Lipidomes in Cadaveric Decomposition and Determination of the Postmortem Interval: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.2. Inclusion Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Exclusion Criteria
2.7. Characteristics of the Studies Included in the Systematic Review
2.8. Risk of Bias Assessment
2.9. Laboratory Methods
3. Results
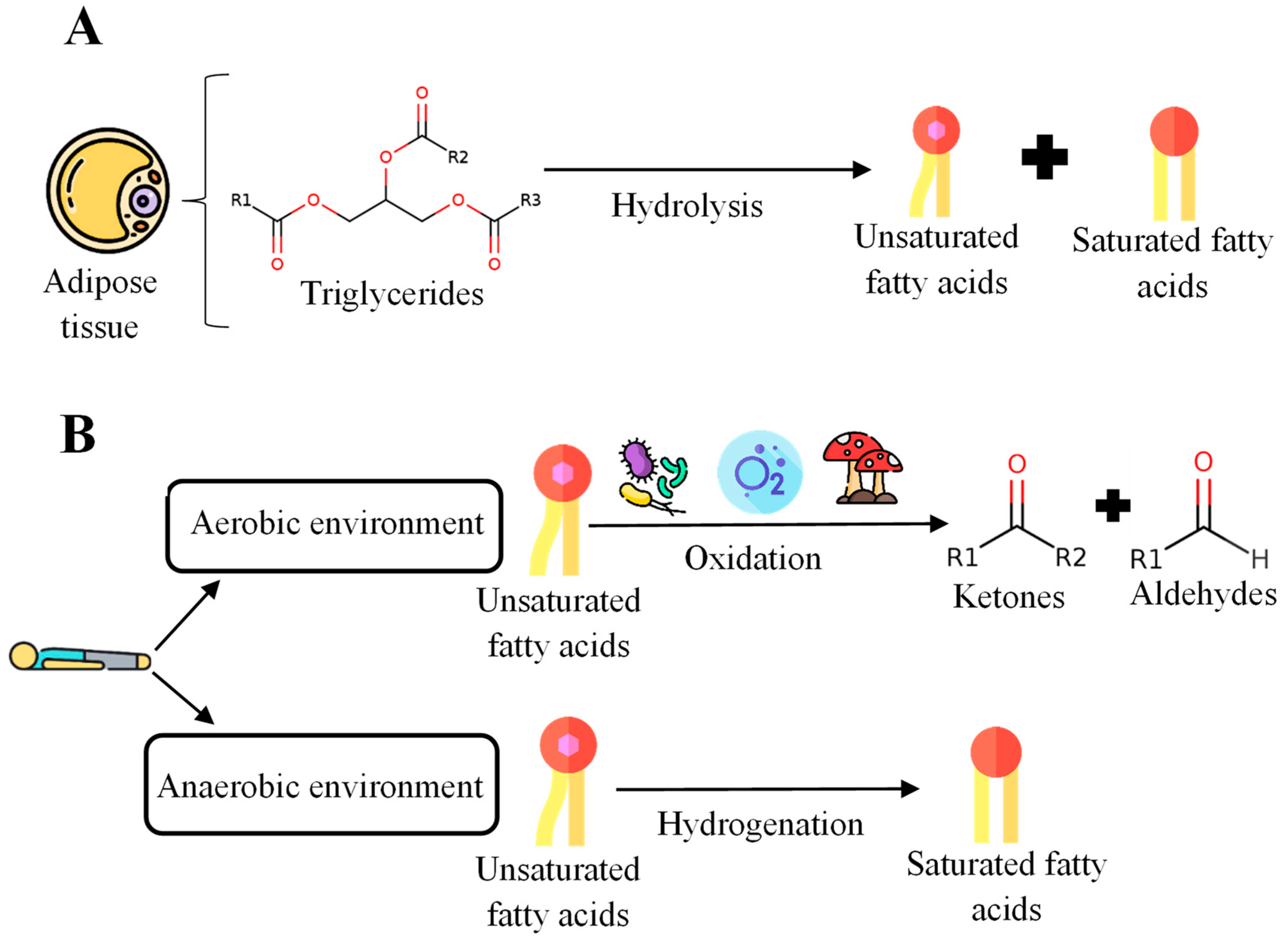
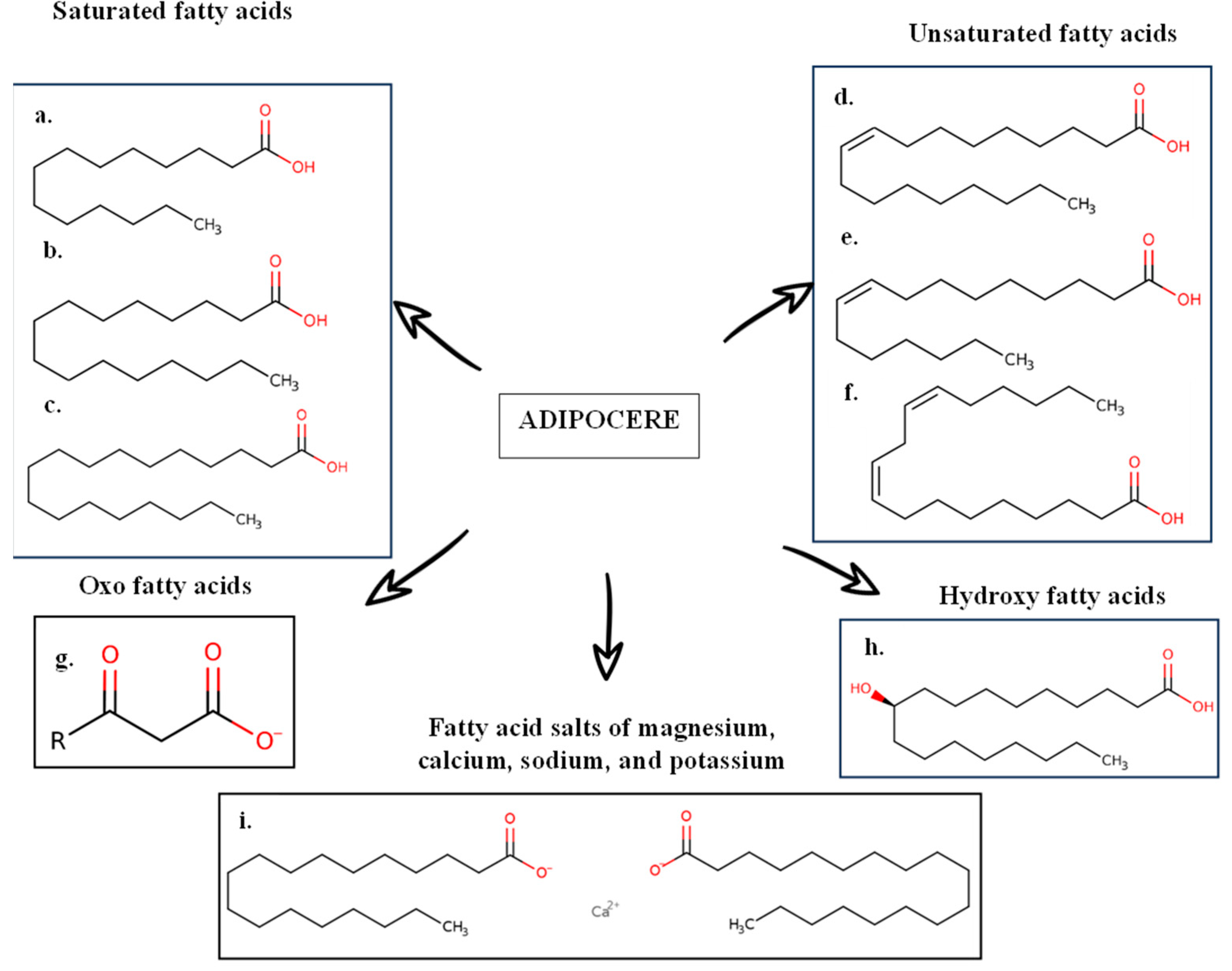
3.1. Corpse Decomposition Process
3.2. PMI
3.3. Lipid Markers for PMI Estimation
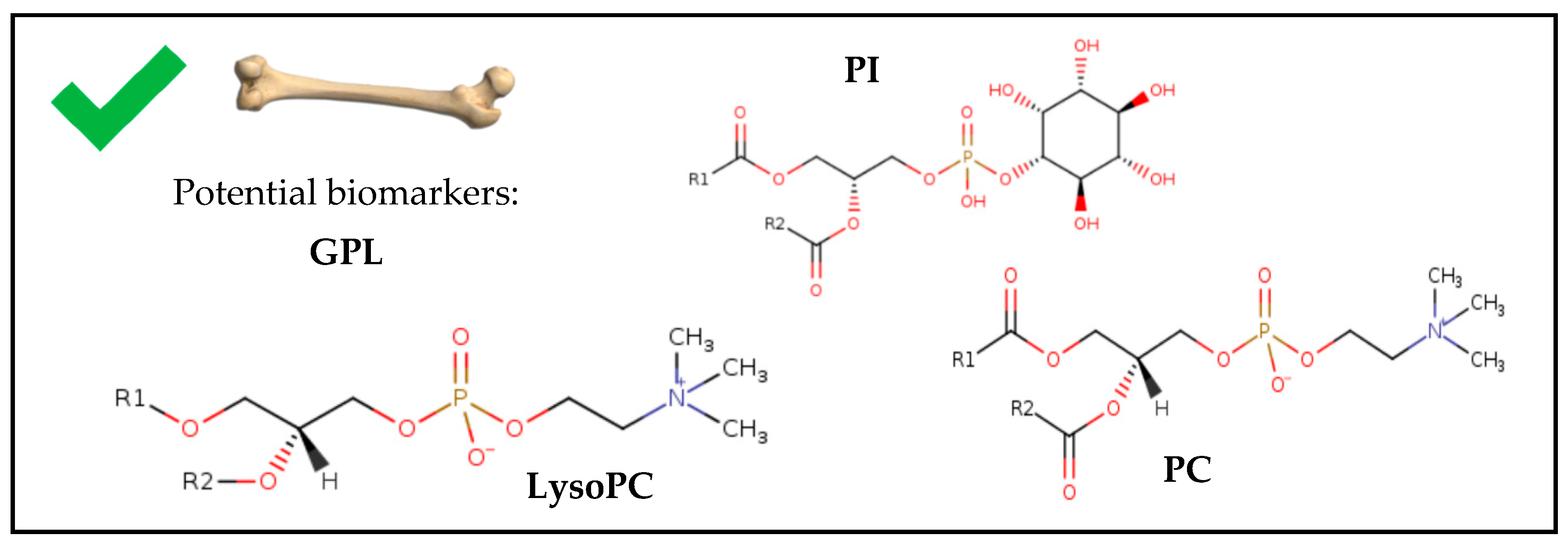
3.3.1. GPL as Markers for PMI Estimation
3.3.2. Human Bone Samples
3.3.3. Samples of Human and Animal Muscle Tissue
3.3.4. FAs and Sterols as Markers for PMI Estimation
3.3.5. Samples of Human and Animal Tissues
3.3.6. Other Types of Samples
3.4. Lipid Markers for Analysis of Burial Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Domínguez, R.; Pateiro, M.; Purriños, L.; Munekata, P.E.S.; Echegaray, N.; Lorenzo, J.M. Chapter 1—Introduction and Classification of Lipids. In Food Lipids; Lorenzo, J.M., Munekata, P.E.S., Pateiro, M., Barba, F.J., Domínguez, R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–16. ISBN 978-0-12-823371-9. [Google Scholar]
