Characterisation of Matrix-Bound Nanovesicles (MBVs) Isolated from Decellularised Bovine Pericardium: New Frontiers in Regenerative Medicine
Abstract
:1. Introduction
2. Results
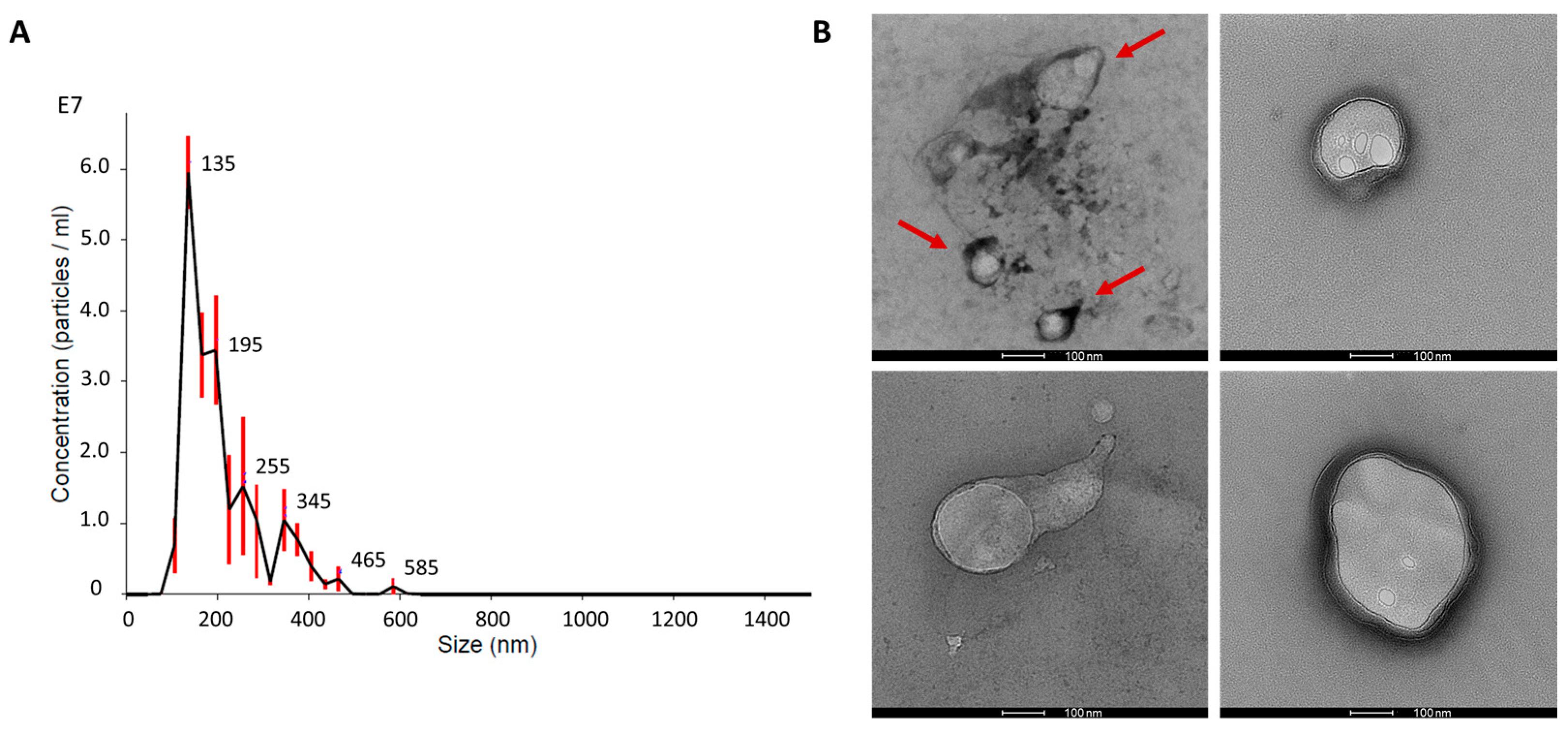
2.1. Matrix-Bound Nanovesicles from Decellularised Bovine Pericardium
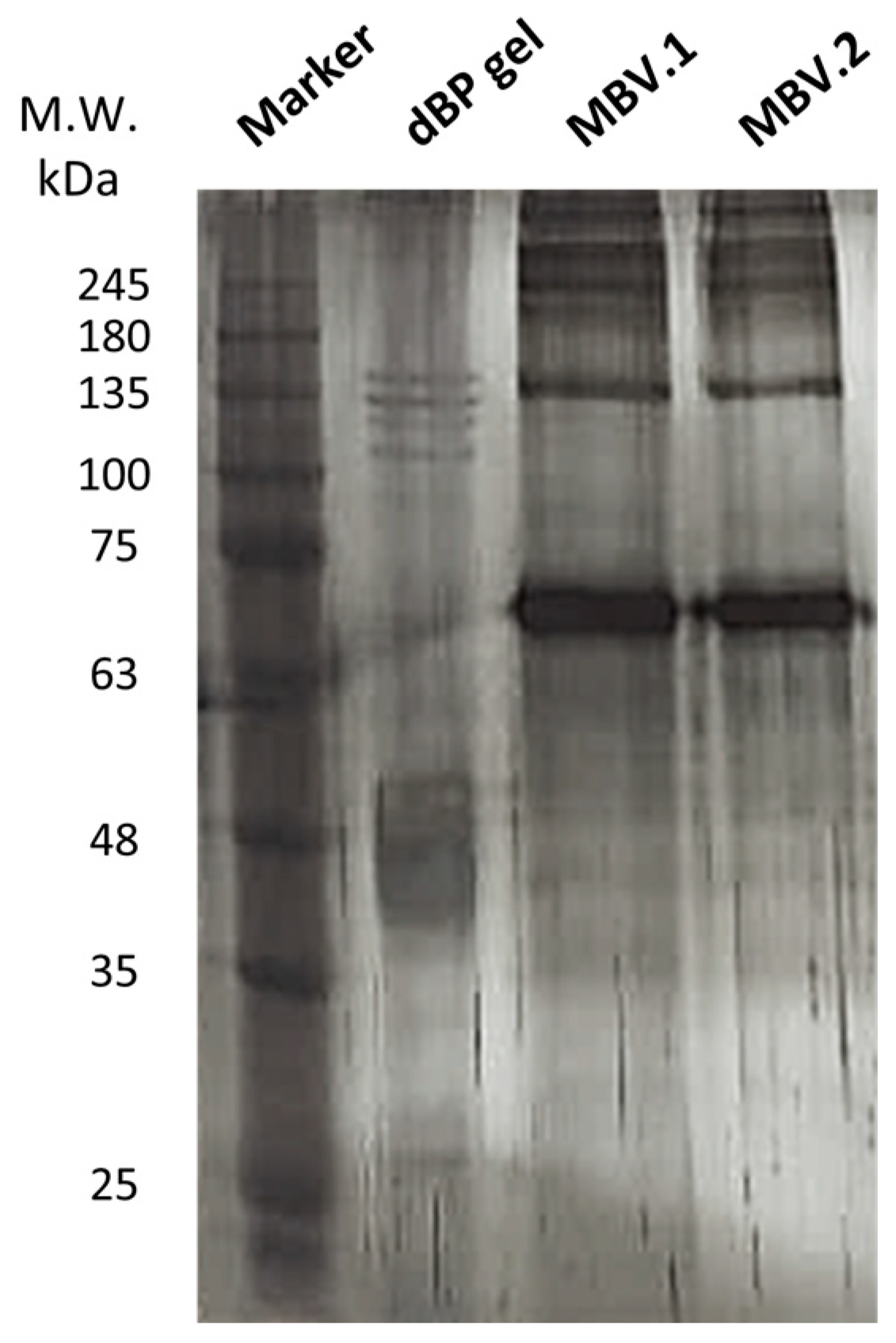
2.2. Matrix-Bound Nanovesicles Protein Content
2.2.1. SDS-PAGE and Silver Staining
2.2.2. Cytokine Antibody Array
2.2.3. Protein Mass Spectrometry
2.3. Cytocompatibility
3. Discussion
4. Materials and Methods
4.1. Matrix-Bound Nanovesicles Isolation
4.2. Nanoparticle Tracking Analysis
4.3. Transmission Electron Microscopy
4.4. Protein Content Characterisation
4.4.1. Protein Extraction and Quantification
4.4.2. SDS-PAGE and Silver Staining
4.4.3. Cytokine Antibody Array
4.4.4. Protein Mass Spectrometry
4.5. Cytocompatibility
4.5.1. Cell Culture
4.5.2. Cell Viability
4.5.3. Immunofluorescence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized Extracellular Matrix: New Promising and Challenging Biomaterials for Regenerative Medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Q.-Y.; Huang, L.-P.; Huang, K.; Xie, H.-Q. Decellularized Scaffold and Its Elicited Immune Response towards the Host: The Underlying Mechanism and Means of Immunomodulatory Modification. Biomater. Sci. 2021, 9, 4803–4820. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Huleihel, L.; Scarritt, M.E.; Badylak, S.F. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 2017, 23, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, B.; Shu, J.; Wang, H.; Han, Y.; Zeng, Q.; Chen, Y.; Xi, J.; Tao, R.; Pei, X.; et al. Human Decellularized Adipose Matrix Derived Hydrogel Assists Mesenchymal Stem Cells Delivery and Accelerates Chronic Wound Healing. J. Biomed. Mater. Res. A 2020, 109, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Seo, Y.; Davaa, G.; Kim, H.-W.; Kim, S.H.; Hyun, J.K. Decellularized Brain Matrix Enhances Macrophage Polarization and Functional Improvements in Rat Spinal Cord Injury. Acta Biomater. 2020, 101, 357–371. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Yu, L.; Kwak, H.-H.; Woo, H.-M. Decellularized Hepatic Extracellular Matrix Hydrogel Attenuates Hepatic Stellate Cell Activation and Liver Fibrosis. Mater. Sci. Eng. C 2020, 116, 111160. [Google Scholar] [CrossRef]
- Wassenaar, J.W.; Gaetani, R.; Garcia, J.J.; Braden, R.L.; Luo, C.G.; Huang, D.; DeMaria, A.N.; Omens, J.H.; Christman, K.L. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Shah, R.; Somala, S.R.R.; Anwar, K.N.; Shen, X.; An, S.; Omidi, M.; Rosenblatt, M.I.; Shokuhfar, T.; Djalilian, A.R. In-Situ Porcine Corneal Matrix Hydrogel as Ocular Surface Bandage. Ocul. Surf. 2021, 21, 27–36. [Google Scholar] [CrossRef]
