Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate
Abstract
1. Introduction
2. Results and Discussion
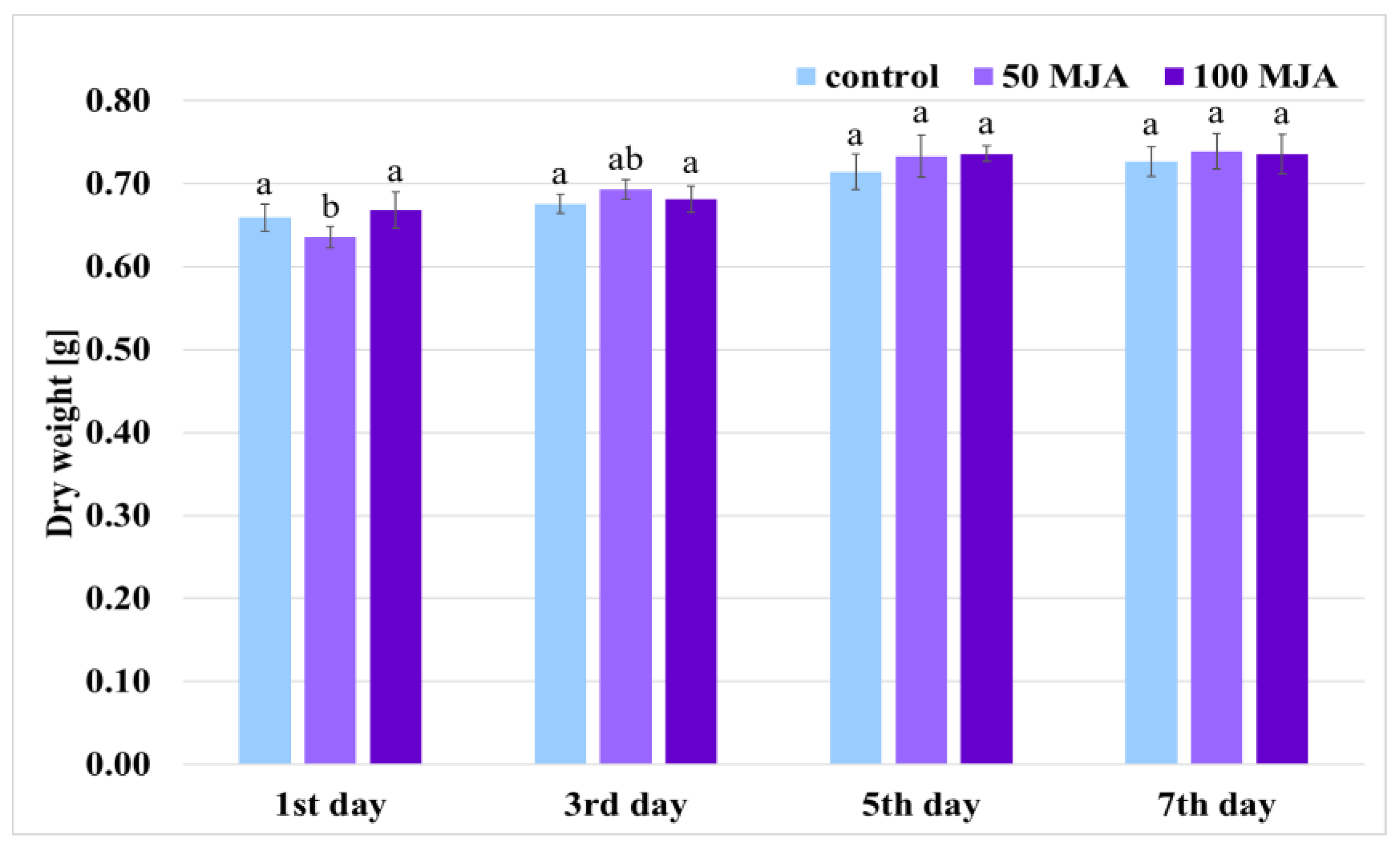
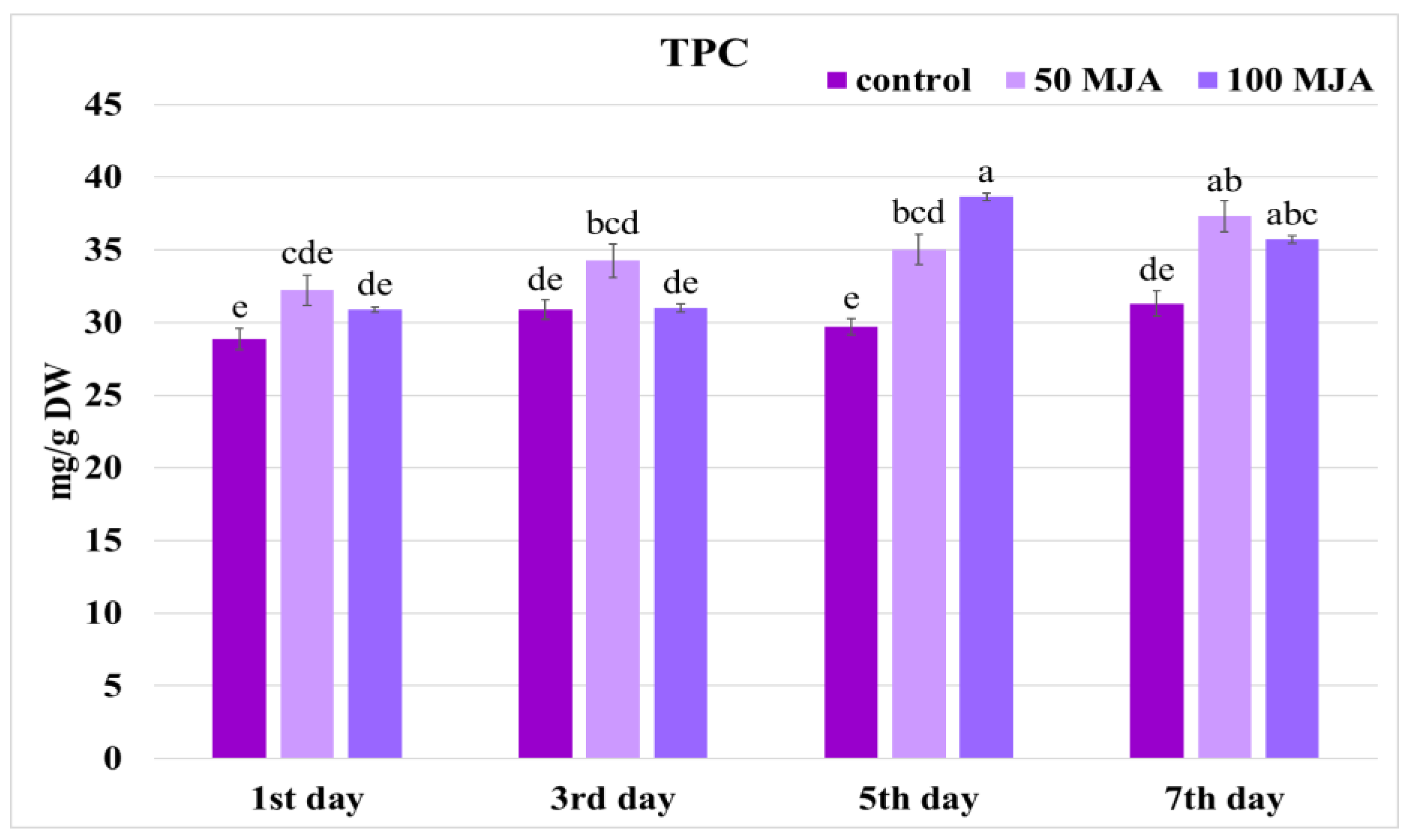
2.1. Effect of MJA on Biomass Accumulation and Phenolic Content in Salvia viridis Hairy Roots
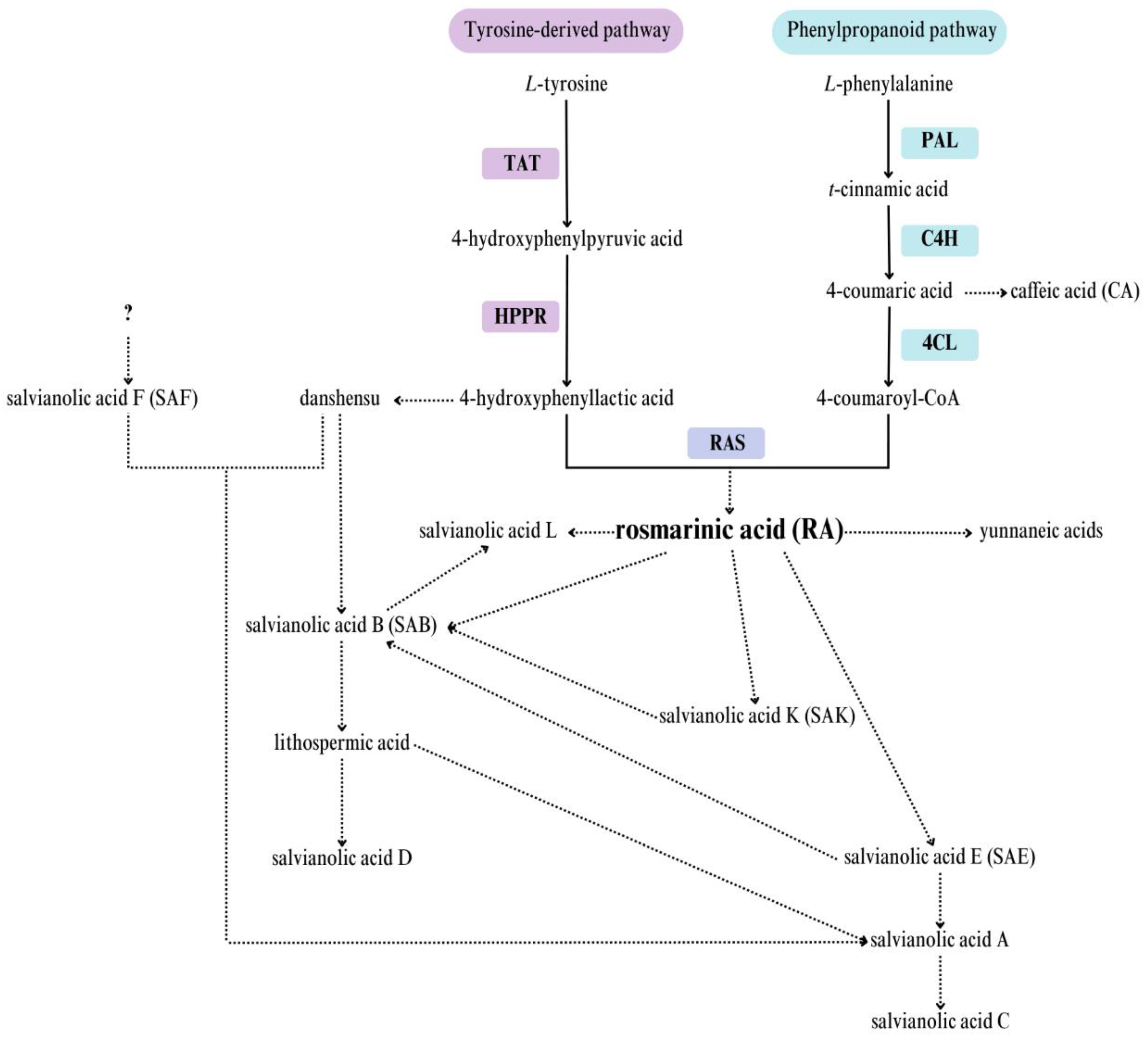
2.2. Gene Expression Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Elicitor Preparation and Treatment
3.3. Preparation of Extracts for Phytochemical Analysis
3.4. Quantitative Analysis
3.5. RNA Extraction and RT-PCR Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheong, J.J.; Do Choi, Y. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Juvik, J.A. Environmental stress and methyl jasmonate-mediated changes in flavonoid concentrations and antioxidant activity in Broccoli Florets and Kale leaf tissues. Hortscience 2013, 48, 996–1002. [Google Scholar] [CrossRef]
- Misra, R.C.; Maiti, P.; Chanotiya, C.S.; Shanker, K.; Ghosh, S. Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant Physiol. 2014, 164, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Repkina, N.; Ignatenko, A.; Holoptseva, E.; MiszalskI, Z.; Kaszycki, P.; Talanova, V. Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants 2021, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss. Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Wongwicha, W.; Tanaka, H.; Shoyama, Y.; Putalun, W. Methyl jasmonate elicitation enhances glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures. Z. Für. Naturforsch. C 2011, 66, 423–428. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.; Lu, B.B.; Kai, G.Y.; Wang, Z.N.; Xia, Y.; Ding, R.X.; Zhang, H.M.; Sun, X.F.; Chen, W.S.; et al. Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing putrescine N-methyltransferase is methyl jasmonate-dependent. Planta 2007, 225, 887–896. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M.A. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Mizukami, H.; Tabira, Y.; Ellis, B.E. Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 1993, 12, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Kuzeva, S.L.; Pavlov, A.I.; Kovacheva, E.G.; Ilieva, M.P. Elicitation of rosmarinic acid by Lavandula vera MM cell suspension culture with abiotic elicitors. World J. Microbiol. Biotechnol. 2007, 23, 301–304. [Google Scholar] [CrossRef]
- Li, W.; Koike, K.; Asada, Y.; Yoshikawa, T.; Nikaido, T. Rosmarinic acid production by Coleus forskohlii hairy root cultures. Plant Cell Tiss. Organ Cult. 2005, 80, 151–155. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Luo, L.; Cao, F.; Yang, B.; Gao, J.; Yan, Y.; Zhang, G.; Peng, L.; Hu, B. Increased phenolic acid and tanshinone production and transcriptional responses of biosynthetic genes in hairy root cultures of Salvia przewalskii Maxim. treated with methyl jasmonate and salicylic acid. Mol. Biol. Rep. 2020, 47, 8565–8578. [Google Scholar] [CrossRef] [PubMed]
- Dowom, S.A.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. after elicitation with Ag+ ions, methyl jasmonate and yeast extract. Ind. Crop. Prod. 2017, 103, 81–88. [Google Scholar] [CrossRef]
- Szabo, E.; Thelen, A.; Petersen, M. Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep. 1999, 18, 485–489. [Google Scholar] [CrossRef]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Garagounis, C.; Papadopoulou, K. Identification and expression profiling of rosmarinic acid biosynthetic genes from Satureja khuzistanica under carbon nanotubes and methyl jasmonate elicitation. Plant Cell Tiss. Organ Cult. 2019, 136, 561–573. [Google Scholar] [CrossRef]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kiss, A.K. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crop. Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Liang, Z.S.; Yang, D.F.; Liang, X.; Zhang, Y.J.; Liu, Y.; Liu, F.H. Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, I.; Wysokińska, H. The effect of methyl jasmonate on production of antioxidant compounds in shoot cultures of Salvia officinalis L. Herba Polonica 2009, 55, 238–243. [Google Scholar]
