Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches
Abstract
:1. Introduction
2. Transcription Factors Regulating Ripening
3. Epigenetic Modifications as Regulators of Ripening
4. Hormonal Control of Ripening
4.1. Auxin Regulation
4.2. Gibberellin Regulation
4.3. Cytokinin Regulation
4.4. Ethylene Regulation
4.5. Brassinosteroid Regulation
4.6. Abscisic Acid Regulation
4.7. Salicylic Acid Regulation
4.8. Jasmonate Regulation
4.9. Hydrogen Sulfide
5. Abiotic Ripening Factors
6. System of Regulation of Tomato Fruit Ripening Process
7. Future Prospects and Challenges
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant 2020, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L. Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, N.; Xiao, Z.; Qiu, Y.; Yang, Y.; Yang, J.; Mao, X.; Wang, Y.; Li, Z.; Guo, H. A Molecular Framework of Ethylene-Mediated Fruit Growth and Ripening Processes in Tomato. Plant Cell 2022, 34, 3280–3300. [Google Scholar] [CrossRef] [PubMed]
- Kamiyoshihara, Y.; Achiha, Y.; Ishikawa, S.; Mizuno, S.; Mori, H.; Tateishi, A.; Huber, D.J.; Klee, H.J. Heteromeric Interactions of Ripening-Related Ethylene Receptors in Tomato Fruit. J. Exp. Bot. 2022, 73, 6773–6783. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.-X. Regulation of Fleshy Fruit Ripening: From Transcription Factors to Epigenetic Modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, W.; Fan, Z.; Zhao, X.; Zhang, Y.; Jing, Y.; Zhu, B.; Zhu, H.; Shan, W.; Chen, J.; et al. Re-Evaluation of the nor Mutation and the Role of the NAC-NOR Transcription Factor in Tomato Fruit Ripening. J. Exp. Bot. 2020, 71, 3560–3574. [Google Scholar] [CrossRef]
- Lelièvre, J.-M.; Latchè, A.; Jones, B.; Bouzayen, M.; Pech, J.-C. Ethylene and Fruit Ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, J.; Yang, Y.-L.; Wang, Z.-H. Functional Characterization of an Invertase Inhibitor Gene Involved in Sucrose Metabolism in Tomato Fruit. J. Zhejiang Univ. Sci. B 2015, 16, 845–856. [Google Scholar] [CrossRef]
- Wang, L.-F.; Qi, X.-X.; Huang, X.-S.; Xu, L.-L.; Jin, C.; Wu, J.; Zhang, S.-L. Overexpression of Sucrose Transporter Gene PbSUT2 from Pyrus Bretschneideri, Enhances Sucrose Content in Solanum lycopersicum Fruit. Plant Physiol. Biochem. 2016, 105, 150–161. [Google Scholar] [CrossRef]
- Cai, Y.; Yin, L.; Tu, W.; Deng, Z.; Yan, J.; Dong, W.; Gao, H.; Xu, J.; Zhang, N.; Wang, J.; et al. Ectopic Expression of VvSUC27 Induces Stenospermocarpy and Sugar Accumulation in Tomato Fruits. Front. Plant Sci. 2021, 12, 759047. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-Y.; Ho, L.-H.; Neuhaus, H.E.; Guo, W.-J. Transporter SlSWEET15 Unloads Sucrose from Phloem and Seed Coat for Fruit and Seed Development in Tomato. Plant Physiol. 2021, 187, 2230–2245. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.-A.; Ruan, Y.-L. Posttranslational Elevation of Cell Wall Invertase Activity by Silencing Its Inhibitor in Tomato Delays Leaf Senescence and Increases Seed Weight and Fruit Hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef] [PubMed]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural Genetic Variation for Expression of a SWEET Transporter among Wild Species of Solanum lycopersicum (Tomato) Determines the Hexose Composition of Ripening Tomato Fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, X.; Yang, J.; Li, H.; Ma, B.; Zhang, K.; Zhang, Y.; Cheng, L.; Ma, F.; Li, M. Heterologous Expression of the Apple Hexose Transporter MdHT2.2 Altered Sugar Concentration with Increasing Cell Wall Invertase Activity in Tomato Fruit. Plant Biotechnol. J. 2020, 18, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Osorio, S.; Gehl, B.; Baxter, C.J.; Kruger, N.J.; Ratcliffe, R.G.; Fernie, A.R.; Sweetlove, L.J. Metabolic Engineering of Tomato Fruit Organic Acid Content Guided by Biochemical Analysis of an Introgression Line. Plant Physiol. 2012, 161, 397–407. [Google Scholar] [CrossRef]
- Bastías, A.; López-Climent, M.; Valcárcel, M.; Rosello, S.; Gómez-Cadenas, A.; Casaretto, J.A. Modulation of Organic Acids and Sugar Content in Tomato Fruits by an Abscisic Acid-regulated Transcription Factor. Physiol. Plant. 2011, 141, 215–226. [Google Scholar] [CrossRef]
- Shi, C.-Y.; Hussain, S.B.; Yang, H.; Bai, Y.-X.; Khan, M.A.; Liu, Y.-Z. CsPH8, a P-Type Proton Pump Gene, Plays a Key Role in the Diversity of Citric Acid Accumulation in Citrus Fruits. Plant Sci. 2019, 289, 110288. [Google Scholar] [CrossRef]
- Imran, M.; Munir, M.Z.; Ialhi, S.; Abbas, F.; Younus, M.; Ahmad, S.; Naeem, M.K.; Waseem, M.; Iqbal, A.; Gul, S.; et al. Identification and Characterization of Malate Dehydrogenases in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2022, 23, 10028. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, B.; Wang, C.; Chen, X.; Ruan, Y.-L.; Yuan, Y.; Ma, F.; Li, M. MdWRKY126 Modulates Malate Accumulation in Apple Fruit by Regulating Cytosolic Malate Dehydrogenase (MdMDH5). Plant Physiol. 2022, 188, 2059–2072. [Google Scholar] [CrossRef]
- Snowden, C.J.; Thomas, B.; Baxter, C.J.; Smith, J.A.C.; Sweetlove, L.J. A Tonoplast Glu/Asp/GABA Exchanger That Affects Tomato Fruit Amino Acid Composition. Plant J. 2015, 81, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated Metabolic Engineering of Γ-aminobutyric Acid Levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient Increase of Ɣ-Aminobutyric Acid (GABA) Content in Tomato Fruits by Targeted Mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Bui, T.P.; Le, N.T.; Nguyen, C.X.; Le, M.T.T.; Dao, N.T.; Phan, Q.; Van Le, T.; To, H.M.T.; Pham, N.B.; et al. Disrupting Sc-uORFs of a Transcription Factor bZIP1 Using CRISPR/Cas9 Enhances Sugar and Amino Acid Contents in Tomato (Solanum lycopersicum). Planta 2023, 257, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, Y.; Hu, H.; Wang, C.; Zhang, W.; Li, H.; Cheng, Z.; Yang, L. Functional Validation of Phytoene Synthase and Lycopene ε-Cyclase Genes for High Lycopene Content in Autumn Olive Fruit (Elaeagnus umbellata). J. Agric. Food Chem. 2020, 68, 11503–11511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Arruabarrena, A.; Lado, J.; González-Arcos, M.; Vidal, S. Targeted Disruption of Tomato Chromoplast-specific Lycopene Β-cyclase (CYC-B) Gene Promotes Early Accumulation of Lycopene in Fruits and Enhanced Postharvest Cold Tolerance. Plant Biotechnol. J. 2023, 21, 2420–2422. [Google Scholar] [CrossRef]
- Diretto, G.; Frusciante, S.; Fabbri, C.; Schauer, N.; Busta, L.; Wang, Z.; Matas, A.J.; Fiore, A.; Rose, J.K.C.; Fernie, A.R.; et al. Manipulation of Β-carotene Levels in Tomato Fruits Results in Increased ABA Content and Extended Shelf Life. Plant Biotechnol. J. 2020, 18, 1185–1199. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Zhang, M.; Yang, M.; Dai, S.; Meng, Q.; Lv, W.; Zhuang, K. ETHYLENE-INSENSITIVE 3-LIKE 2 Regulates β-Carotene and Ascorbic Acid Accumulation in Tomatoes during Ripening. Plant Physiol. 2023, 192, 2067–2080. [Google Scholar] [CrossRef]
- Li, Z.; Peng, R.; Yao, Q. SlMYB14 Promotes Flavonoids Accumulation and Confers Higher Tolerance to 2,4,6-Trichlorophenol in Tomato. Plant Sci. 2021, 303, 110796. [Google Scholar] [CrossRef]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; Ric De Vos, C.H.; van Tunen, A.J.; Verhoeyen, M.E. Overexpression of Petunia Chalcone Isomerase in Tomato Results in Fruit Containing Increased Levels of Flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, Y.; Xie, X.; Li, H.; Li, X.; Zhu, Y.; Zhai, L.; Zhang, C.; Bian, S. A Blueberry MIR156a–SPL12 Module Coordinates the Accumulation of Chlorophylls and Anthocyanins during Fruit Ripening. J. Exp. Bot. 2020, 71, 5976–5989. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, K.; Tang, B.; Su, D.; He, X.; Deng, H.; Wu, M.; Bouzayen, M.; Grierson, D.; Liu, M. The MADS-box Protein SlTAGL1 Regulates a Ripening-associated SlDQD/SDH2 Involved in Flavonoid Biosynthesis and Resistance against Botrytis cinerea in Post-harvest Tomato Fruit. Plant J. 2023, 115, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Chi, C.; Jin, L.-J.; Zhu, J.; Yu, J.-Q.; Zhou, Y.-H. The bZip Transcription Factor HY5 Mediates CRY1a-induced Anthocyanin Biosynthesis in Tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Y.; Lu, M.; Lu, C.; Ludlow, R.A.; Yang, M.; Huang, W.; Liu, Z.; An, H. Gene Expression Profiling in Rosa Roxburghii Fruit and Overexpressing RrGGP2 in Tobacco and Tomato Indicates the Key Control Point of AsA Biosynthesis. Front. Plant Sci. 2023, 13, 1096493. [Google Scholar] [CrossRef]
- Koukounaras, A.; Mellidou, I.; Patelou, E.; Kostas, S.; Shukla, V.; Engineer, C.; Papaefthimiou, D.; Amari, F.; Chatzopoulos, D.; Mattoo, A.K.; et al. Over-Expression of GGP1 and GPP Genes Enhances Ascorbate Content and Nutritional Quality of Tomato. Plant Physiol. Biochem. 2022, 193, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, Y.; Huang, B.; Hu, X.; Tang, Y.; Xu, X.; Wu, M.; Gong, Z.; Luo, Y.; Gong, M.; et al. Control of Fruit Softening and Ascorbic Acid Accumulation by Manipulation of SlIMP3 in Tomato. Plant Biotechnol. J. 2022, 20, 1213–1225. [Google Scholar] [CrossRef]
- Do, J.H.; Park, S.Y.; Park, S.H.; Kim, H.M.; Ma, S.H.; Mai, T.D.; Shim, J.S.; Joung, Y.H. Development of a Genome-Edited Tomato with High Ascorbate Content during Later Stage of Fruit Ripening through Mutation of SlAPX4. Front. Plant Sci. 2022, 13, 836916. [Google Scholar] [CrossRef]
- Grützner, R.; Schubert, R.; Horn, C.; Yang, C.; Vogt, T.; Marillonnet, S. Engineering Betalain Biosynthesis in Tomato for High Level Betanin Production in Fruits. Front. Plant Sci. 2021, 12, 682443. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, J.; Wang, M.; Li, D.; Liu, Y.; Chen, T.H.H.; Yang, X. Genetic Engineering of the Biosynthesis of Glycinebetaine Enhances the Fruit Development and Size of Tomato. Plant Sci. 2019, 280, 355–366. [Google Scholar] [CrossRef]