- Wang, J.; Wang, C.; Han, X. Tutorial on Lipidomics. Anal. Chim. Acta 2019, 1061, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, L.; Zhu, B.; Li, Y. Lipidome in Colorectal Cancer. Oncotarget 2016, 7, 33429–33439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Li, L.; Wei, J.; Xiong, S.; Zhao, Z. High Resolution Mass Spectrometry Coupled with Multivariate Data Analysis Revealing Plasma Lipidomic Alteration in Ovarian Cancer in Asian Women. Talanta 2016, 150, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Proitsi, P.; Kim, M.; Whiley, L.; Pritchard, M.; Leung, R.; Soininen, H.; Kloszewska, I.; Mecocci, P.; Tsolaki, M.; Vellas, B.; et al. Plasma Lipidomics Analysis Finds Long Chain Cholesteryl Esters to Be Associated with Alzheimer’s Disease. Transl. Psychiatry 2015, 5, e494. [Google Scholar] [CrossRef] [PubMed]
- Vodicka, P.; Mo, S.; Tousley, A.; Green, K.M.; Sapp, E.; Iuliano, M.; Sadri-Vakili, G.; Shaffer, S.A.; Aronin, N.; DiFiglia, M.; et al. Mass Spectrometry Analysis of Wild-Type and Knock-in Q140/Q140 Huntington’s Disease Mouse Brains Reveals Changes in Glycerophospholipids Including Alterations in Phosphatidic Acid and