- Grzegorczyk-Karolak, I. Optimization of culture conditions and cultivation phase for the growth of Salvia viridis transformed roots and polyphenolic compound production. Plant Cell Tiss. Organ Cult. 2020, 142, 571–581. [Google Scholar] [CrossRef]
- Yukimune, Y.; Hara, Y.; Nomura, E.; Seto, H.; Yoshida, S. The configuration of methyl jasmonate affects paclitaxel and baccatin III production in Taxus cells. Phytochemistry 2000, 54, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of anthocyanin and associated gene expression in radish sprouts exposed to light and methyl jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag+, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Tsuruga, A.; Matsuno, M.; Mizukami, H. Elicitor-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures: Activities of rosmarinic acid synthase and the final two cytochrome P450-catalyzed hydroxylations. Plant Biotechnol. 2004, 21, 393–396. [Google Scholar] [CrossRef]
- Zhao, S.J.; Zhang, J.J.; Yang, L.; Wang, Z.T.; Hu, Z.B. Determination and biosynthesis of multiple salvianolic acids in hairy roots of Salvia miltiorrhiza. Acta Pharm. Sin. 2011, 46, 1352–1356. [Google Scholar]
- Pesaraklu, A.; Radjabian, T.; Salami, S.A. Methyl jasmonate and Ag+ as effective elicitors for enhancement of phenolic acids contents in Salvia officinalis and Salvia verticillata, as two traditional medicinal plants. S. Afr. J. Bot. 2021, 141, 105–115. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Gong, X.; Yang, M.; Zhang, C.; Li, M. Biosynthesis, chemistry and pharmacology of polyphenols from Chinese Salvia species: A review. Molecules 2019, 24, 155. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Petersen, M. Enzymes of phenylpropanoid metabolism in the important medicinal plant Melissa officinalis L. Planta 2010, 232, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Rizi, M.R.; Azizi, A.; Sayyari, M.; Mirzaie-Asl, A.; Conti, L. Increased phenylpropanoids production in UV-B irradiated Salvia verticillata as a consequence of altered genes expression in young leaves. Plant Physiol. Biochem. 