- Liao, J.; Liu, T.; Xie, L.; Mo, C.; Qiao, J.; Huang, X.; Cui, S.; Jia, X.; Luo, Z.; Ma, X. Heterologous Mogrosides Biosynthesis in Cucumber and Tomato by Genetic Manipulation. Commun. Biol. 2023, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Davod, J.; Fatemeh, D.N.; Honari, H.; Hosseini, R. Constructing and Transient Expression of a Gene Cassette Containing Edible Vaccine Elements and Shigellosis, Anthrax and Cholera Recombinant Antigens in Tomato. Mol. Biol. Rep. 2018, 45, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Tian, Y.; Wu, J.; Gu, Y.; Chen, Z.; Zeng, F.; Liu, J. Construction of a Fusion Anti-caries DNA Vaccine in Transgenic Tomato Plants for PAcA Gene and Cholera Toxin B Subunit. Biotechnol. Appl. Biochem. 2019, 66, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Inam, S.; Abbas, Z.; Noor, S.; Rehman, N.; Adeel Zafar, S.; Ramzan Khan, M.; Ali Kaimkhani, Z.; Al-Misned, F.; Shah, M.; Mahboob, S.; et al. Isolation, Cloning and Transgenic Expression of Hepatitis B Surface Antigen (HBsAg) in Solanum lycopersicum L. Saudi J. Biol. Sci. 2022, 29, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Beihaghi, M.; Marashi, H.; Bagheri, A.; Sankian, M. Transient Expression of CCL21as Recombinant Protein in Tomato. Biotechnol. Rep. 2018, 17, 10–15. [Google Scholar] [CrossRef]
- The Tomato Genome Consortium. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Su, X.; Wang, B.; Geng, X.; Du, Y.; Yang, Q.; Liang, B.; Meng, G.; Gao, Q.; Yang, W.; Zhu, Y.; et al. A High-Continuity and Annotated Tomato Reference Genome. BMC Genom. 2021, 22, 898. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium Tumefaciens-Mediated Transformation of Tomato. In Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 225–234. ISBN 9781493987771. [Google Scholar]
- Sandhya, D.; Jogam, P.; Venkatapuram, A.K.; Savitikadi, P.; Peddaboina, V.; Allini, V.R.; Abbagani, S. Highly Efficient Agrobacterium-Mediated Transformation and Plant Regeneration System for Genome Engineering in Tomato. Saudi J. Biol. Sci. 2022, 29, 103292. [Google Scholar] [CrossRef]
- Honda, C.; Ohkawa, K.; Kusano, H.; Teramura, H.; Shimada, H. A Simple Method for in Planta Tomato Transformation by Inoculating Floral Buds with a Sticky Agrobacterium Tumefaciens Suspension. Plant Biotechnol. 2021, 38, 153–156. [Google Scholar] [CrossRef]
- Ho-Plágaro, T.; Huertas, R.; Tamayo-Navarrete, M.I.; Ocampo, J.A.; García-Garrido, J.M. An Improved Method for Agrobacterium Rhizogenes-Mediated Transformation of Tomato Suitable for the Study of Arbuscular Mycorrhizal Symbiosis. Plant Methods 2018, 14, 34. [Google Scholar] [CrossRef]
- Yuan, S.; Kawasaki, S.; Abdellatif, I.M.Y.; Nishida, K.; Kondo, A.; Ariizumi, T.; Ezura, H.; Miura, K. Efficient Base Editing in Tomato Using a Highly Expressed Transient System. Plant Cell Rep. 2021, 40, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Tor, M.; Barry, C.S.; Vrebalov, J.; Orfila, C.; Jarvis, M.C.; Giovannoni, J.J.; Grierson, D.; Seymour, G.B. Molecular and Genetic Characterization of a Novel Pleiotropic Tomato-Ripening Mutant1. Plant Physiol. 1999, 120, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R. Ripen inginhibitor: A gene with multiple effects on ripening. Rep. Tomato Genet Coop. 1968, 18, 36–37. [Google Scholar]
- Tigchelaar, E.C. A new ripening mutant, non-ripening (nor). Rep. Tomato Genet Coop. 1973, 35, 20. [Google Scholar]
- Rick, C.M. New mutants. Rep. Tomato Genet. Coop. 1956, 6, 22–23. [Google Scholar]
- Kerr, E.A. Mutations of chlorophyll retention in ripe fruit. Rep. Tomato Genet. Coop. 1958, 8, 22. [Google Scholar]
- Kerr, E. Never ripe-2 (Nr-2) a slow ripening mutant resembling Nr an Gr. TGC Rep. 1982, 32, 33. [Google Scholar]
- Kopeliovitch, E.; Rabinowitch, H.D.; Mizrahi, Y.; Kedar, N. Mode of Inheritance of Alcobaca, a Tomato Fruit-Ripening Mutant. Euphytica 1981, 30, 223–225. [Google Scholar] [CrossRef]
- Wang, R.; da Tavano, E.C.R.; Lammers, M.; Martinelli, A.P.; Angenent, G.C.; de Maagd, R.A. Re-Evaluation of Transcription Factor Function in Tomato Fruit Development and Ripening with CRISPR/Cas9-Mutagenesis. Sci. Rep. 2019, 9, 1696. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Chen, S.; Meng, L.; Fu, D. Role of the Tomato TAGL1 Gene in Regulating Fruit Metabolites Elucidated Using RNA Sequence and Metabolomics Analyses. PLoS ONE 2018, 13, e0199083. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Chen, S.; Fu, D.-Q.; Jiang, C.-Z. Metabolomic and Transcriptomic Analyses Reveal That a MADS-Box Transcription Factor TDR4 Regulates Tomato Fruit Quality. Front. Plant Sci. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Shima, Y.; Nakamura, N.; Kotake-Nara, E.; Kawasaki, S.; Toki, S. Re-Evaluation of the Rin Mutation and the Role of RIN in the Induction of Tomato Ripening. Nat. Plants 2017, 3, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.; Ju, Z.; Cao, D.; Zhu, H.; Fu, D.; Grierson, D.; Qin, G.; Luo, Y.; Zhu, B. The RIN-MC Fusion of MADS-Box Transcription Factors Has Transcriptional Activity and Modulates Expression of Many Ripening Genes. Plant Physiol. 2018, 176, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, B.; Pirrello, J.; Xu, C.; Zhang, B.; Bouzayen, M.; Chen, K.; Grierson, D. Roles of RIN and Ethylene in Tomato Fruit Ripening and Ripening-associated Traits. New Phytol. 2020, 226, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Tucker, M.L.; Mattoo, A.K. Ethylene and RIPENING INHIBITOR Modulate Expression of SlHSP17.7A, B Class I Small Heat Shock Protein Genes during Tomato Fruit Ripening. Front. Plant Sci. 2020, 11, 975. [Google Scholar] [CrossRef]
- Ito, Y.; Sekiyama, Y.; Nakayama, H.; Nishizawa-Yokoi, A.; Endo, M.; Shima, Y.; Nakamura, N.; Kotake-Nara, E.; Kawasaki, S.; Hirose, S.; et al. Allelic Mutations in the Ripening-Inhibitor Locus Generate Extensive Variation in Tomato Ripening. Plant Physiol. 2020, 183, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tzeng, D.T.W.; Li, R.; Chen, J.; Zhong, S.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Genome-Wide Identification of Long Non-Coding RNA Targets of the Tomato MADS Box Transcription Factor RIN and Function Analysis. Ann. Bot. 2019, 123, 469–482. [Google Scholar] [CrossRef]
- Kang, J.; Gong, J.; Zhang, L.; Gao, Z.; Xie, Q.; Hu, Z.; Chen, G. A Novel E6-like Gene, E6-2, Affects Fruit Ripening in Tomato. Plant Sci. 2021, 313, 111066. [Google Scholar] [CrossRef]
- Osakabe, K.; Wada, N.; Miyaji, T.; Murakami, E.; Marui, K.; Ueta, R.; Hashimoto, R.; Abe-Hara, C.; Kong, B.; Yano, K.; et al. Genome Editing in Plants Using CRISPR Type I-D Nuclease. Commun. Biol. 2020, 3, 648. [Google Scholar] [CrossRef]
- Niu, Q.; Wu, S.; Li, Y.; Yang, X.; Liu, P.; Xu, Y.; Lang, Z. Expanding the Scope of CRISPR/Cas9-mediated Genome Editing in Plants Using an xCas9 and Cas9-NG Hybrid. J. Integr. Plant Biol. 2020, 62, 398–402. [Google Scholar] [CrossRef]
- Niu, Q.; Wu, S.; Xie, H.; Wu, Q.; Liu, P.; Xu, Y.; Lang, Z. Efficient A·T to G·C Base Conversions in Dicots Using Adenine Base Editors Expressed under the Tomato EF1α Promoter. Plant Biotechnol. J. 2023, 21, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, W.; Zhao, X.; Tan, X.; Fan, Z.; Zhang, Y.; Jing, Y.; Meng, L.; Zhu, B.; Zhu, H.; et al. A NAC Transcription Factor, NOR-Like1, Is a New Positive Regulator of Tomato Fruit Ripening. Hortic. Res. 2018, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, H.; Nonaka, S.; Sato-Izawa, K.; Kusano, M.; Ezura, H. Ethylene Biosynthesis Controlled by NON-RIPENING: A Regulatory Conflict between Wounding and Ripening. Plant Physiol. Biochem. 2018, 132, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-H.; Wang, B.; Li, N.; Tang, Y.; Yang, S.; Yang, T.; Xu, J.; Guo, C.; Yan, P.; Wang, Q.; et al. CRISPR/Cas9-Induced Targeted Mutagenesis and Gene Replacement to Generate Long-Shelf Life Tomato Lines. Sci. Rep. 2017, 7, 11874. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wei, C.; Duan, W.; Gao, Y.; Kuang, J.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Transcriptional and Epigenetic Analysis Reveals That NAC Transcription Factors Regulate Fruit Flavor Ester Biosynthesis. Plant J. 2021, 106, 785–800. [Google Scholar] [CrossRef]
- Jian, W.; Zheng, Y.; Yu, T.; Cao, H.; Chen, Y.; Cui, Q.; Xu, C.; Li, Z. SlNAC6, A NAC Transcription Factor, Is Involved in Drought Stress Response and Reproductive Process in Tomato. J. Plant Physiol. 2021, 264, 153483. [Google Scholar] [CrossRef]
- Gong, J.; Zeng, Y.; Meng, Q.; Guan, Y.; Li, C.; Yang, H.; Zhang, Y.; Ampomah-Dwamena, C.; Liu, P.; Chen, C.; et al. Red Light-Induced Kumquat Fruit Coloration Is Attributable to Increased Carotenoid Metabolism Regulated by FcrNAC22. J. Exp. Bot. 2021, 72, 6274–6290. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, Z.-Q.; Zhang, Q.; Li, H.-L.; Liu, G.-S.; Jing, Y.; Zhang, Y.-P.; Zhu, B.-Z.; Zhu, H.-L.; Chen, J.-Y.; et al. A Tomato NAC Transcription Factor, SlNAM1, Positively Regulates Ethylene Biosynthesis and the Onset of Tomato Fruit Ripening. Plant J. 2021, 108, 1317–1331. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vishwakarma, A.; Kenea, H.D.; Galsurker, O.; Cohen, H.; Aharoni, A.; Arazi, T. CRISPR/Cas9 Mutants of Tomato MICRORNA164 Genes Uncover Their Functional Specialization in Development. Plant Physiol. 2021, 187, 1636–1652. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, X.; Qi, B.; Gao, Z.; Tian, P.; Li, Z.; Lin, Z.; Zhang, Y.; Huang, T. SlMIR164A Regulates Fruit Ripening and Quality by Controlling SlNAM2 and SlNAM3 in Tomato. Plant Biotechnol. J. 2022, 20, 1456–1469. [Google Scholar] [CrossRef]
- Dong, Y.; Tang, M.; Huang, Z.; Song, J.; Xu, J.; Ahammed, G.J.; Yu, J.; Zhou, Y. The miR164a-NAM3 Module Confers Cold Tolerance by Inducing Ethylene Production in Tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Turečková, V.; Xue, G.-P.; Fernie, A.R.; Mueller-Roeber, B.; Balazadeh, S. The NAC Transcription Factor SlNAP2 Regulates Leaf Senescence and Fruit Yield in Tomato. Plant Physiol. 2018, 177, 1286–1302. [Google Scholar] [CrossRef] [PubMed]