- Changchen, W.; Hongquan, W.; Bo, Z.; Leilei, X.; Haiyue, J.; Bo, P. The Characterization, Cytotoxicity, Macrophage Response and Tissue Regeneration of Decellularized Cartilage in Costal Cartilage Defects. Acta Biomater. 2021, 136, 147–158. [Google Scholar] [CrossRef]
- Farnebo, S.; Woon, C.Y.L.; Schmitt, T.; Joubert, L.-M.; Kim, M.; Pham, H.; Chang, J. Design and Characterization of an Injectable Tendon Hydrogel: A Novel Scaffold for Guided Tissue Regeneration in the Musculoskeletal System. Tissue Eng. Part A 2014, 20, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, M.; Papa, V.; Deluca, G.; Purpura, V.; Ruscelli, P.; Melandri, D.; Capirossi, D.; Nigrisoli, E.; Minghetti, P.; Bondioli, E.; et al. Histological and Ultrastructural Evaluation of Human Decellularized Matrix as a Hernia Repair Device. Ultrastruct. Pathol. 2018, 42, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical Applications of Decellularized Extracellular Matrices for Tissue Engineering and Regenerative Medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Qin, Z.; Chen, G.; Song, B.; Zhang, Z. Decellularized Extracellular Matrix (d-ECM): The Key Role of the Inflammatory Process in Pre-Regeneration after Implantation. Biomater. Sci. 2023, 11, 1215–1235. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, W.; Liu, X.; Wang, H.; Shen, J.; Xiao, H.; Mei, J.; Chai, Y.; Wen, G. Extracellular Matrix Scaffold-Immune Microenvironment Modulates Tissue Regeneration. Compos. Part B Eng. 2022, 230, 109524. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, 1801578. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Leal, R.A.; Healey, G.D.; Corradetti, B. Biomimetic Immunomodulation Strategies for Effective Tissue Repair and Restoration. Adv. Drug Deliv. Rev. 2021, 179, 113913. [Google Scholar] [CrossRef]
- Petrosyan, A.; Da Sacco, S.; Tripuraneni, N.; Kreuser, U.; Lavarreda-Pearce, M.; Tamburrini, R.; De Filippo, R.E.; Orlando, G.; Cravedi, P.; Perin, L. A Step towards Clinical Application of Acellular Matrix: A Clue from Macrophage Polarization. Matrix Biol. 2017, 57–58, 334–346. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Matrikines for Therapeutic and Biomedical Applications. Life Sci. 2018, 214, 22–33. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the Retention of Tissue Niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.J.; et al. Macrophage Phenotype in Response to ECM Bioscaffolds. Semin. Immunol. 2017, 29, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-Bound Nanovesicles within ECM Bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef] [PubMed]