2021, 167, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, S.; Cui, G.; Chen, S.; Wei, J.; Huang, L. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza. Mol. Biol. Rep. 2010, 37, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yi, B.; Duan, Y.; Sun, L.; Yu, X.; Guo, J.; Chen, W. Characterization and expression profiling of tyrosine aminotransferase gene from Salvia miltiorrhiza (Dan-shen) in rosmarinic acid biosynthesis pathway. Mol. Biol. Rep. 2008, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Ellis, B.E. Rosmarinic acid formation and differential expression of tyrosine aminotransferase isoforms in Anchusa officinalis cell suspension cultures. Plant Cell Rep. 1991, 10, 321–324. [Google Scholar] [CrossRef]
- Fooladi Vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef]
- Sumaryono, W.; Proksch, P.; Hartmann, T.; Nimtz, M.; Wray, V. Induction of rosmarinic acid accumulation in cell suspension cultures of Orthosiphon aristatus after treatment with yeast extract. Phytochemistry 1991, 30, 3267–3271. [Google Scholar] [CrossRef]
- Xing, B.; Yang, D.; Guo, W.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots. Molecules 2014, 20, 309–324. [Google Scholar] [CrossRef]
- Chen, I.G.J.; Lee, M.S.; Lin, M.K.; Ko, C.Y.; Chang, W.T. Blue light decreases tanshinone IIA content in Salvia miltiorrhiza hairy roots via genes regulation. J. Photochem. Photobiol. B 2018, 183, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Xiao, Y.; Gao, S.; Di, P.; Chen, J.; Chen, W.; Zhang, L. Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol. Plant. 2009, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]


| TAT | PAL | HPPR | C4H | 4CL | RAS | ||||||||||||||||||||
| TPC | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | |
| 1 | 0.7856 | 0.4091 | 0.9528 | 0.5104 | 0.9101 | 0.9956 | 0.9319 | 0.9790 | 0.9856 | 0.6551 | 0.3923 | −0.731 | 0.6487 | 0.8569 | 0.9602 | 0.2202 | 0.0232 | 0.9377 | −0.0917 | 0.5705 | 0.6303 | 0.3792 | 0.9503 | 0.8026 | |
| 3 | 0.2956 | 0.9614 | 0.9337 | 0.5140 | 0.2345 | 0.9542 | 0.7155 | 0.8410 | 0.8810 | 0.1021 | 0.6320 | 0.9242 | 0.5866 | −0.2107 | 0.5505 | 0.0783 | 0.9698 | 0.9365 | |||||||
| 5 | 0.9876 | 0.9226 | 0.9221 | 0.7904 | 0.7932 | 0.9947 | 0.9992 | 0.9777 | −0.7183 | 0.4463 | 0.9979 | 0.9096 | |||||||||||||
| 7 | 0.8691 | 0.9617 | 0.9998 | 0.7550 | −0.1262 | 0.7391 | |||||||||||||||||||
| RA | 1 | 0.9142 | 0.5058 | 0.9332 | 0.4048 | 0.9843 | 0.9999 | 0.9090 | 0.9479 | 0.9968 | 0.7333 | 0.3374 | −0.8068 | 0.8168 | 0.9078 | 0.9421 | 0.1029 | −0.2248 | 0.8944 | −0.1502 | 0.6639 | 0.8028 | 0.4774 | 0.9303 | 0.7263 |
| 3 | 0.3976 | 0.9435 | 0.8846 | 0.6041 | 0.1768 | 0.9120 | 0.7872 | 0.8077 | 0.9309 | 0.2096 | 0.5853 | 0.8724 | 0.4951 | −0.2679 | 0.6457 | 0.1862 | 0.9537 | 0.8883 | |||||||
| 5 | 0.9767 | 0.8704 | 0.8977 | 0.8575 | 0.7559 | 0.9754 | 0.9998 | 0.9957 | −0.7581 | 0.5493 | 0.9999 | 0.8538 | |||||||||||||
| 7 | 0.8043 | 0.9224 | 0.9904 | 0.6719 | −0.0076 | 0.6539 | |||||||||||||||||||
| SAE | 1 | −0.6799 | −0.8209 | 0.4484 | 0.7826 | −0.8346 | 0.0922 | 0.3918 | 0.0689 | −0.9474 | −0.6211 | −0.3839 | 0.7754 | −0.5226 | −0.3478 | 0.4712 | 0.9364 | −0.1783 | 0.5152 | −0.7765 | −0.8912 | −0.5021 | −0.8391 | 0.4414 | 0.4818 |
| 3 | −0.8840 | 0.4748 | 0.2278 | −0.7477 | −0.5316 | 0.1666 | −0.5539 | 0.2006 | −0.589 | −0.9586 | −0.1128 | 0.2527 | 0.9046 | −0.8465 | −0.9019 | −0.9652 | 0.5033 | 0.2201 | |||||||
| 5 | 0.5780 | 0.2567 | 0.3676 | −0.7143 | 0.1186 | −0.0332 | 0.7270 | −0.3415 | −0.9996 | −0.9473 | 0.7432 | 0.2880 | |||||||||||||
| 7 | 0.3719 | 0.1408 | −0.1160 | 0.5470 | −0.9656 | 0.5668 | |||||||||||||||||||
| SAF I | 1 | 0.2843 | 0.8608 | 0.8761 | −0.072 | 0.0484 | 0.8672 | 0.9045 | 0.6876 | −0.2076 | 0.9711 | 0.9373 | −0.9897 | 0.4671 | 0.9973 | 0.8635 | −0.3759 | −0.9374 | 0.5651 | 0.6592 | 0.9372 | 0.4881 | 0.8438 | 0.8799 | 0.3186 |