- Forlani, S.; Cozzi, C.; Rosa, S.; Tadini, L.; Masiero, S.; Mizzotti, C. HEBE, a Novel Positive Regulator of Senescence in Solanum lycopersicum. Sci. Rep. 2020, 10, 11021. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, N.; Zhu, X.; Wu, M.; Jiang, C.-Z.; Grierson, D.; Luo, Y.; Shen, W.; Zhong, S.; Fu, D.-Q.; et al. Diversity and Redundancy of the Ripening Regulatory Networks Revealed by the fruitENCODE and the New CRISPR/Cas9 CNR and NOR Mutants. Hortic. Res. 2019, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zeng, J.; Li, Z.; Song, Y.; Yan, H.; He, J.; Jiang, Y.; Duan, X. Redox Regulation of the NOR Transcription Factor Is Involved in the Regulation of Fruit Ripening in Tomato. Plant Physiol. 2020, 183, 671–685. [Google Scholar] [CrossRef]
- Lai, T.; Wang, X.; Ye, B.; Jin, M.; Chen, W.; Wang, Y.; Zhou, Y.; Blanks, A.M.; Gu, M.; Zhang, P.; et al. Molecular and Functional Characterization of the SBP-Box Transcription Factor SPL-CNR in Tomato Fruit Ripening and Cell Death. J. Exp. Bot. 2020, 71, 2995–3011. [Google Scholar] [CrossRef]
- Yin, W.; Hu, Z.; Cui, B.; Guo, X.; Hu, J.; Zhu, Z.; Chen, G. Suppression of the MADS-Box Gene SlMBP8 Accelerates Fruit Ripening of Tomato (Solanum lycopersicum). Plant Physiol. Biochem. 2017, 118, 235–244. [Google Scholar] [CrossRef]
- Yin, W.; Yu, X.; Chen, G.; Tang, B.; Wang, Y.; Liao, C.; Zhang, Y.; Hu, Z. Suppression of SlMBP15 Inhibits Plant Vegetative Growth and Delays Fruit Ripening in Tomato. Front. Plant Sci. 2018, 9, 938. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, C.; Li, H.; Zhang, J.; Ye, Z. Silencing GRAS2 Reduces Fruit Weight in Tomato. J. Integr. Plant Biol. 2018, 60, 498–513. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Su, D.; Lu, W.; Li, Z. SlGRAS4 Accelerates Fruit Ripening by Regulating Ethylene Biosynthesis Genes and SlMADS1 in Tomato. Hortic. Res. 2021, 8, 3. [Google Scholar] [CrossRef]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a Tomato miR171 Target Gene SlGRAS24 Impacts Multiple Agronomical Traits via Regulating Gibberellin and Auxin Homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Fan, S.; Wen, T.; Wang, M.; Zhang, L.; Zhao, L. The Transcription Factor WRKY32 Affects Tomato Fruit Colour by Regulating YELLOW FRUITED-TOMATO 1, a Core Component of Ethylene Signal Transduction. J. Exp. Bot. 2021, 72, 4269–4282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.-L.; Wang, L.; Tian, Y.; Jia, N.; Chen, S.; Shi, N.-B.; Huang, X.; Zhou, C.; Yu, Y.; et al. Regulation of Ethylene-Responsive SlWRKYs Involved in Color Change during Tomato Fruit Ripening. Sci. Rep. 2017, 7, 16674. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Jiang, L.; Ji, D. Epigenetic Regulation in Tomato Fruit Ripening. Front. Plant Sci. 2023, 14, 1269090. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, A. Recent Advances in Epigenetic Triggering of Climacteric Fruit Ripening. Plant Physiol. 2023, 192, 1711–1717. [Google Scholar] [CrossRef]
- Liang, Z.; Riaz, A.; Chachar, S.; Ding, Y.; Du, H.; Gu, X. Epigenetic Modifications of mRNA and DNA in Plants. Mol. Plant 2020, 13, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J. Gene Regulation in Climacteric Fruit Ripening. Curr. Opin. Plant Biol. 2021, 63, 102042. [Google Scholar] [CrossRef]
- Lang, Z.; Wang, Y.; Tang, K.; Tang, D.; Datsenka, T.; Cheng, J.; Zhang, Y.; Handa, A.K.; Zhu, J.-K. Critical Roles of DNA Demethylation in the Activation of Ripening-Induced Genes and Inhibition of Ripening-Repressed Genes in Tomato Fruit. Proc. Natl. Acad. Sci. USA 2017, 114, E4511–E4519. [Google Scholar] [CrossRef]
- Li, Z.; Pi, Y.; Fan, J.; Yang, X.; Zhai, C.; Chen, H.; Wang, F.; Ding, J.; Gu, T.; Li, Y.; et al. High Mobility Group A3 Enhances Transcription of the DNA Demethylase Gene SlDML2 to Promote Tomato Fruit Ripening. Plant Physiol. 2022, 189, 315–328. [Google Scholar] [CrossRef]
- Hollwey, E.; Out, S.; Watson, M.R.; Heidmann, I.; Meyer, P. TET3-Mediated Demethylation in Tomato Activates Expression of a CETS Gene That Stimulates Vegetative Growth. Plant Direct 2017, 1, e00022. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, K.; Datsenka, T.U.; Liu, W.; Lv, S.; Lang, Z.; Wang, X.; Gao, J.; Wang, W.; Nie, W.; et al. Critical Function of DNA Methyltransferase 1 in Tomato Development and Regulation of the DNA Methylome and Transcriptome. J. Integr. Plant Biol. 2019, 61, 1224–1242. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Chen, W.; Kong, J.; Zhang, X.; Shi, N.; Zhong, S.; Ma, P.; Gallusci, P.; Jackson, S.; Liu, Y.; et al. METHYLTRANSFERASE1 and Ripening Modulate Vivipary during Tomato Fruit Development. Plant Physiol. 2020, 183, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.X.; Wang, J.Y.; Zhu, H.H.; Han, G.H.; Huang, R.N.; Huang, L.; Hong, Y.G.; Zheng, S.J.; Yang, J.L.; Chen, W.W. Potential Role of Domains Rearranged Methyltransferase7 in Starch and Chlorophyll Metabolism to Regulate Leaf Senescence in Tomato. Front. Plant Sci. 2022, 13, 836015. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Jia, H.; Lu, S.; Zhang, Z.; Su, Z.; Sadeghnezhad, E.; Li, T.; Xiao, X.; Wang, M.; Pervaiz, T.; et al. DNA and Histone Methylation Regulates Different Types of Fruit Ripening by Transcriptome and Proteome Analyses. J. Agric. Food Chem. 2022, 70, 3541–3556. [Google Scholar] [CrossRef] [PubMed]
- Corem, S.; Doron-Faigenboim, A.; Jouffroy, O.; Maumus, F.; Arazi, T.; Bouché, N. Redistribution of CHH Methylation and Small Interfering RNAs across the Genome of Tomato Ddm1 Mutants. Plant Cell 2018, 30, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, J.; Chen, Z.; Qiao, J.; Zhang, Y.; Shen, H.; Hu, Z. CRISPR/Cas9-Targeted Mutagenesis of SlCMT4 Causes Changes in Plant Architecture and Reproductive Organs in Tomato. Hortic. Res. 2022, 9, uhac081. [Google Scholar] [CrossRef]
- Hu, G.; Huang, B.; Wang, K.; Frasse, P.; Maza, E.; Djari, A.; Benhamed, M.; Gallusci, P.; Li, Z.; Zouine, M.; et al. Histone Posttranslational Modifications Rather than DNA Methylation Underlie Gene Reprogramming in Pollination-dependent and Pollination-independent Fruit Set in Tomato. New Phytol. 2021, 229, 902–919. [Google Scholar] [CrossRef]
- Bvindi, C.; Tang, L.; Lee, S.; Patrick, R.M.; Yee, Z.R.; Mengiste, T.; Li, Y. Histone Methyltransferases SDG33 and SDG34 Regulate Organ-Specific Nitrogen Responses in Tomato. Front. Plant Sci. 2022, 13, 1005077. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, G.; Liu, X.; Ding, X.; Zhang, D.; Wang, X.; Zhou, Y.; Yan, H.; Li, T.; Wu, K.; et al. Histone Demethylase SlJMJ6 Promotes Fruit Ripening by Removing H3K27 Methylation of Ripening-related Genes in Tomato. New Phytol. 2020, 227, 1138–1156. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, D.; Gu, D.; Li, Z.; Liang, H.; Zhu, H.; Jiang, Y.; Duan, X. The Histone H3K27 Demethylase SlJMJ4 Promotes Dark- and ABA-Induced Leaf Senescence in Tomato. Hortic. Res. 2022, 9, uhab077. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, Y.; Zhang, H.; Zhu, W.; Nie, W.-F. The Histone Variant Sl_H2A.Z Regulates Carotenoid Biosynthesis and Gene Expression during Tomato Fruit Ripening. Hortic. Res. 2021, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-E.; Hu, Z.; Li, F.; Zhang, L.; Yu, X.; Tang, B.; Chen, G. Silencing of Histone Deacetylase SlHDT3 Delays Fruit Ripening and Suppresses Carotenoid Accumulation in Tomato. Plant Sci. 2017, 265, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-E.; Hu, Z.; Zhu, M.; Li, F.; Zhu, Z.; Lu, Y.; Chen, G. The Tomato Histone Deacetylase SlHDA1 Contributes to the Repression of Fruit Ripening and Carotenoid Accumulation. Sci. Rep. 2017, 7, 7930. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-E. Histone Deacetylase Gene SlHDT1 Regulates Tomato Fruit Ripening by Affecting Carotenoid Accumulation and Ethylene Biosynthesis. Plant Sci. 2022, 318, 111235. [Google Scholar] [CrossRef] [PubMed]
- Hawar, A.; Xiong, S.; Yang, Z.; Sun, B. Histone Acetyltransferase SlGCN5 Regulates Shoot Meristem and Flower Development in Solanum lycopersicum. Front. Plant Sci. 2022, 12, 805879. [Google Scholar] [CrossRef]
- Bollier, N.; Sicard, A.; Leblond, J.; Latrasse, D.; Gonzalez, N.; Gévaudant, F.; Benhamed, M.; Raynaud, C.; Lenhard, M.; Chevalier, C.; et al. At-MINI ZINC FINGER2 and Sl-INHIBITOR OF MERISTEM ACTIVITY, a Conserved Missing Link in the Regulation of Floral Meristem Termination in Arabidopsis and Tomato. Plant Cell 2018, 30, 83–100. [Google Scholar] [CrossRef]
- Verma, S.; Attuluri, V.P.S.; Robert, H.S. An Essential Function for Auxin in Embryo Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039966. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in Root Development. Cold Spring Harb. Perspect. Biol. 2021, 14, a039933. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and Its Role in Plant Development: Structure, Signalling, Regulation and Response Mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Mano, Y.; Nemoto, K. The Pathway of Auxin Biosynthesis in Plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A Role for Flavin Monooxygenase-like Enzymes in Auxin Biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Huang, R.; Zhang, D.; Li, M.; Li, G.; Li, W.; Ahiakpa, J.K.; Wang, Y.; Hong, Z.; Zhang, J. SlGH3.15, a Member of the GH3 Gene Family, Regulates Lateral Root Development and Gravitropism Response by Modulating Auxin Homeostasis in Tomato. Plant Sci. 2023, 330, 111638. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, H.; Li, Y.; Xiang, H.; Liu, Y.; He, Y.; Qi, M.; Li, T. Tomato YABBY2b Controls Plant Height through Regulating Indole-3-Acetic Acid-Amido Synthetase (GH3.8) Expression. Plant Sci. 2020, 297, 110530. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, D.; Yang, X.; Ji, M.; Wang, S.; Gu, M.; Chen, A.; Xu, G. Three Cis-Regulatory Motifs, AuxRE, MYCRS1 and MYCRS2, Are Required for Modulating the Auxin- and Mycorrhiza-Responsive Expression of a Tomato GH3 Gene. Plant Cell Physiol. 2017, 58, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Yan, A.; Zhao, Q.; Xie, K.; Li, Y.; et al. Auxin-mediated Regulation of Arbuscular Mycorrhizal Symbiosis: A Role of SlGH3.4 in Tomato. Plant Cell Environ. 2022, 45, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Sravankumar, T.; Akash; Naik, N.; Kumar, R. A Ripening-Induced SlGH3-2 Gene Regulates Fruit Ripening via Adjusting Auxin-Ethylene Levels in Tomato (Solanum lycopersicum L.). Plant Mol. Biol. 2018, 98, 455–469. [Google Scholar] [CrossRef]