- Piening, L.M.; Wachs, R.A. Matrix-Bound Nanovesicles: What Are They and What Do They Do? Cells Tissues Organs 2022, 212, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Quijano, L.M.; Hussey, G.S.; Jiang, P.; Badylak, S.F. Matrix Bound Nanovesicles Have Tissue-Specific Characteristics That Suggest a Regulatory Role. Tissue Eng. Part A 2022, 28, 879–892. [Google Scholar] [CrossRef]
- Hussey, G.; Pineda, C.; Cramer, M.; Tyurina, Y.; Tyurin, V.; Lee, Y.; El-Mossier, S.; Murdock, M.; Timashev, P.; Kagan, V.; et al. Lipidomics and RNA Sequencing Reveal a Novel Subpopulation of Nanovesicle within Extracellular Matrix Biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory Matrix-Bound Nanovesicles Mitigate Acute and Chronic Pristane-Induced Rheumatoid Arthritis. NPJ Regen. Med. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Crum, R.J.; Capella-Monsonís, H.; Chang, J.; Dewey, M.J.; Kolich, B.D.; Hall, K.T.; El-Mossier, S.O.; Nascari, D.G.; Hussey, G.S.; Badylak, S.F. Biocompatibility and Biodistribution of Matrix-Bound Nanovesicles in Vitro and in Vivo. Acta Biomater. 2023, 155, 113–122. [Google Scholar] [CrossRef]
- Crum, R.J.; Huckestien, B.R.; Dwyer, G.; Mathews, L.; Nascari, D.G.; Hussey, G.S.; Turnquist, H.R.; Alcorn, J.F.; Badylak, S.F. Mitigation of Influenza-Mediated Inflammation by Immunomodulatory Matrix-Bound Nanovesicles. Sci. Adv. 2023, 9, eadf9016. [Google Scholar] [CrossRef]
- Huleihel, L.; Bartolacci, J.G.; Dziki, J.L.; Vorobyov, T.; Arnold, B.; Scarritt, M.E.; Pineda Molina, C.; LoPresti, S.T.; Brown, B.N.; Naranjo, J.D.; et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng. Part A 2017, 23, 1283–1294. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Lee, Y.C.; Bartolacci, J.G.; Behun, M.; Turnquist, H.R.; Badylak, S.F. Matrix Bound Nanovesicle-Associated IL-33 Activates a pro-Remodeling Macrophage Phenotype via a Non-Canonical, ST2-Independent Pathway. J. Immunol. Regen. Med. 2019, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, Y.; Faust, A.; Sakallı, E.; Westrick, C.; Hussey, G.; Conner, I.; Fu, V.; Badylak, S.; Steketee, M. Matrix-Bound Nanovesicles Prevent Ischemia-Induced Retinal Ganglion Cell Axon Degeneration and Death and Preserve Visual Function. Sci. Rep. 2019, 9, 3482. [Google Scholar] [CrossRef] [PubMed]
- Faust, A.; Kandakatla, A.; van der Merwe, Y.; Ren, T.; Huleihel, L.; Hussey, G.; Naranjo, J.D.; Johnson, S.; Badylak, S.; Steketee, M. Urinary Bladder Extracellular Matrix Hydrogels and Matrix-Bound Vesicles Differentially Regulate Central Nervous System Neuron Viability and Axon Growth and Branching. J. Biomater. Appl. 2017, 31, 1277–1295. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.; Pineda Molina, C.; Hussey, G.; Turnquist, H.R.; Badylak, S.F. Transcriptomic Regulation of Macrophages by Matrix-Bound Nanovesicle-Associated Interleukin-33. Tissue Eng. Part A 2022, 28, 867–878. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishida, N.; Hashimoto, Y.; Negishi, J.; Saga, H.; Sasaki, Y.; Akiyoshi, K.; Kimura, T.; Kishida, A. Extraction and Biological Evaluation of Matrix-Bound Nanovesicles (MBVs) from High-Hydrostatic Pressure-Decellularized Tissues. Int. J. Mol. Sci. 2022, 23, 8868. [Google Scholar] [CrossRef]
- Patel, N.J.; Ashraf, A.; Chung, E.J. Extracellular Vesicles as Regulators of the Extracellular Matrix. Bioengineering 2023, 10, 136. [Google Scholar] [CrossRef]
- Quijano, L.M.; Naranjo, J.D.; El-Mossier, S.O.; Turner, N.J.; Pineda Molina, C.; Bartolacci, J.; Zhang, L.; White, L.; Li, H.; Badylak, S.F. Matrix-Bound Nanovesicles: The Effects of Isolation Method upon Yield, Purity, and Function. Tissue Eng. Part C Methods 2020, 26, 528–540. [Google Scholar] [CrossRef]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; Giorgio, D.R.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Umashankar, P.R.; Arun, T.; Kumary, T.V. Effect of Chronic Inflammation and Immune Response on Regeneration Induced by Decellularized Bovine Pericardium. J. Biomed. Mater. Res. Part A 2013, 101A, 2202–2209. [Google Scholar] [CrossRef]
- Heuschkel, M.A.; Leitolis, A.; Roderjan, J.G.; Suss, P.H.; Luzia, C.A.O.; da Costa, F.D.A.; Correa, A.; Stimamiglio, M.A. In Vitro Evaluation of Bovine Pericardium after a Soft Decellularization Approach for Use in Tissue Engineering. Xenotransplantation 2019, 26, e12464. [Google Scholar] [CrossRef]
- Botes, L.; Laker, L.; Dohmen, P.M.; van den Heever, J.J.; Jordaan, C.J.; Lewies, A.; Smit, F.E. Advantages of Decellularized Bovine Pericardial Scaffolds Compared to Glutaraldehyde Fixed Bovine Pericardial Patches Demonstrated in a 180-Day Implant Ovine Study. Cell Tissue Bank 2022, 23, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Bozso, S.J.; Kang, J.J.H.; El-Andari, R.; Boe, D.; Hedtke, H.; Moon, M.C.; Freed, D.H.; Nagendran, J.; Nagendran, J. Recellularized Bovine Pericardium with Autologous Mesenchymal Stem Cells Reduces Immune Activation. Xenotransplantation 2022, 29, e12774. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, D.; Bertani, F.; Fusaro, L.; Clemente, N.; Carton, F.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Regenerative Potential of A Bovine ECM-Derived Hydrogel for Biomedical Applications. Biomolecules 2022, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Di Francesco, D.; Fusaro, L.; Zanella, E.; Apostolo, C.; Oltolina, F.; Cotella, D.; Prat, M.; Boccafoschi, F. Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium. Int. J. Mol. Sci. 2021, 22, 9406. [Google Scholar] [CrossRef]