Lyso-Phosphatidic Acid. J. Huntingtons. Dis. 2015, 4, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-Targeted Lipidomics of CSF and Frontal Cortex Grey and White Matter in Control, Mild Cognitive Impairment, and Alzheimer’s Disease Subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Medicherla, S.; Sheikh, N.; Terry, B.; Phillipps, A.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment (MCI) and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 537–546. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Palacios, G.; Gao, J.; Zhang, N.; Li, G.; Lu, J.; Song, T.; Zhang, Y.; Lv, H. Plasma Metabolomics Reveals Biomarkers of the Atherosclerosis. J. Sep. Sci. 2010, 33, 2776–2783. [Google Scholar] [CrossRef]
- LIPID MAPS. Available online: https://www.lipidmaps.org/ (accessed on 15 January 2023).
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS Comprehensive Classification System for Lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Holčapek, M.; Liebisch, G.; Ekroos, K. Lipidomic Analysis. Anal. Chem. 2018, 90, 4249–4257. [Google Scholar] [CrossRef]
- Bonicelli, A.; Mickleburgh, H.L.; Chighine, A.; Locci, E.; Wescott, D.J.; Procopio, N. The ‘ForensOMICS’ Approach for Postmortem Interval Estimation from Human Bone by Integrating Metabolomics, Lipidomics, and Proteomics. eLife 2022, 11, e83658. [Google Scholar] [CrossRef]
- Comstock, J. Elucidation of the Lipid Degradation Process in Soft Tissue and Fluid during Decomposition, in the Presence and Absence of Insects. Ph.D. Dissertation, Ontario Tech University, Oshawa, ON, Canada, 2014. [Google Scholar]
- Sousa Queirós, S.; von der Lühe, B.; Silva-Bessa, A.; Machado Brito-da-Costa, A.; Caldas, I.M.; Dawson, L.; Madureira-Carvalho, Á. Lipidic Compounds Found in Soils Surrounding Human Decomposing Bodies and Its Use in Forensic Investigations—A Narrative Review. Sci. Justice 2023, 63, 303–312. [Google Scholar] [CrossRef]
- Fiedler, S.; Graw, M. Decomposition of Buried Corpses, with Special Reference to the Formation of Adipocere. Naturwissenschaften 2003, 90, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F.; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data: The PRISMA-IPD Statement. J. Am. Med. Assoc. 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- CASP Checklists—Critical Appraisal Skills Programme. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 16 June 2023).