- Shi, Z.; Jiang, Y.; Han, X.; Liu, X.; Cao, R.; Qi, M.; Xu, T.; Li, T. SlPIN1 Regulates Auxin Efflux to Affect Flower Abscission Process. Sci. Rep. 2017, 7, 14919. [Google Scholar] [CrossRef]
- Li, A.; Chen, G.; Yu, X.; Zhu, Z.; Zhang, L.; Zhou, S.; Hu, Z. The Tomato MADS-Box Gene SlMBP9 Negatively Regulates Lateral Root Formation and Apical Dominance by Reducing Auxin Biosynthesis and Transport. Plant Cell Rep. 2019, 38, 951–963. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, Q.; Qin, L.; Pan, C.; Lamin-Samu, A.T.; Lu, G. Tomato AUXIN RESPONSE FACTOR 5 Regulates Fruit Set and Development via the Mediation of Auxin and Gibberellin Signaling. Sci. Rep. 2018, 8, 2971. [Google Scholar] [CrossRef]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an Auxin Response Factor, Is Involved in Chlorophyll and Sugar Accumulation during Tomato Fruit Development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef] [PubMed]
- Israeli, A.; Capua, Y.; Shwartz, I.; Tal, L.; Meir, Z.; Levy, M.; Bar, M.; Efroni, I.; Ori, N. Multiple Auxin-Response Regulators Enable Stability and Variability in Leaf Development. Curr. Biol. 2019, 29, 1746–1759.e5. [Google Scholar] [CrossRef] [PubMed]
- Abe-Hara, C.; Yamada, K.; Wada, N.; Ueta, R.; Hashimoto, R.; Osakabe, K.; Osakabe, Y. Effects of the Sliaa9 Mutation on Shoot Elongation Growth of Tomato Cultivars. Front. Plant Sci. 2021, 12, 627832. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tian, Z.; Zhang, J. Functional Dissection of Auxin Response Factors in Regulating Tomato Leaf Shape Development. Front. Plant Sci. 2018, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, S.; Corem, S.; Gupta, S.K.; Arazi, T. Tuning of SlARF10A Dosage by sly-miR160a Is Critical for Auxin-mediated Compound Leaf and Flower Development. Plant J. 2018, 96, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the Mevalonate and Methylerythritol Phosphate Pathways to the Biosynthesis of Gibberellins inArabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef]
- Lange, T.; Pimenta Lange, M.J. The Multifunctional Dioxygenases of Gibberellin Synthesis. Plant Cell Physiol. 2020, 61, 1869–1879. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin Biosynthesis and Its Regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Lange, T. Molecular Biology of Gibberellin Synthesis. Planta 1998, 204, 409–419. [Google Scholar] [CrossRef]
- Helliwell, C.A.; Sullivan, J.A.; Mould, R.M.; Gray, J.C.; Peacock, W.J.; Dennis, E.S. A Plastid Envelope Location of Arabidopsis Ent-kaurene Oxidase Links the Plastid and Endoplasmic Reticulum Steps of the Gibberellin Biosynthesis Pathway. Plant J. 2001, 28, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hou, M.; Liu, L.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.-C. The Maize DWARF1 Encodes a Gibberellin 3-Oxidase and Is Dual Localized to the Nucleus and Cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 Genome Editing in Tomato to Create a Gibberellin-responsive Dominant Dwarf DELLA Allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Ezura, K.; Hu, J.; Okabe, Y.; Bénard, C.; Prodhomme, D.; Gibon, Y.; Sun, T.-P.; Ezura, H.; Ariizumi, T. Identification and Functional Study of a Mild Allele of SlDELLA Gene Conferring the Potential for Improved Yield in Tomato. Sci. Rep. 2018, 8, 12043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yin, S.; Tu, Y.; Mei, H.; Wang, Y.; Yang, Y. SlCAND1, Encoding Cullin-Associated Nedd8-Dissociated Protein 1, Regulates Plant Height, Flowering Time, Seed Germination, and Root Architecture in Tomato. Plant Mol. Biol. 2020, 102, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Illouz-Eliaz, N.; Ramon, U.; Shohat, H.; Blum, S.; Livne, S.; Mendelson, D.; Weiss, D. Multiple Gibberellin Receptors Contribute to Phenotypic Stability under Changing Environments. Plant Cell 2019, 31, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Shohat, H.; Panizel, I.; Olszewski, N.; Aharoni, A.; Weiss, D. The Tomato DELLA Protein PROCERA Acts in Guard Cells to Promote Stomatal Closure. Plant Cell 2017, 29, 3186–3197. [Google Scholar] [CrossRef]
- Shohat, H.; Illouz-Eliaz, N.; Kanno, Y.; Seo, M.; Weiss, D. The Tomato DELLA Protein PROCERA Promotes Abscisic Acid Responses in Guard Cells by Upregulating an Abscisic Acid Transporter. Plant Physiol. 2020, 184, 518–528. [Google Scholar] [CrossRef]
- Silva, G.F.F.; Silva, E.M.; Correa, J.P.O.; Vicente, M.H.; Jiang, N.; Notini, M.M.; Junior, A.C.; De Jesus, F.A.; Castilho, P.; Carrera, E.; et al. Tomato Floral Induction and Flower Development Are Orchestrated by the Interplay between Gibberellin and Two Unrelated microRNA-controlled Modules. New Phytol. 2019, 221, 1328–1344. [Google Scholar] [CrossRef]
- Naeem, M.; Waseem, M.; Zhu, Z.; Zhang, L. Downregulation of SlGRAS15 Manipulates Plant Architecture in Tomato (Solanum lycopersicum). Dev. Genes Evol. 2020, 230, 1–12. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, H.; Chen, G.; Li, F.; Wang, Y.; Liao, C.; Hu, Z. The bHLH Transcription Factor SlPRE2 Regulates Tomato Fruit Development and Modulates Plant Response to Gibberellin. Plant Cell Rep. 2019, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, G.; Guo, X.; Yin, W.; Yu, X.; Hu, J.; Hu, Z. Overexpression of SlPRE2, an Atypical bHLH Transcription Factor, Affects Plant Morphology and Fruit Pigment Accumulation in Tomato. Sci. Rep. 2017, 7, 5786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, J.; Zhang, L.; Shen, H.; Chen, G.; Xie, Q.; Hu, Z. Overexpression of SlPRE5, an Atypical bHLH Transcription Factor, Affects Plant Morphology and Chlorophyll Accumulation in Tomato. J. Plant Physiol. 2022, 273, 153698. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Malka, S.K. Gibberellin Delays Metabolic Shift during Tomato Ripening by Inducing Auxin Signaling. Front. Plant Sci. 2022, 13, 1045761. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and Function in Plant Adaptation to Environmental Stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as Central Regulators during Plant Growth and Stress Response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Terceros, G.C.; Resentini, F.; Cucinotta, M.; Manrique, S.; Colombo, L.; Mendes, M.A. The Importance of Cytokinins during Reproductive Development in Arabidopsis and Beyond. Int. J. Mol. Sci. 2020, 21, 8161. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinin Biosynthesis and Regulation. In Plant Hormones; Elsevier: Amsterdam, The Netherlands, 2005; pp. 271–287. ISBN 9780127098722. [Google Scholar]
- Glanz-Idan, N.; Lach, M.; Tarkowski, P.; Vrobel, O.; Wolf, S. Delayed Leaf Senescence by Upregulation of Cytokinin Biosynthesis Specifically in Tomato Roots. Front. Plant Sci. 2022, 13, 922106. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Tu, Y.; Cheng, W.; Yang, Y. Tomato (Solanum lycopersicum) SlIPT4, Encoding an Isopentenyltransferase, Is Involved in Leaf Senescence and Lycopene Biosynthesis during Fruit Ripening. BMC Plant Biol. 2018, 18, 107. [Google Scholar] [CrossRef]
- Gan, L.; Song, M.; Wang, X.; Yang, N.; Li, H.; Liu, X.; Li, Y. Cytokinins Are Involved in Regulation of Tomato Pericarp Thickness and Fruit Size. Hortic. Res. 2022, 9, uhab041. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.; Teixeira, R.; Agudelo-Romero, P. Complex Interplay of Hormonal Signals during Grape Berry Ripening. Molecules 2015, 20, 9326–9343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Chen, J.; He, Q.; Wang, Y.; Shen, H.; Sun, L. DNA Methylation Is Involved in the Regulation of Pepper Fruit Ripening and Interacts with Phytohormones. J. Exp. Bot. 2020, 71, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Amagai, A. Ethylene as a Potent Inducer of Sexual Development. Dev. Growth Differ. 2011, 53, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Fröhlich, K.; García-Ramírez, G.X.; Trapp, M.A.; Hirt, H. Ethylene: A Master Regulator of Plant–Microbe Interactions under Abiotic Stresses. Cells 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Du, P.; Ding, N.; Zhang, Q.; Xing, S.; Wei, L.; Zhao, Y.; Mao, W.; Li, J.; Li, B.; et al. Two FERONIA-like Receptor Kinases Regulate Apple Fruit Ripening by Modulating Ethylene Production. Front. Plant Sci. 2017, 8, 1406. [Google Scholar] [CrossRef]
- Yanping, Z.; Yuqing, H.; Chen, W.; Qian, M.; Songtao, J.; Xudong, Z.; Ting, Z.; Kekun, Z.; Haifeng, J.; Tariq, P.; et al. Characterization and Identification of PpEIN3 during the Modulation of Fruit Ripening Process by Ectopic Expressions in Tomato. Plant Genome 2019, 12, 180089. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Liang, F.; Cong, L.; Song, L.; Li, X.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; et al. PbEIL1 Acts Upstream of PbCysp1 to Regulate Ovule Senescence in Seedless Pear. Hortic. Res. 2021, 8, 59. [Google Scholar] [CrossRef]