- Cramer, M.C.; D’Angelo, W.A.; Dewey, M.J.; Manuel, A.M.; Mullett, S.J.; Wendell, S.G.; Napierala, D.; Jiang, P.; Badylak, S.F. Extracellular Vesicles Present in Bone, Blood and Extracellular Matrix Have Distinctive Characteristics and Biologic Roles. J. Immunol. Regen. Med. 2022, 18, 100066. [Google Scholar] [CrossRef]
- Bason, C.; Gallorini, M.; Berardi, A.C. The Extracellular Matrix, Growth Factors and Morphogens in Biomaterial Design and Tissue Engineering. In Extracellular Matrix for Tissue Engineering and Biomaterials; Stem Cell Biology and Regenerative Medicine; Berardi, A.C., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–26. ISBN 978-3-319-77023-9. [Google Scholar]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- De Jong, O.G.; Van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular Vesicles: Potential Roles in Regenerative Medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W.; Day, R. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast Growth Factors: Key Players in Regeneration and Tissue Repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef]
- Nederlof, R.; Reidel, S.; Spychala, A.; Gödecke, S.; Heinen, A.; Lautwein, T.; Petzsch, P.; Köhrer, K.; Gödecke, A. Insulin-Like Growth Factor 1 Attenuates the Pro-Inflammatory Phenotype of Neutrophils in Myocardial Infarction. Front. Immunol. 2022, 13, 908023. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhong, J.; Dong, L. The Role of Decorin in Autoimmune and Inflammatory Diseases. J. Immunol. Res. 2022, 2022, 1283383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin Is a Pivotal Effector in the Extracellular Matrix and Tumour Microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ghorai, S.M. Role of Cytokines as Immunomodulators. In Immunomodulators and Human Health; Kesharwani, R.K., Keservani, R.K., Sharma, A.K., Eds.; Springer Nature: Singapore, 2022; pp. 371–414. ISBN 9789811663796. [Google Scholar]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- McInnes, I.B.; Gracie, J.A. Interleukin-15: A New Cytokine Target for the Treatment of Inflammatory Diseases. Curr. Opin. Pharmacol. 2004, 4, 392–397. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A Double-Edged Sword with Therapeutic Potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Karin, N.; Razon, H. Chemokines beyond Chemo-Attraction: CXCL10 and Its Significant Role in Cancer and Autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef]