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading Quality of Evidence and Strength of Recommendations. Br. Med. J. 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Stuart, B.; Ueland, M. The Use of Lipids from Textiles as Soft-Tissue Biomarkers of Human Decomposition. Forensic Sci. Int. 2023, 343, 111547. [Google Scholar] [CrossRef]
- Dudzik, B.; Jantz, L.M.; Langley, N.R.; Wood, P. Postmortem Interval Determination from Bone: A Metabolomics and Lipidomics Approach; Office of Justice Programs: Washington, DC, USA, 2022. [Google Scholar]
- Donnelly, L.J.; Cassella, J.; Pirrie, D.; Dawson, L.; Blom, G.; Davidson, A.; Arnold, P.; Harrison, M.; Hope, C. Analysis of Soil Following a Police-Led Open Area Search and the Recovery of a Cold-Case Homicide Grave. Geol. Soc. Spec. Publ. 2021, 492, 65–86. [Google Scholar] [CrossRef]
- Ueland, M.; Collins, S.; Maestrini, L.; Forbes, S.L.; Luong, S. Fresh vs. Frozen Human Decomposition—A Preliminary Investigation of Lipid Degradation Products as Biomarkers of Postmortem Interval. Forensic Chem. 2021, 24, 100335. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, H.; Liu, Y.; Wu, H.; Cai, W.; Wei, X.; Liu, R.; Wang, G.; Sun, Q.; Wang, Z. Adipose Tissue Estimates the Postmortem Interval Based on ATR-FTIR Spectroscopy. Microchem. J. 2021, 164, 105977. [Google Scholar] [CrossRef]
- Ismail, S.S.; Chock, X.; Kholidan, M.A.; Jemain, S.F.P. Lipid Distributions Associated with Cadaver Decomposition in Mangrove and Oil Palm Plantation Soils under Tropical Climate. Malays. J. Anal. Sci. 2020, 24, 339–349. [Google Scholar]
- Pesko, B.K.; Weidt, S.; McLaughlin, M.; Wescott, D.J.; Torrance, H.; Burgess, K.; Burchmore, R. Postmortomics: The Potential of Untargeted Metabolomics to Highlight Markers for Time Since Death. OMICS 2020, 24, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Langley, N.; Wood, P.; Herring, P.; Steadman, D. Forensic Postmortem Interval Estimation from Skeletal Muscle Tissue: A Lipidomics Approach. Forensic Anthropol. 2019, 2, 152–157. [Google Scholar] [CrossRef]
- Luong, S.; Forbes, S.L.; Wallman, J.F.; Roberts, R.G. Monitoring the Extent of Vertical and Lateral Movement of Human Decomposition Products through Sediment Using Cholesterol as a Biomarker. Forensic Sci. Int. 2018, 285, 93–104. [Google Scholar] [CrossRef]
- Ueland, M.; Forbes, S.L.; Stuart, B.H. Seasonal Variation of Fatty Acid Profiles from Textiles Associated with Decomposing Pig Remains in a Temperate Australian Environment. Forensic Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, X.; Chen, F.; Dai, X.; Ye, Y.; Yan, Y.; Liao, L. Estimation of Early Postmortem Interval in Rats by GC–MS-Based Metabolomics. Leg. Med. 2018, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vass, A.A. The Use of Volatile Fatty Acid Biomarkers to Estimate the Postmortem Interval. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment; Schotsmans, E.M.J., Márquez-Grant, N., Forbes, S.L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 385–393. ISBN 978-1-118-95335-8. [Google Scholar]
- Von Der Lühe, B.; Fiedler, S.; Mayes, R.W.; Dawson, L. Temporal Fatty Acid Profiles of Human Decomposition Fluid in Soil. Org. Geochem. 2017, 111, 26–33. [Google Scholar] [CrossRef]