- Althiab-Almasaud, R.; Chen, Y.; Maza, E.; Djari, A.; Frasse, P.; Mollet, J.-C.; Mazars, C.; Jamet, E.; Chervin, C. Ethylene Signaling Modulates Tomato Pollen Tube Growth through Modifications of Cell Wall Remodeling and Calcium Gradient. Plant J. 2021, 107, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, G.; Rodriguez, C.; Liu, M.; Binder, B.M.; Chervin, C. Roles of SlETR7, a Newly Discovered Ethylene Receptor, in Tomato Plant and Fruit Development. Hortic. Res. 2020, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted Base Editing in Rice and Tomato Using a CRISPR-Cas9 Cytidine Deaminase Fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Kashojiya, S.; Lu, Y.; Takayama, M.; Komatsu, H.; Minh, L.H.T.; Nishida, K.; Shirasawa, K.; Miura, K.; Nonaka, S.; Masuda, J.-I.; et al. Modification of Tomato Breeding Traits and Plant Hormone Signaling by Target-AID, the Genome-Editing System Inducing Efficient Nucleotide Substitution. Hortic. Res. 2022, 9, uhab004. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Mao, M.; Deng, Y.; Sun, L.; Chen, R.; Cao, P.; Lai, J.; Zhang, Y.; Wang, C.; Li, C.; et al. Multi-Omics Analysis Reveals That SlERF.D6 Synergistically Regulates SGAs and Fruit Development. Front. Plant Sci. 2022, 13, 860577. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, P.; Singh, V.; Parida, A.; Raghuvanshi, U.; Kumar, R.; Sharma, A.K. Ethylene Response Factor ERF.D7 Activates Auxin Response Factor 2 Paralogs to Regulate Tomato Fruit Ripening. Plant Physiol. 2022, 190, 2775–2796. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, M.; Zhao, Y.; Chen, Y.; Wu, L.; Yin, H.; Yang, J.; Xiong, S.; Wang, S.; Wang, J.; et al. LcERF19, an AP2/ERF Transcription Factor from Litsea Cubeba, Positively Regulates Geranial and Neral Biosynthesis. Hortic. Res. 2022, 9, uhac093. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Zhang, C.; Ai, G.; Zhang, D.; Wei, J.; Cai, L.; Li, C.; Zhu, W.; Larkin, R.M.; et al. L2, a Chloroplast Metalloproteinase, Regulates Fruit Ripening by Participating in Ethylene Autocatalysis under the Control of Ethylene Response Factors. J. Exp. Bot. 2021, 72, 7035–7048. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, P.; Tang, B.; Hu, Z.; Xie, Q.; Zhou, S.; Chen, G. The AP2/ERF Transcription Factor SlERF.F5 Functions in Leaf Senescence in Tomato. Plant Cell Rep. 2022, 41, 1181–1195. [Google Scholar] [CrossRef]
- Hu, C.; Wu, S.; Li, J.; Dong, H.; Zhu, C.; Sun, T.; Hu, Z.; Foyer, C.H.; Yu, J. Herbivore-induced Ca2+ Signals Trigger a Jasmonate Burst by Activating ERF16-mediated Expression in Tomato. New Phytol. 2022, 236, 1796–1808. [Google Scholar] [CrossRef]
- Sun, H.; Hu, K.; Wei, S.; Yao, G.; Zhang, H. ETHYLENE RESPONSE FACTORS 4.1/4.2 with an EAR Motif Repress Anthocyanin Biosynthesis in Red-Skinned Pears. Plant Physiol. 2023, 192, 1892–1912. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-C.; Cheng, C.-P. Pathogen-induced ERF68 Regulates Hypersensitive Cell Death in Tomato. Mol. Plant Pathol. 2017, 18, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, L.; Guo, W.; Wu, W. Transcription Factor TERF1 Promotes Seed Germination under Osmotic Conditions by Activating Gibberellin Acid Signaling. Plant Sci. 2022, 322, 111350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Tang, B.; Li, F.; Xie, Q.; Chen, G.; Hu, Z. The AP2/ERF Transcription Factor SlERF.J2 Functions in Hypocotyl Elongation and Plant Height in Tomato. Plant Cell Rep. 2022, 42, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, X.; Tang, B.; Xie, Q.; Chen, G.; Chen, X.; Hu, Z. SlERF.J2 Reduces Chlorophyll Accumulation and Inhibits Chloroplast Biogenesis and Development in Tomato Leaves. Plant Sci. 2023, 328, 111578. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Upadhyay, R.K.; Prabhakar, R.; Tiwari, N.; Garg, R.; Sane, V.A.; Sane, A.P. SlDREB3, a Negative Regulator of ABA Responses, Controls Seed Germination, Fruit Size and the Onset of Ripening in Tomato. Plant Sci. 2022, 319, 111249. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Haubrick, L.L.; Assmann, S.M. Brassinosteroids and Plant Function: Some Clues, More Puzzles. Plant Cell Environ. 2006, 29, 446–457. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Asami, T.; Nakano, T.; Fujioka, S. Plant Brassinosteroid Hormones. In Plant Hormones; Elsevier: Amsterdam, The Netherlands, 2005; pp. 479–504. ISBN 9780127098722. [Google Scholar]
- Fang, P.; Wang, Y.; Wang, M.; Wang, F.; Chi, C.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; et al. Crosstalk between Brassinosteroid and Redox Signaling Contributes to the Activation of CBF Expression during Cold Responses in Tomato. Antioxidants 2021, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.-J.; Wang, K.-X.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Zhou, J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Sang, K.; Li, J.; Qian, X.; Yu, J.; Zhou, Y.; Xia, X. The APETALA2a/DWARF/BRASSINAZOLE-RESISTANT 1 Module Contributes to Carotenoid Synthesis in Tomato Fruits. Plant J. 2022, 112, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, L.; Li, S.; Shao, Z.; Meng, F.; Liu, H.; Duan, W.; Liang, D.; Zhu, C.; Xu, T.; et al. Regulation of Fruit Ripening by the Brassinosteroid Biosynthetic Gene SlCYP90B3 via an Ethylene-Dependent Pathway in Tomato. Hortic. Res. 2020, 7, 163. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Cheng, D.; Liu, J.; Lv, S.; Li, Q.; Wang, X. Enhancing Brassinosteroid Signaling via Overexpression of Tomato (Solanum lycopersicum) SlBRI1 Improves Major Agronomic Traits. Front. Plant Sci. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Zhao, T.; Du, C.; Nie, S.; Zhang, Y.; Lv, S.; Huang, S.; Wang, X. Modification of Threonine-1050 of SlBRI1 Regulates BR Signalling and Increases Fruit Yield of Tomato. BMC Plant Biol. 2019, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Z.; Wu, M.; Wang, W.; Wang, Y.; Nie, S. Enhanced Brassinosteroid Signaling via the Overexpression of SlBRI1 Positively Regulates the Chilling Stress Tolerance of Tomato. Plant Sci. 2022, 320, 111281. [Google Scholar] [CrossRef]
- Khan, M.; Luo, B.; Hu, M.; Fu, S.; Liu, J.; Jiang, M.; Zhao, Y.; Huang, S.; Wang, S.; Wang, X. Brassinosteroid Signaling Downstream Suppressor BIN2 Interacts with SLFRIGIDA-LIKE to Induce Early Flowering in Tomato. Int. J. Mol. Sci. 2022, 23, 11264. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Lee, H.-Y.; Jeong, B.; Heo, T.-Y.; Hyun, T.K.; Kim, K.; Je, B.I.; Lee, H.; Shim, D.; et al. Brassinosteroids Facilitate Xylem Differentiation and Wood Formation in Tomato. Planta 2019, 249, 1391–1403. [Google Scholar] [CrossRef]
- Mori, K.; Lemaire-Chamley, M.; Jorly, J.; Carrari, F.; Conte, M.; Asamizu, E.; Mizoguchi, T.; Ezura, H.; Rothan, C. The Conserved Brassinosteroid-Related Transcription Factor BIM1a Negatively Regulates Fruit Growth in Tomato. J. Exp. Bot. 2021, 72, 1181–1197. [Google Scholar] [CrossRef]
- Yu, T.; Ai, G.; Xie, Q.; Wang, W.; Song, J.; Wang, J.; Tao, J.; Zhang, X.; Hong, Z.; Lu, Y.; et al. Regulation of Tomato Fruit Elongation by Transcription Factor BZR1.7 through Promotion of SUN Gene Expression. Hortic. Res. 2022, 9, uhac121. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Dong, H.; Yin, Y.; Song, X.; Gu, X.; Sang, K.; Zhou, J.; Shi, K.; Zhou, Y.; Foyer, C.H.; et al. Brassinosteroid Signaling Integrates Multiple Pathways to Release Apical Dominance in Tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2004384118. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, L.; Liang, D.; Zhang, M.; Jia, C.; Qi, M.; Liu, Y.; Shao, Z.; Meng, F.; Hu, S.; et al. SlBES1 Promotes Tomato Fruit Softening through Transcriptional Inhibition of PMEU1. iScience 2021, 24, 102926. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Orozco, E.; Shekasteband, R.; Illa-Berenguer, E.; Snouffer, A.; van der Knaap, E.; Lee, T.G.; Hutton, S.F. Identification and Characterization of GLOBE, a Major Gene Controlling Fruit Shape and Impacting Fruit Size and Marketability in Tomato. Hortic. Res. 2021, 8, 138. [Google Scholar] [CrossRef]
- Hou, J.; Sun, Q.; Li, J.; Ahammed, G.J.; Yu, J.; Fang, H.; Xia, X. Glutaredoxin S25 and Its Interacting TGACG Motif-Binding Factor TGA2 Mediate Brassinosteroid-Induced Chlorothalonil Metabolism in Tomato Plants. Environ. Pollut. 2019, 255, 113256. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Q.; Zhou, Y.; Ahammed, G.J.; Zhou, Y.; Yu, J.; Fang, H.; Xia, X. Glutaredoxin GRXS16 Mediates Brassinosteroid-Induced Apoplastic H2O2 Production to Promote Pesticide Metabolism in Tomato. Environ. Pollut. 2018, 240, 227–234. [Google Scholar] [CrossRef]
- Gupta, K.; Wani, S.H.; Razzaq, A.; Skalicky, M.; Samantara, K.; Gupta, S.; Pandita, D.; Goel, S.; Grewal, S.; Hejnak, V.; et al. Abscisic Acid: Role in Fruit Development and Ripening. Front. Plant Sci. 2022, 13, 817500. [Google Scholar] [CrossRef]
- Milborrow, B.V. The Pathway of Biosynthesis of Abscisic Acid in Vascular Plants: A Review of the Present State of Knowledge of ABA Biosynthesis. J. Exp. Bot. 2001, 52, 1145–1164. [Google Scholar] [CrossRef]
- Seo, M.; Marion-Poll, A. Abscisic Acid Metabolism and Transport. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–49. ISBN 9780081026205. [Google Scholar]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Rock, C.D. Abscisic Acid Biosynthesis and Response. Arabidopsis Book 2002, 1, e0058. [Google Scholar] [CrossRef]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of Abscisic Acid Synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Ruiz, K.B.; Ravaglia, D.; Costa, G.; Torrigiani, P. ABA May Promote or Delay Peach Fruit Ripening through Modulation of Ripening- and Hormone-Related Gene Expression Depending on the Developmental Stage. Plant Physiol. Biochem. 2013, 64, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Li, D.; Bu, J.; Jiang, Y.; Khan, Z.U.; Luo, Z.; Mao, L.; Ying, T. Comprehensive Analysis of ABA Effects on Ethylene Biosynthesis and Signaling during Tomato Fruit Ripening. PLoS ONE 2016, 11, e0154072. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.H.; Wareing, P.F.; Robinson, P.M. Chemistry and Physiology of ‘Dormins’ in Sycamore: Action of the Sycamore ‘Dormin’ as a Gibberellin Antagonist. Nature 1965, 205, 1270–1272. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid Signaling Positively Regulates Abscisic Acid Biosynthesis in Response to Chilling Stress in Tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Sun, Y.; Wang, J.; Zheng, Y.; Zhang, W.; Xu, Y.; Li, Q.; Leng, P. Tomato Protein Phosphatase 2C Influences the Onset of Fruit Ripening and Fruit Glossiness. J. Exp. Bot. 2021, 72, 2403–2418. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhang, W.; Zheng, Y.; Yuan, B.; Li, Q.; Leng, P. Tomato SlPP2C5 Is Involved in the Regulation of Fruit Development and Ripening. Plant Cell Physiol. 2021, 62, 1760–1769. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Jiang, L.; Kai, W.; Liang, B.; Wang, J.; Du, Y.; Zhai, X.; Wang, J.; Zhang, Y.; et al. Suppressing Type 2C Protein Phosphatases Alters Fruit Ripening and the Stress Response in Tomato. Plant Cell Physiol. 2018, 59, 142–154. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, K.; Liang, B.; Du, Y.; Jiang, L.; Wang, J.; Kai, W.; Zhang, Y.; Zhai, X.; Chen, P.; et al. Suppressing ABA Uridine Diphosphate Glucosyltransferase (SlUGT75C1) Alters Fruit Ripening and the Stress Response in Tomato. Plant J. 2017, 91, 574–589. [Google Scholar] [CrossRef]