- Wang, C.; Yin, Q.; Patterson, T.A.; Liu, S.; Zhang, X.; Liu, F.; Paule, M.G.; Slikker, W. Chapter 2—Neural Cell Adhesion Molecules in Normal and Abnormal Neural Development. In Handbook of Developmental Neurotoxicology, 2nd ed.; Slikker, W., Paule, M.G., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 17–22. ISBN 978-0-12-809405-1. [Google Scholar]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.D.; Rodriguez-Menocal, L.; Candanedo, A.; Guzman, W.; Garcia-Contreras, M.; Badiavas, E.V. Dual Mechanism of Type VII Collagen Transfer by Bone Marrow Mesenchymal Stem Cell Extracellular Vesicles to Recessive Dystrophic Epidermolysis Bullosa Fibroblasts. Biochimie 2018, 155, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Alanazi, Y.F.; Jowitt, T.A.; Roseman, A.M.; Baldock, C. Elastic Fibre Proteins in Elastogenesis and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4087. [Google Scholar] [CrossRef] [PubMed]
- Deckx, S.; Heymans, S.; Papageorgiou, A.-P. The Diverse Functions of Osteoglycin: A Deceitful Dwarf, or a Master Regulator of Disease? FASEB J. 2016, 30, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Otoupalova, E.; Hough, K.P.; Locy, M.L.; Bernard, K.; Deshane, J.S.; Sanderson, R.D.; Mobley, J.A.; Thannickal, V.J. Fibronectin on the Surface of Extracellular Vesicles Mediates Fibroblast Invasion. Am. J. Respir. Cell Mol. Biol. 2019, 60, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, R.; Kemper, S.; Brigstock, D.R. Structural and Functional Characterization of Fibronectin in Extracellular Vesicles From Hepatocytes. Front. Cell Dev. Biol. 2021, 9, 640667. [Google Scholar] [CrossRef]
- Bin, B.-H.; Kim, D.-K.; Kim, N.-H.; Choi, E.-J.; Bhin, J.; Kim, S.T.; Gho, Y.S.; Lee, A.-Y.; Lee, T.R.; Cho, E.-G. Fibronectin-Containing Extracellular Vesicles Protect Melanocytes against Ultraviolet Radiation-Induced Cytotoxicity. J. Investig. Dermatol. 2016, 136, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.J.; Stewart, S.E.; Moreau, K. Unconventional Secretion of Annexins and Galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50. [Google Scholar] [CrossRef]
- Parvanian, S.; Yan, F.; Su, D.; Coelho-Rato, L.S.; Venu, A.P.; Yang, P.; Zou, X.; Jiu, Y.; Chen, H.; Eriksson, J.E.; et al. Exosomal Vimentin from Adipocyte Progenitors Accelerates Wound Healing. Cytoskeleton 2020, 77, 399–413. [Google Scholar] [CrossRef]
- Singh, A.; Verma, S.; Modak, S.B.; Chaturvedi, M.M.; Purohit, J.S. Extra-Nuclear Histones: Origin, Significance and Perspectives. Mol. Cell Biochem. 2022, 477, 507–524. [Google Scholar] [CrossRef]