| 3 | 0.7928 | 0.8614 | 0.5624 | 0.9149 | 0.8666 | 0.6129 | 0.9875 | 0.9713 | 0.9935 | 0.6576 | 0.9972 | 0.5410 | 0.0115 | 0.5641 | 0.9285 | 0.6394 | 0.8444 | 0.5688 | |||||||
| 5 | 0.7935 | 0.5375 | 0.9154 | 0.9987 | 0.9877 | 0.7580 | 0.6585 | 0.9228 | 0.0103 | 0.8772 | 0.6404 | 0.5097 | |||||||||||||
| 7 | 0.4314 | 0.6333 | 0.8095 | 0.2457 | 0.4625 | 0.2225 | |||||||||||||||||||
| SAF II | 1 | −0.8292 | −0.6768 | 0.8963 | 0.3962 | −0.6726 | 0.3068 | 0.8669 | 0.9449 | −0.4620 | −0.4355 | 0.2498 | −0.8123 | −0.9228 | −0.1352 | 0.9074 | 0.0936 | 0.9449 | 0.6895 | −0.2402 | 0.6709 | −0.9318 | −0.7004 | 0.8928 | 0.7197 |
| 3 | −0.7610 | 0.9091 | 0.8802 | −0.5851 | 0.0859 | 0.9081 | −0.3593 | 0.7502 | 0.9343 | −0.8736 | 0.5085 | 0.8678 | 0.9757 | −0.3551 | 0.6528 | −0.8850 | 0.9222 | 0.8839 | |||||||
| 5 | 0.9529 | 0.8657 | 0.8536 | 0.8623 | 0.6928 | 0.9733 | 0.9939 | 0.9965 | −0.8146 | 0.5571 | 0.9963 | 0.8489 | |||||||||||||
| 7 | 0.7987 | 0.9188 | 0.9890 | 0.6649 | 0.0017 | 0.6469 | |||||||||||||||||||
| PLS | 1 | 0.9857 | 0.9680 | 0.9526 | 0.8997 | 0.9975 | 0.6956 | 0.9317 | 0.9294 | 0.9467 | 0.9992 | 0.3917 | −0.2342 | 0.9334 | 0.9380 | 0.9600 | 0.7211 | −0.4571 | 0.3148 | −0.0923 | 0.0237 | 0.9246 | 0.9593 | 0.9501 | 0.9985 |
| 3 | 0.9305 | 0.9612 | 0.9765 | 0.9909 | 0.2339 | 0.9611 | 0.9925 | 0.8407 | 0.4746 | 0.8404 | 0.6315 | 0.9817 | −0.2656 | −0.2113 | −0.0005 | 0.8272 | 0.9697 | 0.9748 | |||||||
| 5 | 0.9875 | 0.9825 | 0.9219 | 0.3222 | 0.7928 | 0.8869 | 0.9992 | 0.7001 | −0.7188 | −0.1206 | 0.9980 | 0.9880 | |||||||||||||
| 7 | 0.9978 | 0.9536 | 0.8455 | 0.9913 | −0.6518 | 0.9879 | |||||||||||||||||||
| CA | 1 | 0.9264 | 0.4431 | 0.9997 | 0.8452 | 0.9893 | 0.9984 | 0.9962 | 0.9648 | 0.9938 | 0.6830 | 0.6339 | −0.3414 | 0.8344 | 0.8757 | 1 | 0.6391 | −0.2551 | 0.9240 | 0.1905 | 0.1354 | 0.8210 | 0.4137 | 0.9995 | 0.9860 |
| 3 | 0.3313 | 0.9999 | 0.9945 | 0.5459 | 0.4970 | 0.9860 | 0.7413 | 0.9588 | 0.5701 | 0.1394 | 0.8236 | 0.9968 | 0.5557 | 0.0711 | 0.1114 | 0.1158 | 0.9993 | 0.9936 | |||||||