- Kai, W.; Wang, J.; Liang, B.; Fu, Y.; Zheng, Y.; Zhang, W.; Li, Q.; Leng, P. PYL9 Is Involved in the Regulation of ABA Signaling during Tomato Fruit Ripening. J. Exp. Bot. 2019, 70, 6305–6319. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.-Y.; Pu, D.; Liu, B.-Z.; Meng, X.-M.; Shang, Q.-M.; Duan, Y.-D.; Zhang, F.; Zhang, M.-X.; Dong, C.-J. ABA-responsive AREB1/ABI3-1/ABI5 Cascade Regulates IAA Oxidase Gene SlDAO2 to Inhibit Hypocotyl Elongation in Tomato. Plant Cell Environ. 2023, 46, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shang, L.; Wang, X.; Xing, Y.; Xu, W.; Zhang, Y.; Wang, T.; Li, H.; Zhang, J.; Ye, Z. MAPK11 Regulates Seed Germination and ABA Signaling in Tomato by Phosphorylating SnRKs. J. Exp. Bot. 2021, 72, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Wang, Y.; Zhou, G.; Wang, C.; Hussain, S.; Adnan; Lin, R.; Wang, T.; Wang, S. SlEAD1, an EAR Motif-Containing ABA down-Regulated Novel Transcription Repressor Regulates ABA Response in Tomato. GM Crops Food 2020, 11, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic Acid beyond Defence: Its Role in Plant Growth and Development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arabidopsis Book 2011, 9, e0156. [Google Scholar] [CrossRef]
- Changwal, C.; Shukla, T.; Hussain, Z.; Singh, N.; Kar, A.; Singh, V.P.; Abdin, M.Z.; Arora, A. Regulation of Postharvest Tomato Fruit Ripening by Endogenous Salicylic Acid. Front. Plant Sci. 2021, 12, 663943. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Parsons, B.L.; Liu, D.; Mattoo, A.K. Accumulation of Wound-Inducible ACC Synthase Transcript in Tomato Fruit Is Inhibited by Salicylic Acid and Polyamines. Plant Mol. Biol. 1992, 18, 477–487. [Google Scholar] [CrossRef]
- Lemaire-Chamley, M.; Koutouan, C.; Jorly, J.; Assali, J.; Yoshida, T.; Nogueira, M.; Tohge, T.; Ferrand, C.; Peres, L.E.P.; Asamizu, E.; et al. A Chimeric TGA Repressor Slows down Fruit Maturation and Ripening in Tomato. Plant Cell Physiol. 2022, 63, 120–134. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, Y.; Zhang, C.; Dou, M.; Weng, K.; Wang, Y.; Xu, Y. VvHDZ28 Positively Regulate Salicylic Acid Biosynthesis during Seed Abortion in Thompson Seedless. Plant Biotechnol. J. 2021, 19, 1824–1838. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Shao, X.; Hu, Z.; Li, J.; Wang, P.; Wang, A.; Yu, J.; Shi, K. Transcriptomic and Genetic Approaches Reveal an Essential Role of the NAC Transcription Factor SlNAP1 in the Growth and Defense Response of Tomato. Hortic. Res. 2020, 7, 209. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Zhu, B.; Zhu, H.; Luo, Y.; Wang, Q.; Zuo, J. Integrative Analysis of Long Non-Coding RNA Acting as ceRNAs Involved in Chilling Injury in Tomato Fruit. Gene 2018, 667, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, S.; Yang, P.; Yang, J.; Yang, S.; Wu, K. Identification and Expression Analysis of Snf2 Family Proteins in Tomato (Solanum lycopersicum). Int. J. Genom. 2019, 2019, 5080935. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Y.; Chen, D.; Zang, Y.; Zhang, Q.; Yi, Y.; Qu, G. Genome-Wide Identification, Classification, Characterization, and Expression Analysis of the Wall-Associated Kinase Family during Fruit Development and under Wound Stress in Tomato (Solanum lycopersicum L.). Genes 2020, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, J. Molecular and Biochemical Characterization of LeCRK1, a Ripening-Associated Tomato CDPK-Related Kinase. J. Exp. Bot. 2004, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, H.; Whitaker, B.D.; Conway, W.S. Characterization of a Calcium/Calmodulin-Regulated SR/CAMTA Gene Family during Tomato Fruit Development and Ripening. BMC Plant Biol. 2012, 12, 19. [Google Scholar] [CrossRef]
- Peng, H.; Yang, T.; Ii, W. Calmodulin Gene Expression in Response to Mechanical Wounding and Botrytis cinerea Infection in Tomato Fruit. Plants 2014, 3, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, H.; Whitaker, B.D.; Jurick, W.M. Differential Expression of Calcium/Calmodulin-regulated SlSRs in Response to Abiotic and Biotic Stresses in Tomato Fruit. Physiol. Plant. 2013, 148, 445–455. [Google Scholar] [CrossRef]
- Wang, C.-J.; Chan, Y.-L.; Shien, C.H.; Yeh, K.-W. Molecular Characterization of Fruit-Specific Class III Peroxidase Genes in Tomato (Solanum lycopersicum). J. Plant Physiol. 2015, 177, 83–92. [Google Scholar] [CrossRef]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional Analysis of a Tomato Salicylic Acid Methyl Transferase and Its Role in Synthesis of the Flavor Volatile Methyl Salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Ding, C.-K.; Yi Wang, C. The Dual Effects of Methyl Salicylate on Ripening and Expression of Ethylene Biosynthetic Genes in Tomato Fruit. Plant Sci. 2003, 164, 589–596. [Google Scholar] [CrossRef]
- Frick, E.M.; Sapkota, M.; Pereira, L.; Wang, Y.; Hermanns, A.; Giovannoni, J.J.; van der Knaap, E.; Tieman, D.M.; Klee, H.J. A Family of Methyl Esterases Converts Methyl Salicylate to Salicylic Acid in Ripening Tomato Fruit. Plant Physiol. 2023, 191, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Zhao, J.; Li, Y.; Wang, J.; Guo, R.; Gan, S.; Liu, C.-J.; Zhang, K. S5H/DMR6 Encodes a Salicylic Acid 5-Hydroxylase That Fine-Tunes Salicylic Acid Homeostasis. Plant Physiol. 2017, 175, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, F.; Xue, J.; Yang, X.; Fan, J.; Chen, H.; Li, Y.; Wu, H. Identification and Expression Analysis of Hormone Biosynthetic and Metabolism Genes in the 2OGD Family for Identifying Genes That May Be Involved in Tomato Fruit Ripening. Int. J. Mol. Sci. 2020, 21, 5344. [Google Scholar] [CrossRef] [PubMed]
- de Thomazella, D.P.T.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of Function of a DMR6 Ortholog in Tomato Confers Broad-Spectrum Disease Resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Last, R.L.; Pichersky, E. Degradation of Salicylic Acid to Catechol in Solanaceae by SA 1-Hydroxylase. Plant Physiol. 2021, 185, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J.; et al. Salicylic Acid Targets Protein Phosphatase 2A to Attenuate Growth in Plants. Curr. Biol. 2020, 30, 381–395.e8. [Google Scholar] [CrossRef] [PubMed]
- Breitel, D.A.; Chappell-Maor, L.; Meir, S.; Panizel, I.; Puig, C.P.; Hao, Y.; Yifhar, T.; Yasuor, H.; Zouine, M.; Bouzayen, M.; et al. AUXIN RESPONSE FACTOR 2 Intersects Hormonal Signals in the Regulation of Tomato Fruit Ripening. PLoS Genet. 2016, 12, e1005903. [Google Scholar] [CrossRef]
- Hu, Z.; Shao, S.; Zheng, C.; Sun, Z.; Shi, J.; Yu, J.; Qi, Z.; Shi, K. Induction of Systemic Resistance in Tomato against Botrytis cinerea by N-Decanoyl-Homoserine Lactone via Jasmonic Acid Signaling. Planta 2018, 247, 1217–1227. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Xu, Z.; Wang, L.; Chen, C.; Ren, Z. Overexpression of SlMYB75 Enhances Resistance to Botrytis cinerea and Prolongs Fruit Storage Life in Tomato. Plant Cell Rep. 2021, 40, 43–58. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, K.; Sun, S.; Zhou, J.; Yu, J.-Q. COP9 Signalosome CSN4 and CSN5 Subunits Are Involved in Jasmonate-Dependent Defense against Root-Knot Nematode in Tomato. Front. Plant Sci. 2019, 10, 1223. [Google Scholar] [CrossRef]
- Wang, G.; Hu, C.; Zhou, J.; Liu, Y.; Cai, J.; Pan, C.; Wang, Y.; Wu, X.; Shi, K.; Xia, X.; et al. Systemic Root-Shoot Signaling Drives Jasmonate-Based Root Defense against Nematodes. Curr. Biol. 2019, 29, 3430–3438.e4. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Li, Z.; Min, D.; Zhang, X.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Li, F.; Li, X. CRISPR/Cas9-Mediated SlMYC2 Mutagenesis Adverse to Tomato Plant Growth and MeJA-Induced Fruit Resistance to Botrytis cinerea. J. Agric. Food Chem. 2020, 68, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Li, F.; Cui, X.; Zhou, J.; Li, J.; Ai, W.; Shu, P.; Zhang, X.; Li, X.; Meng, D.; et al. SlMYC2 Are Required for Methyl Jasmonate-Induced Tomato Fruit Resistance to Botrytis cinerea. Food Chem. 2020, 310, 125901. [Google Scholar] [CrossRef]
- Sun, Z.; Zang, Y.; Zhou, L.; Song, Y.; Chen, D.; Zhang, Q.; Liu, C.; Yi, Y.; Zhu, B.; Fu, D.; et al. A Tomato Receptor-like Cytoplasmic Kinase, SlZRK1, Acts as a Negative Regulator in Wound-Induced Jasmonic Acid Accumulation and Insect Resistance. J. Exp. Bot. 2021, 72, 7285–7300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Su, H.; Guo, L.; Zhang, J.; Li, Y.; Xu, J.; Zhang, X.; Guo, Y.-D.; Zhang, N. Jasmonate and Aluminum Crosstalk in Tomato: Identification and Expression Analysis of WRKYs and ALMTs during JA/Al-Regulated Root Growth. Plant Physiol. Biochem. 2020, 154, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, H.; Wang, J.; Wang, X.; Xu, B.; Yao, X.; Sun, L.; Yang, R.; Wang, J.; Sun, A.; et al. Jasmonic Acid Enhances Osmotic Stress Responses by MYC2-mediated Inhibition of Protein Phosphatase 2C1 and Response Regulators 26 Transcription Factor in Tomato. Plant J. 2023, 113, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Zhang, S. Sedoheptulose-1,7-Bisphosphatase Is Involved in Methyl Jasmonate- and Dark-Induced Leaf Senescence in Tomato Plants. Int. J. Mol. Sci. 2018, 19, 3673. [Google Scholar] [CrossRef]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, Signaling, and Transport of Jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar] [CrossRef]
- Yuan, H.-M.; Liu, W.-C.; Lu, Y.-T. CATALASE2 Coordinates SA-Mediated Repression of Both Auxin Accumulation and JA Biosynthesis in Plant Defenses. Cell Host Microbe 2017, 21, 143–155. [Google Scholar] [CrossRef]
- Agrawal, R.; Sharma, M.; Dwivedi, N.; Maji, S.; Thakur, P.; Junaid, A.; Fajkus, J.; Laxmi, A.; Thakur, J.K. MEDIATOR SUBUNIT17 Integrates Jasmonate and Auxin Signaling Pathways to Regulate Thermomorphogenesis. Plant Physiol. 2022, 189, 2259–2280. [Google Scholar] [CrossRef]