- Ismail, S.S.; Saminadan, S.; Daud, N.A. A Study of Cadaver Decomposition in Sandy Soil of East Coast of Peninsular Malaysia. ESTEEM Acad. J. 2017, 13, 139–146. [Google Scholar]
- Von Der Lühe, B.; Dawson, L.A.; Mayes, R.W.; Forbes, S.L.; Fiedler, S. Investigation of Sterols as Potential Biomarkers for the Detection of Pig (S. s. Domesticus) Decomposition Fluid in Soils. Forensic Sci. Int. 2013, 230, 68–73. [Google Scholar] [CrossRef]
- Wood, P.L. Lipidomics Analysis of Postmortem Interval: Preliminary Evaluation of Human Skeletal Muscle. Metabolomics 2012, 3, 127. [Google Scholar] [CrossRef]
- Algarra, M.; Rodríguez-Borges, J.E.; Esteves Da Silva, J.C.G. LC-MS Identification of Derivatized Free Fatty Acids from Adipocere in Soil Samples: Liquid Chromatography. J. Sep. Sci. 2010, 33, 143–154. [Google Scholar] [CrossRef]
- Stuart, B.H.; Craft, L.; Forbes, S.L.; Dent, B.B. Studies of Adipocere Using Attenuated Total Reflectance Infrared Spectroscopy. Forensic Sci. Med. Pathol. FSMP 2005, 1, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.L.; Stuart, B.H.; Dadour, I.R.; Dent, B.B. A Preliminary Investigation of the Stages of Adipocere Formation. J. Forensic Sci. 2004, 49, 566–574. [Google Scholar] [CrossRef]
- Forbes, S.L.; Keegan, J.; Stuart, B.H.; Dent, B.B. A Gas Chromatography-mass Spectrometry Method for the Detection of Adipocere in Grave Soils. Eur. J. Lipid Sci. Technol. 2003, 105, 761–768. [Google Scholar] [CrossRef]
- Forbes, S.L.; Stuart, B.H.; Dent, B.B. The Identification of Adipocere in Grave Soils. Forensic Sci. Int. 2002, 127, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H.; Forbes, S.; Dent, B.B.; Hodgson, G. Studies of Adipocere Using Diffuse Reflectance Infrared Spectroscopy. Vib. Spectrosc. 2000, 24, 233–242. [Google Scholar] [CrossRef]
- Stuart, B. Decomposition Chemistry: Overview, Analysis, and Interpretation. In Encyclopedia of Forensic Sciences; Elsevier: Amsterdam, The Netherlands, 2013; pp. 11–15. ISBN 978-0-12-382166-9. [Google Scholar]
- Notter, S.J.; Stuart, B.H.; Rowe, R.; Langlois, N. The Initial Changes of Fat Deposits During the Decomposition of Human and Pig Remains. J. Forensic Sci. 2009, 54, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.A.; Lawn, J.; Guareschi, E.E. A Practical Review of Adipocere: Key Findings, Case Studies and Operational Considerations from Crime Scene to Autopsy. J. Forensic Leg. Med. 2021, 78, 102109. [Google Scholar] [CrossRef]
- Johari, I.S. Lipidomic Characterisation and Profiling of Soil for Forensic Investigations. Ph.D. Dissertation, University of Bristol, Bristol, UK, 2019. [Google Scholar]
- Fiedler, S.; Buegger, F.; Klaubert, B.; Zipp, K.; Dohrmann, R.; Witteyer, M.; Zarei, M.; Graw, M. Adipocere Withstands 1600 Years of Fluctuating Groundwater Levels in Soil. J. Archaeol. Sci. 2009, 36, 1328–1333. [Google Scholar] [CrossRef]
- Wq, S. The Vitreous Humour: Postmortem Potassium Changes. Lancet 1963, 1, 807–808. [Google Scholar] [CrossRef]
- Chemical Entities of Biological Interest (ChEBI). Available online: https://www.ebi.ac.uk/chebi/init.do (accessed on 5 July 2023).