| 5 | 0.9921 | 0.9972 | 0.9935 | 0.4261 | 0.9319 | 0.9330 | 0.9480 | 0.7756 | −0.4951 | −0.0088 | 0.9403 | 0.9991 | |||||||||||||
| 7 | 0.9989 | 0.9813 | 0.8999 | 0.9704 | −0.5629 | 0.9644 | |||||||||||||||||||
| Gene Name | Primers | References | PCR Product Length (bp) |
|---|---|---|---|
| TAT | CGCCGACTACCATCACCATTAAGG GCAGAGCCTCCACAACACCTTC | [14] | 151 |
| HPPR | GACTCCAGAAACAACCCACATT CCCAGACGACCCTCCACAAGA | [43] | 138 |
| PAL | GGCGGCGATTGAGAGCAGGA ATCAGCAGATAGGAAGAGGAGCACC | [40] | 564 |
| C4H | CCAGGAGTCCAAATAACAGAGCC GAGCCACCAAGCGTTCACCAA | [43] | 186 |
| 4CL | CGCCAAATACGACCTTTCCTC TGCTTCAGTCATCCCATACCCA | [43] | 133 |
| RAS | CGCCCTAGTTGAGTTCTACCCTTACGC TCGGATAGGTGGTGCTCGTTTGC | [40] | 282 |
| β-ACTIN | AGGAACCACCGATCCAGACA GGTGCCCTGAGGTCCTGTT | [14] | 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorczyk-Karolak, I.; Krzemińska, M.; Grąbkowska, R.; Gomulski, J.; Żekanowski, C.; Gaweda-Walerych, K. Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate. Int. J. Mol. Sci. 2024, 25, 764. https://doi.org/10.3390/ijms25020764
Grzegorczyk-Karolak I, Krzemińska M, Grąbkowska R, Gomulski J, Żekanowski C, Gaweda-Walerych K. Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate. International Journal of Molecular Sciences. 2024; 25(2):764. https://doi.org/10.3390/ijms25020764
Chicago/Turabian StyleGrzegorczyk-Karolak, Izabela, Marta Krzemińska, Renata Grąbkowska, Jan Gomulski, Cezary Żekanowski, and Katarzyna Gaweda-Walerych. 2024. "Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate" International Journal of Molecular Sciences 25, no. 2: 764. https://doi.org/10.3390/ijms25020764
APA StyleGrzegorczyk-Karolak, I., Krzemińska, M., Grąbkowska, R., Gomulski, J., Żekanowski, C., & Gaweda-Walerych, K. (2024). Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate. International Journal of Molecular Sciences, 25(2), 764. https://doi.org/10.3390/ijms25020764