- Campos, M.L.; de Almeida, M.; Rossi, M.L.; Martinelli, A.P.; Litholdo Junior, C.G.; Figueira, A.; Rampelotti-Ferreira, F.T.; Vendramim, J.D.; Benedito, V.A.; Pereira Peres, L.E. Brassinosteroids Interact Negatively with Jasmonates in the Formation of Anti-Herbivory Traits in Tomato. J. Exp. Bot. 2009, 60, 4347–4361. [Google Scholar] [CrossRef] [PubMed]
- Khatun, K.; Robin, A.H.K.; Park, J.-I.; Nath, U.K.; Kim, C.K.; Lim, K.-B.; Nou, I.S.; Chung, M.-Y. Molecular Characterization and Expression Profiling of Tomato GRF Transcription Factor Family Genes in Response to Abiotic Stresses and Phytohormones. Int. J. Mol. Sci. 2017, 18, 1056. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ding, L.; Yu, H. Crosstalk between GA and JA Signaling Mediates Plant Growth and Defense. Plant Cell Rep. 2013, 32, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, J.; Zhang, M.; Zhang, L.; Li, C.; Wang, Q. Ethylene Independent Induction of Lycopene Biosynthesis in Tomato Fruits by Jasmonates. J. Exp. Bot. 2012, 63, 5751–5761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; Mu, A.; Jiang, Z.; Kim, J.-M.; To, T.K.; Li, W.; et al. Derepression of Ethylene-Stabilized Transcription Factors (EIN3/EIL1) Mediates Jasmonate and Ethylene Signaling Synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; di Rienzo, V.; Boss, P.K.; Davies, C. Jasmonic Acid-isoleucine Formation in Grapevine (Vitis Vinifera L.) by Two Enzymes with Distinct Transcription Profiles. J. Integr. Plant Biol. 2015, 57, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Cao, Y.; Dai, J.; Li, G.; Manzoor, M.A.; Chen, C.; Deng, H. The Multifaceted Roles of MYC2 in Plants: Toward Transcriptional Reprogramming and Stress Tolerance by Jasmonate Signaling. Front. Plant Sci. 2022, 13, 868874. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ Family of Repressors Is the Missing Link in Jasmonate Signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ Repressor Proteins Are Targets of the SCFCOI1 Complex during Jasmonate Signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- You, Y.; Zhai, Q.; An, C.; Li, C. LEUNIG_HOMOLOG Mediates MYC2-Dependent Transcriptional Activation in Cooperation with the Coactivators HAC1 and MED25. Plant Cell 2019, 31, 2187–2205. [Google Scholar] [CrossRef]
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA Connects the Co-Repressor TOPLESS to Jasmonate Signalling. Nature 2010, 464, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, H.; Wang, H.; Yu, L.; Yang, Z.; Meng, X.; Hu, P.; Fan, H.; Yu, Y.; Cui, N. SlMYC2 Interacted with the SlTOR Promoter and Mediated JA Signaling to Regulate Growth and Fruit Quality in Tomato. Front. Plant Sci. 2022, 13, 1013445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Deng, L.; Shen, J.; Fang, M.; Chen, Q.; Lu, Y.; Wang, Q.; Li, C.; Zhai, Q. MYC2 Regulates the Termination of Jasmonate Signaling via an Autoregulatory Negative Feedback Loop. Plant Cell 2019, 31, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic Acid and Sucrose Regulate Tomato and Strawberry Fruit Ripening through the Abscisic Acid-stress-ripening Transcription Factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid during Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Kausch, K.D.; Sobolev, A.P.; Goyal, R.K.; Fatima, T.; Laila-Beevi, R.; Saftner, R.A.; Handa, A.K.; Mattoo, A.K. Methyl Jasmonate Deficiency Alters Cellular Metabolome, Including the Aminome of Tomato (Solanum lycopersicum L.) Fruit. Amino Acids 2012, 42, 843–856. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Mattoo, A.K. Genome-Wide Identification of Tomato (Solanum lycopersicum L.) Lipoxygenases Coupled with Expression Profiles during Plant Development and in Response to Methyl-Jasmonate and Wounding. J. Plant Physiol. 2018, 231, 318–328. [Google Scholar] [CrossRef]
- Beaudoin, N.; Rothstein, S.J. Developmental regulation of two tomato lipoxygenase promoters in transgenic tobacco and tomato. Plant Mol. Biol. 1997, 33, 835–846. [Google Scholar] [CrossRef]
- Tyagi, K.; Sunkum, A.; Rai, M.; Yadav, A.; Sircar, S.; Sreelakshmi, Y.; Sharma, R. Seeing the Unseen: A Trifoliate (MYB117) Mutant Allele Fortifies Folate and Carotenoids in Tomato Fruits. Plant J. 2022, 112, 38–54. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, X.; Pi, Y.; Li, Z.; Xue, J.; Chen, H.; Li, Y.; Wu, H. Genome-Wide Identification and Expression Analysis of Extensin Genes in Tomato. Genomics 2020, 112, 4348–4360. [Google Scholar] [CrossRef]
- Almeida, J.; Asís, R.; Molineri, V.N.; Sestari, I.; Lira, B.S.; Carrari, F.; Peres, L.E.P.; Rossi, M. Fruits from Ripening Impaired, Chlorophyll Degraded and Jasmonate Insensitive Tomato Mutants Have Altered Tocopherol Content and Composition. Phytochemistry 2015, 111, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jones, A.D.; Howe, G.A. Constitutive Activation of the Jasmonate Signaling Pathway Enhances the Production of Secondary Metabolites in Tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Li, Z.; Ai, W.; Li, J.; Zhou, J.; Zhang, X.; Mu, D.; Li, F.; Li, X.; Guo, Y. The Co-Regulation of Ethylene Biosynthesis and Ascorbate–Glutathione Cycle by Methy Jasmonate Contributes to Aroma Formation of Tomato Fruit during Postharvest Ripening. J. Agric. Food Chem. 2020, 68, 10822–10832. [Google Scholar] [CrossRef] [PubMed]
- Rausch, T.; Wachter, A. Sulfur Metabolism: A Versatile Platform for Launching Defence Operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-D.; Zhang, X.-Y.; Yao, G.-F.; Rong, Y.-L.; Ding, C.; Tang, J.; Yang, F.; Huang, Z.-Q.; Xu, Z.-M.; Chen, X.-Y.; et al. A Nuclear-Localized Cysteine Desulfhydrase Plays a Role in Fruit Ripening in Tomato. Hortic. Res. 2020, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Hu, K.-D.; Yao, G.-F.; Wang, S.-Y.; Peng, X.-J.; Zhang, H. A D-Cysteine Desulfhydrase, SlDCD2, Participates in Tomato Fruit Ripening by Modulating ROS Homoeostasis and Ethylene Biosynthesis. Hortic. Res. 2023, 10, uhad014. [Google Scholar] [CrossRef]
- Sun, C.; Yao, G.-F.; Li, L.-X.; Li, T.-T.; Zhao, Y.-Q.; Hu, K.-D.; Zhang, C.; Zhang, H. E3 Ligase BRG3 Persulfidation Delays Tomato Ripening by Reducing Ubiquitination of the Repressor WRKY71. Plant Physiol. 2023, 192, 616–632. [Google Scholar] [CrossRef]
- Hu, K.; Peng, X.; Yao, G.; Zhou, Z.; Yang, F.; Li, W.; Zhao, Y.; Li, Y.; Han, Z.; Chen, X.; et al. Roles of a Cysteine Desulfhydrase LCD1 in Regulating Leaf Senescence in Tomato. Int. J. Mol. Sci. 2021, 22, 13078. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-Regulated Transcriptional Networks in Higher Plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating Colors: Regulation of Carotenoid Biosynthesis and Accumulation by Light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; D’Andrea, L.; Rodríguez-Concepción, M. Evolutionary Recycling of Light Signaling Components in Fleshy Fruits: New Insights on the Role of Pigments to Monitor Ripening. Front. Plant Sci. 2016, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Choi, G. Decoding of Light Signals by Plant Phytochromes and Their Interacting Proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Yang, S.; Choi, G. Phytochrome Regulates Translation of mRNA in the Cytosol. Proc. Natl. Acad. Sci. USA 2012, 109, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.; Viczián, A.; Kircher, S.; Schäfer, E.; Nagy, F. Molecular Mechanisms for Mediating Light-dependent Nucleo/Cytoplasmic Partitioning of Phytochrome Photoreceptors. New Phytol. 2015, 206, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Ernesto Bianchetti, R.; Silvestre Lira, B.; Santos Monteiro, S.; Demarco, D.; Purgatto, E.; Rothan, C.; Rossi, M.; Freschi, L. Fruit-Localized Phytochromes Regulate Plastid Biogenesis, Starch Synthesis, and Carotenoid Metabolism in Tomato. J. Exp. Bot. 2018, 69, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Muqarab, R.; Waseem, M. The Solanum Melongena COP1 Delays Fruit Ripening and Influences Ethylene Signaling in Tomato. J. Plant Physiol. 2019, 240, 152997. [Google Scholar] [CrossRef]
- Hunziker, J.; Nishida, K.; Kondo, A.; Kishimoto, S.; Ariizumi, T.; Ezura, H. Multiple Gene Substitution by Target-AID Base-Editing Technology in Tomato. Sci. Rep. 2020, 10, 20471. [Google Scholar] [CrossRef]
- Gramegna, G.; Rosado, D.; Sánchez Carranza, A.P.; Cruz, A.B.; Simon-Moya, M.; Llorente, B.; Rodríguez-Concepcíon, M.; Freschi, L.; Rossi, M. PHYTOCHROME-INTERACTING FACTOR 3 Mediates Light-dependent Induction of Tocopherol Biosynthesis during Tomato Fruit Ripening. Plant Cell Environ. 2019, 42, 1328–1339. [Google Scholar] [CrossRef]
- Rosado, D.; Trench, B.; Bianchetti, R.; Zuccarelli, R.; Rodrigues Alves, F.R.; Purgatto, E.; Segal Floh, E.I.; Silveira Nogueira, F.T.; Freschi, L.; Rossi, M. Downregulation of PHYTOCHROME-INTERACTING FACTOR 4 Influences Plant Development and Fruit Production. Plant Physiol. 2019, 181, 1360–1370. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Ali, M.; Ye, L.; Pan, C.; Li, M.; Zhao, X.; Yu, F.; Zhao, X.; Lu, G. Phytochrome Interacting Factor 3 Regulates Pollen Mitotic Division through Auxin Signalling and Sugar Metabolism Pathways in Tomato. New Phytol. 2022, 234, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Meng, L.-H.; Gao, Y.; Grierson, D.; Fu, D.-Q. Manipulation of Light Signal Transduction Factors as a Means of Modifying Steroidal Glycoalkaloids Accumulation in Tomato Leaves. Front. Plant Sci. 2018, 9, 437. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Zhang, Y.; Yan, J.; Ahammed, G.J.; Bu, X.; Sun, X.; Liu, Y.; Xu, T.; Qi, H.; et al. SlFHY3 and SlHY5 Act Compliantly to Enhance Cold Tolerance through the Integration of Myo-inositol and Light Signaling in Tomato. New Phytol. 2022, 233, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, C.; Dong, H.; Liu, X.; Guo, H.; Tong, B.; Fang, F.; Zhao, Y.; Yu, Y.; Liu, Y.; et al. Activation and Negative Feedback Regulation of SlHY5 Transcription by the SlBBX20/21–SlHY5 Transcription Factor Module in UV-B Signaling. Plant Cell 2022, 34, 2038–2055. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, H.; Li, D.; Yu, B.; Hui, Q.; Yan, S.; Huang, Z.; Cui, X.; Cao, B. Identification of Candidate HY5-Dependent and -Independent Regulators of Anthocyanin Biosynthesis in Tomato. Plant Cell Physiol. 2019, 60, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Balderrama, D.; Barnwell, S.; Carlson, K.D.; Salido, E.; Guevara, R.; Nguyen, C.; Madlung, A. Phytochrome F Mediates Red Light Responsiveness Additively with Phytochromes B1 and B2 in Tomato. Plant Physiol. 2023, 191, 2353–2366. [Google Scholar] [CrossRef]