- Hernandez, M.J.; Yakutis, G.E.; Zelus, E.I.; Hill, R.C.; Dzieciatkowska, M.; Hansen, K.C.; Christman, K.L. Manufacturing Considerations for Producing and Assessing Decellularized Extracellular Matrix Hydrogels. Methods 2020, 171, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tiruvayipati, S.; Wolfgeher, D.; Yue, M.; Duan, F.; Andrade, J.; Jiang, H.; Schuger, L. Variability in Protein Cargo Detection in Technical and Biological Replicates of Exosome-Enriched Extracellular Vesicles. PLoS ONE 2020, 15, e0228871. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Hill, R.C.; Dzieciatkowska, M.; Nigam, V.; Behfar, A.; Christman, K.L.; Hansen, K.C. Quantification of Decellularized Human Myocardial Matrix: A Comparison of Six Patients. Proteom.-Clin. Appl. 2016, 10, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Reis, R.L.; Motta, A.; Khang, G. Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1250, ISBN 9789811532610. [Google Scholar]
- Hortensius, R.A.; Harley, B.A. Naturally Derived Biomaterials for Addressing Inflammation in Tissue Regeneration. Exp. Biol. Med. 2016, 241, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.J.; Capella-Monsonís, H.; Badylak, S.F.; Hussey, G.S. Extracellular Vesicles for Regenerative Medicine Applications. Appl. Sci. 2022, 12, 7472. [Google Scholar] [CrossRef]
- Subedi, P.; Schneider, M.; Philipp, J.; Azimzadeh, O.; Metzger, F.; Moertl, S.; Atkinson, M.J.; Tapio, S. Comparison of Methods to Isolate Proteins from Extracellular Vesicles for Mass Spectrometry-Based Proteomic Analyses. Anal. Biochem. 2019, 584, 113390. [Google Scholar] [CrossRef]
- Manfredi, M.; Martinotti, S.; Gosetti, F.; Ranzato, E.; Marengo, E. The Secretome Signature of Malignant Mesothelioma Cell Lines. J. Proteom. 2016, 145, 3–10. [Google Scholar] [CrossRef]


| Protein | MBV.1 | MBV.2 |
|---|---|---|
| Total | 45 | 23 |
| Transthyretin | ✔ | |
| Keratocan | ✔ | |
| Prothrombin | ✔ | |
| Protein AMBP | ✔ | |
| Collagen alpha-1(I) chain | ✔ | ✔ |
| Collagen alpha-2(I) chain | ✔ | ✔ |
| Fibrinogen beta chain | ✔ | |
| Albumin | ✔ | ✔ |
| Annexin A2 | ✔ | ✔ |
| Fibronectin | ✔ | ✔ |
| Heat shock 70 kDa protein 1-like | ✔ | ✔ |
| Galectin-1 | ✔ | |
| Alpha-2-HS-glycoprotein | ✔ | ✔ |
| Apolipoprotein A-I | ✔ | |
| Mimecan | ✔ | ✔ |
| Decorin | ✔ | ✔ |
| Protein-lysine 6-oxidase | ✔ | |
| Alpha-1-antiproteinase | ✔ | |
| Vimentin | ✔ | ✔ |
| Transforming growth factor-beta-induced protein | ✔ | |
| Actin | ✔ | ✔ |
| Ras-related protein Rap-1b | ✔ | |
| Histone H4 | ✔ | ✔ |
| 14-3-3 protein zeta/delta | ✔ | |
| Elongation factor 1-alpha 1 | ✔ | |
| Tubulin alpha-4A chain | ✔ | |
| Fibrillin-1 | ✔ | ✔ |
| Endoplasmic reticulum chaperone BiP | ✔ | |
| Myosin-10 | ✔ | |
| Latent-transforming growth factor beta-binding protein 2 | ✔ | |
| Complement component C6 | ✔ | ✔ |
| Tubulin beta-5 chain | ✔ | ✔ |
| Complement C3 | ✔ | |
| Thrombospondin-4 | ✔ | |
| Gelsolin | ✔ | |
| Heat shock protein beta-1 | ✔ | |
| Asporin | ✔ | ✔ |
| Fibulin-5 | ✔ | ✔ |
| Tropomyosin alpha-3 chain | ✔ | |
