orbitrap High-Resolution Mass Spectrometry. Molecules 2020, 25, 2321. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhai, H.; Wu, D.; Chu, X. Traditional Chinese Herb Couples Mitigate Diabetic Macrovascular Disease via the AGE-RAGE Signaling Pathway. Chem. Biodivers. 2025, e202500531. [Google Scholar] [CrossRef]
- Joss, N.; Paterson, K.R.; Deighan, C.J.; Simpson, K.; Boulton-Jones, J.M. Diabetic nephropathy: How effective is treatment in clinical practice? QJM 2002, 95, 41–49. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Zhang, S.L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Chan, J.S. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 2002, 143, 2975–2985. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Fustier, P.; Zhang, S.L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Fantus, I.G.; Hamet, P.; Chan, J.S. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology 2003, 144, 4338–4349. [Google Scholar] [CrossRef]
- Wolff, S.P.; Jiang, Z.Y.; Hunt, J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991, 10, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Munoz, L.M.; Vidal-Vanaclocha, F.; Lampreabe, I. Enalaprilat inhibits hydrogen peroxide production by murine mesangial cells exposed to high glucose concentrations. Nephrol. Dial. Transplant. 1997, 12, 456–464. [Google Scholar] [CrossRef]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Tran, S.; Sachetelli, S.; Zhang, S.L.; Guo, D.F.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007, 71, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lo, C.S.; Chenier, I.; Maachi, H.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Overexpression of catalase prevents hypertension and tubulointerstitial fibrosis and normalization of renal angiotensin-converting enzyme-2 expression in Akita mice. Am. J. Physiol. Renal Physiol. 2013, 304, F1335–F1346. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.; Shi, Y.; Otoukesh, A.; Ghosh, A.; Lo, C.S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 2014, 63, 3483–3496. [Google Scholar] [CrossRef]
- Ohashi, N.; Urushihara, M.; Satou, R.; Kobori, H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species--ERK/JNK pathways. Hypertens. Res. 2010, 33, 1174–1181. [Google Scholar] [CrossRef]
- Pat, B.K.; Cuttle, L.; Watters, D.; Yang, T.; Johnson, D.W.; Gobe, G.C. Fibrogenic stresses activate different mitogen-activated protein kinase pathways in renal epithelial, endothelial or fibroblast cell populations. Nephrology 2003, 8, 196–204. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333 Pt 2, 291–300. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Guo, W.; Yu, L. Potential role of ghrelin in the regulation of inflammation. FASEB J. 2022, 36, e22508. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC technology: From drug development to probe technology for target deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.; Chen, X.; Neamati, N. Discovery of a Napabucasin PROTAC as an Effective Degrader of the E3 Ligase ZFP91. J. Med. Chem. 2021, 64, 1626–1648. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Ookawa, M.; Saito, R.; Tange, N.; Hashizume, M.; Matsunaga, M.; Yokota, R.; Yoshihara, A.; Iwamoto, T. Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion in Mesangial Cells. Curr. Top. Nutraceutical Res. 2024, 22, 624–628. [Google Scholar]
- Xiao, Y.; Deng, J.; Li, C.; Gong, X.; Gui, Z.; Huang, J.; Zhang, Y.; Liu, Y.; Ye, X.; Li, X. Epiberberine ameliorated diabetic nephropathy by inactivating the angiotensinogen (Agt) to repress TGFbeta/Smad2 pathway. Phytomedicine 2021, 83, 153488. [Google Scholar] [CrossRef]
| Isoflavone | Mean ± Standard Error (%) |
|---|---|
| Daidzein | 66.3 ± 0.8 |
| Equol | 69.9 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiyama, M.; Adachi, H.; Ogiwara, M.; Ishikawa, M.; Inoue, S.; Iwata, M.; Urushibata, H.; Ono, S.; Kato, H.; Iwamoto, T. Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion. Int. J. Mol. Sci. 2025, 26, 4029. https://doi.org/10.3390/ijms26094029
Kamiyama M, Adachi H, Ogiwara M, Ishikawa M, Inoue S, Iwata M, Urushibata H, Ono S, Kato H, Iwamoto T. Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion. International Journal of Molecular Sciences. 2025; 26(9):4029. https://doi.org/10.3390/ijms26094029
Chicago/Turabian StyleKamiyama, Masumi, Haruna Adachi, Mau Ogiwara, Madoka Ishikawa, Shieri Inoue, Miho Iwata, Hinano Urushibata, Shiho Ono, Hiyori Kato, and Tamami Iwamoto. 2025. "Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion" International Journal of Molecular Sciences 26, no. 9: 4029. https://doi.org/10.3390/ijms26094029
APA StyleKamiyama, M., Adachi, H., Ogiwara, M., Ishikawa, M., Inoue, S., Iwata, M., Urushibata, H., Ono, S., Kato, H., & Iwamoto, T. (2025). Isoflavones Inhibit Hydrogen Peroxide-Induced Angiotensinogen Secretion. International Journal of Molecular Sciences, 26(9), 4029. https://doi.org/10.3390/ijms26094029