- Dong, H.; Hu, C.; Liu, C.; Wang, J.; Zhou, Y.; Yu, J. ELONGATED HYPOCOTYL 5 Mediates Blue Light-Induced Starch Degradation in Tomato. J. Exp. Bot. 2021, 72, 2627–2641. [Google Scholar] [CrossRef]
- Zhi, J.; Liu, X.; Li, D.; Huang, Y.; Yan, S.; Cao, B.; Qiu, Z. CRISPR/Cas9-Mediated SlAN2 Mutants Reveal Various Regulatory Models of Anthocyanin Biosynthesis in Tomato Plant. Plant Cell Rep. 2020, 39, 799–809. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-Type Transcription Factor, Promotes Anthocyanin Accumulation and Enhances Volatile Aroma Production in Tomato Fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef]
- Heo, J.; Bang, W.Y.; Jeong, J.C.; Park, S.-C.; Lee, J.M.; Choi, S.; Lee, B.; Lee, Y.K.; Kim, K.; Park, S.J. The Comparisons of Expression Pattern Reveal Molecular Regulation of Fruit Metabolites in S. Nigrum and S. Lycopersicum. Sci. Rep. 2022, 12, 5001. [Google Scholar] [CrossRef]
- Cerqueira, J.V.A.; Zhu, F.; Mendes, K.; Nunes-Nesi, A.; Martins, S.C.V.; Benedito, V.; Fernie, A.R.; Zsögön, A. Promoter Replacement of ANT1 Induces Anthocyanin Accumulation and Triggers the Shade Avoidance Response through Developmental, Physiological and Metabolic Reprogramming in Tomato. Hortic. Res. 2023, 10, uhac254. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, H.; Sun, C.; Li, Q.; Jiang, H.; Du, M.; Li, C.-B.; Li, C. Efficient Generation of Pink-Fruited Tomatoes Using CRISPR/Cas9 System. J. Genet. Genom. 2018, 45, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.-B.; Li, C. A Transcriptional Network Promotes Anthocyanin Biosynthesis in Tomato Flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, N.; Huang, Z.; Li, D.; Zhi, J.; Yu, B.; Liu, X.; Cao, B.; Qiu, Z. Anthocyanin Fruit Encodes an R2R3-MYB Transcription Factor, SlAN2-like, Activating the Transcription of SlMYBATV to Fine-tune Anthocyanin Content in Tomato Fruit. New Phytol. 2020, 225, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Colanero, S.; Perata, P.; Gonzali, S. The Atroviolacea Gene Encodes an R3-MYB Protein Repressing Anthocyanin Synthesis in Tomato Plants. Front. Plant Sci. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Danilo, B.; Perrot, L.; Botton, E.; Nogué, F.; Mazier, M. The DFR Locus: A Smart Landing Pad for Targeted Transgene Insertion in Tomato. PLoS ONE 2018, 13, e0208395. [Google Scholar] [CrossRef]
- Luo, D.; Xiong, C.; Lin, A.; Zhang, C.; Sun, W.; Zhang, J.; Yang, C.; Lu, Y.; Li, H.; Ye, Z.; et al. SlBBX20 Interacts with the COP9 Signalosome Subunit SlCSN5-2 to Regulate Anthocyanin Biosynthesis by Activating SlDFR Expression in Tomato. Hortic. Res. 2021, 8, 163. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and Hormonal Mechanisms Regulating Fleshy Fruit Ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Wang, R.; Angenent, G.C.; Seymour, G.; de Maagd, R.A. Revisiting the Role of Master Regulators in Tomato Ripening. Trends Plant Sci. 2020, 25, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Liu, H.; Zhang, M.; Liao, W. DNA Methylation in Tomato Fruit Ripening. Physiol. Plant. 2022, 174, e13627. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, X.; Jiang, G.; Li, Z.; Song, Y.; Zhang, D.; Jiang, Y.; Duan, X. SlJMJ7 Orchestrates Tomato Fruit Ripening via Crosstalk between H3K4me3 and DML2-mediated DNA Demethylation. New Phytol. 2022, 233, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, R.; Bellora, N.; de Haro, L.A.; Zuccarelli, R.; Rosado, D.; Freschi, L.; Rossi, M.; Bermudez, L. Phytochrome-Mediated Light Perception Affects Fruit Development and Ripening through Epigenetic Mechanisms. Front. Plant Sci. 2022, 13, 870974. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Deng, H.; Li, Y.; Liu, Z.; Shu, P.; Fu, R.; Zhang, Y.; Pirrello, J.; Zhang, Y.; Grierson, D.; et al. Like Heterochromatin Protein 1b Represses Fruit Ripening via Regulating the H3K27me3 Levels in Ripening-related Genes in Tomato. New Phytol. 2020, 227, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Mu, J.; Grierson, D.; Wang, Y.; Gao, L.; Zhao, X.; Zhu, B.; Luo, Y.; Shi, K.; Wang, Q.; et al. Noncoding RNAs: Functional Regulatory Factors in Tomato Fruit Ripening. Züchter Genet. Breed. Res. 2020, 133, 1753–1762. [Google Scholar] [CrossRef]
- Li, R.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. CRISPR/Cas9-mediated Mutagenesis of lncRNA1459 Alters Tomato Fruit Ripening. Plant J. 2018, 94, 513–524. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, Y.; Li, R.; Fu, D.; Wen, L.; Luo, Y.; Zhu, H. RNA Sequencing and Functional Analysis Implicate the Regulatory Role of Long Non-Coding RNAs in Tomato Fruit Ripening. J. Exp. Bot. 2015, 66, 4483–4495. [Google Scholar] [CrossRef]
- de Correa, J.P.O.; Silva, E.M.; Nogueira, F.T.S. Molecular Control by Non-Coding RNAs during Fruit Development: From Gynoecium Patterning to Fruit Ripening. Front. Plant Sci. 2018, 9, 1760. [Google Scholar] [CrossRef]
- Zuo, J.; Grierson, D.; Courtney, L.T.; Wang, Y.; Gao, L.; Zhao, X.; Zhu, B.; Luo, Y.; Wang, Q.; Giovannoni, J.J. Relationships between Genome Methylation, Levels of Non-coding RNAs, mRNAs and Metabolites in Ripening Tomato Fruit. Plant J. 2020, 103, 980–994. [Google Scholar] [CrossRef]
- Liu, Y.; Andersson, M.; Granell, A.; Cardi, T.; Hofvander, P.; Nicolia, A. Establishment of a DNA-Free Genome Editing and Protoplast Regeneration Method in Cultivated Tomato (Solanum lycopersicum). Plant Cell Rep. 2022, 41, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Li, Q.; Peng, W.; Zhang, Z.; Chu, P.; Guo, S.; Fan, Y.; Lyu, S. AtGCS Promoter-Driven Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Highly Efficiently Generates Homozygous/Biallelic Mutations in the Transformed Roots by Agrobacterium Rhizogenes–Mediated Transformation. Front. Plant Sci. 2022, 13, 952428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Roberts, H.M.; Van Eck, J.; Martin, G.B. Generation and Molecular Characterization of CRISPR/Cas9-Induced Mutations in 63 Immunity-Associated Genes in Tomato Reveals Specificity and a Range of Gene Modifications. Front. Plant Sci. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, T.; Doan, D.T.H.; Tran, M.T.; Sung, Y.W.; Song, Y.J.; Kim, J.-Y. Improvement of the LbCas12a-crRNA System for Efficient Gene Targeting in Tomato. Front. Plant Sci. 2021, 12, 722552. [Google Scholar] [CrossRef]
- Kirke, J.; Kaplan, N.; Velez, S.; Jin, X.-L.; Vichyavichien, P.; Zhang, X.-H. Tissue-Preferential Activity and Induction of the Pepper Capsaicin Synthase PUN1 Promoter by Wounding, Heat and Metabolic Pathway Precursor in Tobacco and Tomato Plants. Mol. Biotechnol. 2018, 60, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, T.; Sivankalyani, V.; Kim, E.-J.; Doan, D.T.H.; Tran, M.T.; Kim, J.; Sung, Y.W.; Park, M.; Kang, Y.J.; Kim, J.-Y. Highly Efficient Homology-directed Repair Using CRISPR/Cpf1-geminiviral Replicon in Tomato. Plant Biotechnol. J. 2020, 18, 2133–2143. [Google Scholar] [CrossRef]
- de Maagd, R.A.; Loonen, A.; Chouaref, J.; Pelé, A.; Meijer-Dekens, F.; Fransz, P.; Bai, Y. CRISPR/Cas Inactivation of RECQ4 Increases Homeologous Crossovers in an Interspecific Tomato Hybrid. Plant Biotechnol. J. 2020, 18, 805–813. [Google Scholar] [CrossRef]
- Wada, N.; Osakabe, K.; Osakabe, Y. Type I-D CRISPR System-Mediated Genome Editing in Plants. In Methods in Molecular Biology; Springer: New York, NY, USA, 2023; pp. 21–38. ISBN 9781071631300. [Google Scholar]
- Veillet, F.; Perrot, L.; Guyon-Debast, A.; Kermarrec, M.-P.; Chauvin, L.; Chauvin, J.-E.; Gallois, J.-L.; Mazier, M.; Nogué, F. Expanding the CRISPR Toolbox in P. Patens Using SpCas9-NG Variant and Application for Gene and Base Editing in Solanaceae Crops. Int. J. Mol. Sci. 2020, 21, 1024. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Sretenovic, S.; Liu, S.; Cheng, Y.; Han, Y.; Liu, G.; Bao, Y.; Fang, Q.; Zheng, X.; et al. CRISPR-BETS: A Base-editing Design Tool for Generating Stop Codons. Plant Biotechnol. J. 2022, 20, 499–510. [Google Scholar] [CrossRef]
- Matsumoto, A.; Schlüter, T.; Melkonian, K.; Takeda, A.; Nakagami, H.; Mine, A. A Versatile Tn7 Transposon-Based Bioluminescence Tagging Tool for Quantitative and Spatial Detection of Bacteria in Plants. Plant Commun. 2022, 3, 100227. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental Impacts of Genetically Modified Plants: A Review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Caradus, J.R. Intended and Unintended Consequences of Genetically Modified Crops—Myth, Fact and/or Manageable Outcomes? N. Z. J. Agric. Res. 2023, 66, 519–619. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamil, A.; Munawar, N. GMOs or Non-GMOs? The CRISPR Conundrum. Front. Plant Sci. 2023, 14, 1232938. [Google Scholar] [CrossRef] [PubMed]
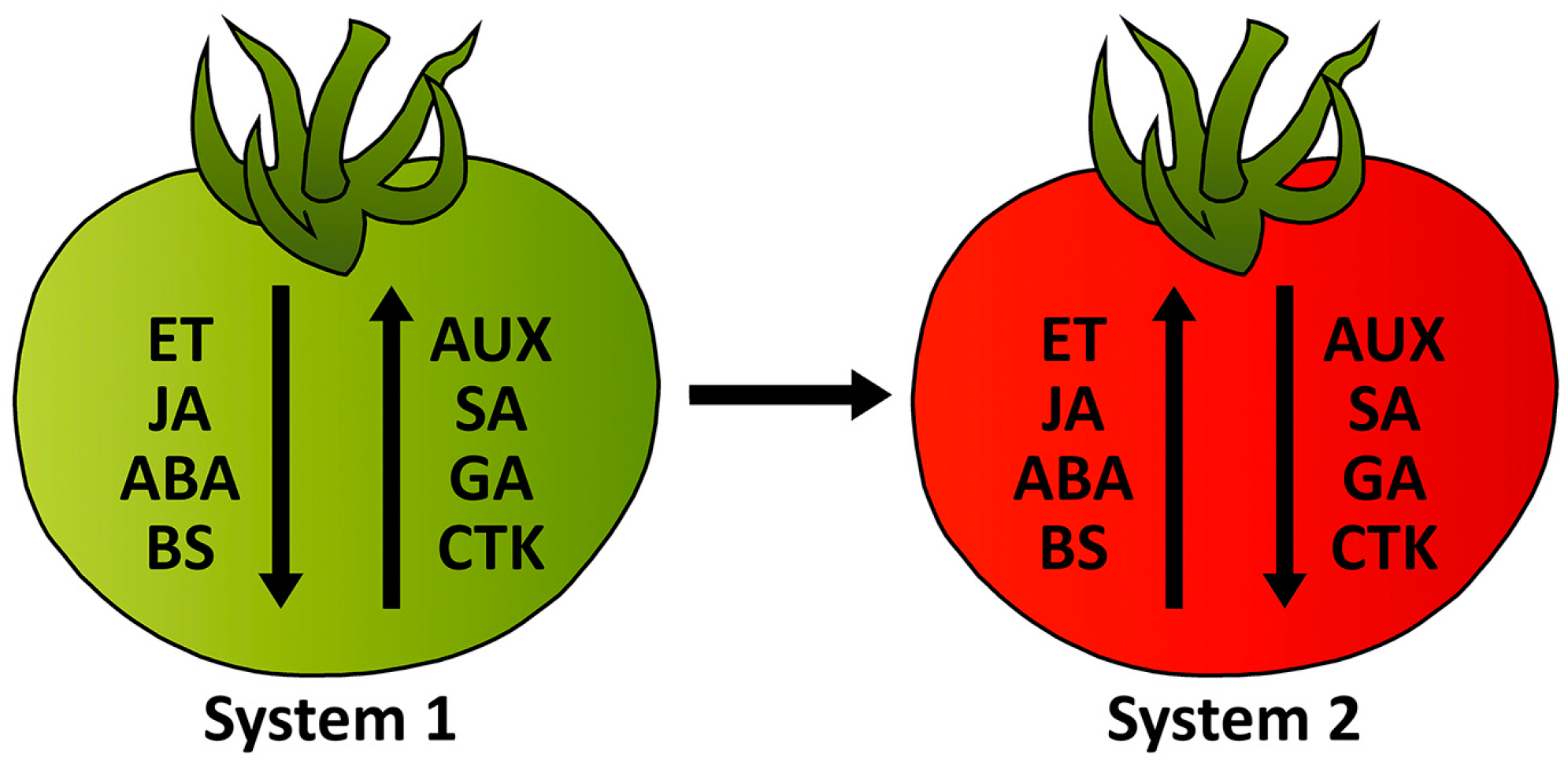



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranov, D.; Timerbaev, V. Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches. Int. J. Mol. Sci. 2024, 25, 760. https://doi.org/10.3390/ijms25020760
Baranov D, Timerbaev V. Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches. International Journal of Molecular Sciences. 2024; 25(2):760. https://doi.org/10.3390/ijms25020760
Chicago/Turabian StyleBaranov, Denis, and Vadim Timerbaev. 2024. "Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches" International Journal of Molecular Sciences 25, no. 2: 760. https://doi.org/10.3390/ijms25020760
APA StyleBaranov, D., & Timerbaev, V. (2024). Recent Advances in Studying the Regulation of Fruit Ripening in Tomato Using Genetic Engineering Approaches. International Journal of Molecular Sciences, 25(2), 760. https://doi.org/10.3390/ijms25020760