| Small ribosomal subunit protein eS12 | ✔ | |
| Heat shock protein HSP 90-beta | ✔ | |
| Alpha-2-macroglobulin | ✔ | |
| Junction plakoglobin | ✔ | |
| Prolargin | ✔ | ✔ |
| Myocilin | ✔ | |
| Collagen alpha-1(III) chain | ✔ | |
| LIM and SH3 domain protein 1 | ✔ | |
| Fibrinogen gamma-B chain | ✔ | |
| Cadherin-13 | ✔ | |
| Poly(rC)-binding protein 1 | ✔ |
| POS | POS | NEG | NEG | aFGF | Angiopoietin-1 | bFGF | CD40 Ligand (TNFSF5) | Decorin | GASP-1 (WFIKKNRP) | IFN alpha | IFN beta |
| POS | POS | NEG | NEG | aFGF | Angiopoietin-1 | bFGF | CD40 Ligand (TNFSF5) | Decorin | GASP-1 (WFIKKNRP) | IFN alpha | IFN beta |
| IFN-gamma | IGF-1 | IL-1 alpha (IL-1 F1) | IL-1 beta (IL-1 F2) | IL-36 Ra (IL-1 F5) | IL-10 | IL-13 | IL-15 | IL-17A | IL-18 | IL-2 | IL-21 |
| IFN-gamma | IGF-1 | IL-1 alpha (IL-1 F1) | IL-1 beta (IL-1 F2) | IL-36 Ra (IL-1 F5) | IL-10 | IL-13 | IL-15 | IL-17A | IL-18 | IL-2 | IL-21 |
| IL-4 | IP-10 (CXCL10) | LIF | MCP-1 (CCL2) | MIG (CXCL9) | MIP-1 beta (CCL4) | NCAM-1 (CD56) | RANTES (CCL5) | TNF alpha | VEGF-A | BLANK | POS |
| IL-4 | IP-10 (CXCL10) | LIF | MCP-1 (CCL2) | MIG (CXCL9) | MIP-1 beta (CCL4) | NCAM-1 (CD56) | RANTES (CCL5) | TNF alpha | VEGF-A | BLANK | POS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Francesco, D.; Di Varsavia, C.; Casarella, S.; Donetti, E.; Manfredi, M.; Mantovani, D.; Boccafoschi, F. Characterisation of Matrix-Bound Nanovesicles (MBVs) Isolated from Decellularised Bovine Pericardium: New Frontiers in Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 740. https://doi.org/10.3390/ijms25020740
Di Francesco D, Di Varsavia C, Casarella S, Donetti E, Manfredi M, Mantovani D, Boccafoschi F. Characterisation of Matrix-Bound Nanovesicles (MBVs) Isolated from Decellularised Bovine Pericardium: New Frontiers in Regenerative Medicine. International Journal of Molecular Sciences. 2024; 25(2):740. https://doi.org/10.3390/ijms25020740
Chicago/Turabian StyleDi Francesco, Dalila, Carolina Di Varsavia, Simona Casarella, Elena Donetti, Marcello Manfredi, Diego Mantovani, and Francesca Boccafoschi. 2024. "Characterisation of Matrix-Bound Nanovesicles (MBVs) Isolated from Decellularised Bovine Pericardium: New Frontiers in Regenerative Medicine" International Journal of Molecular Sciences 25, no. 2: 740. https://doi.org/10.3390/ijms25020740
APA StyleDi Francesco, D., Di Varsavia, C., Casarella, S., Donetti, E., Manfredi, M., Mantovani, D., & Boccafoschi, F. (2024). Characterisation of Matrix-Bound Nanovesicles (MBVs) Isolated from Decellularised Bovine Pericardium: New Frontiers in Regenerative Medicine. International Journal of Molecular Sciences, 25(2), 740. https://doi.org/10.3390/ijms25020740