- Dorninger, F.; Brodde, A.; Braverman, N.E.; Moser, A.B.; Just, W.W.; Forss-Petter, S.; Brügger, B.; Berger, J. Homeostasis of Phospholipids—The Level of Phosphatidylethanolamine Tightly Adapts to Changes in Ethanolamine Plasmalogens. Biochim. Biophys. Acta 2015, 1851, 117–128. [Google Scholar] [CrossRef]
- Saito, K. The Occurrence of 10-Hydroxystearic Acid in Adipocere. J. Biochem. 1966, 59, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Takatori, T.; Yamaoka, A. The Mechanism of Adipocere Formation 1. Identification and Chemical Properties of Hydroxy Fatty Acids in Adipocere. Forensic Sci. 1977, 9, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Agudelo, M.E.; Rojas López, M. La necrosis, un mecanismo regulado de muerte celular. Iatreia 2010, 23, 166–177. [Google Scholar] [CrossRef]
- Evershed, R.P. Chemical Composition of a Bog Body Adipocere. Archaeometry 1992, 34, 253–265. [Google Scholar] [CrossRef]
- Larizza, M.; Forbes, S.L. Detection of Fatty Acids in the Lateral Extent of the Cadaver Decomposition Island. In Environmental and Criminal Geoforensics; Pirrie, D., Ruffell, A., Dawson, L.A., Eds.; Geological Society of London: London, UK, 2013; Volume 384, pp. 209–219. ISBN 978-1-86239-366-0. [Google Scholar]
- Von der Lühe, B.; Prost, K.; Birk, J.; Fiedler, S. Steroids Aid in Human Decomposition Fluid Identification in Soils of Temporary Mass Graves from World War II. J. Archaeol. Sci. Rep. 2020, 32, 102431. [Google Scholar] [CrossRef]
- Wallace, M.; Morris, C.; O’Grada, C.M.; Ryan, M.; Dillon, E.T.; Coleman, E.; Gibney, E.R.; Gibney, M.J.; Roche, H.M.; Brennan, L. Relationship between the Lipidome, Inflammatory Markers and Insulin Resistance. Mol. Biosyst. 2014, 10, 1586–1595. [Google Scholar] [CrossRef]
- Audano, M.; Maldini, M.; De Fabiani, E.; Mitro, N.; Caruso, D. Gender-Related Metabolomics and Lipidomics: From Experimental Animal Models to Clinical Evidence. J. Proteom. 2018, 178, 82–91. [Google Scholar] [CrossRef]
- Malcom, G.T.; Bhattacharyya, A.K.; Velez-Duran, M.; Guzman, M.A.; Oalmann, M.C.; Strong, J.P. Fatty Acid Composition of Adipose Tissue in Humans: Differences between Subcutaneous Sites. Am. J. Clin. Nutr. 1989, 50, 288–291. [Google Scholar] [CrossRef]
- Calder, P.C.; Harvey, D.J.; Pond, C.M.; Newsholme, E.A. Site-Specific Differences in the Fatty Acid Composition of Human Adipose Tissue. Lipids 1992, 27, 716–720. [Google Scholar] [CrossRef]
- Davenel, A.; Riaublanc, A.; Marchal, P.; Gandemer, G. Quality of Pig Adipose Tissue: Relationship between Solid Fat Content and Lipid Composition. Meat Sci. 1999, 51, 73–79. [Google Scholar] [CrossRef]
- Dai, X.; Fan, F.; Ye, Y.; Lu, X.; Chen, F.; Wu, Z.; Liao, L. An Experimental Study on Investigating the Postmortem Interval in Dichlorvos Poisoned Rats by GC/MS-Based Metabolomics. Leg. Med. 2019, 36, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, G.J.; Bloch, K. The Stereospecific Conversion of Stearic Acid to Oleic Acid. J. Biol. Chem. 1965, 240, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tamm, C. Transformation of Organic Compounds by Microbial Enzymes. FEBS Lett. 1974, 48, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.; Cross, P.A.; Adlam, R.E.; Moffatt, C. The Influence of Insects on Decomposition Rate in Buried and Surface Remains: The Influence Of Insects On Decomposition Rate. J. Forensic Sci. 2010, 55, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.F.; Lowry, M.A.; Micozzi, M.S. Experimental Observations on Adipocere Formation. J. Forensic Sci. 1993, 38, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; McNally, R.; Kontanis, E.J.; Sadik, O.A. Preliminary Quantitative Investigation of Postmortem Adipocere Formation. J. Forensic Sci. 2001, 46, 609–614. [Google Scholar] [CrossRef]
- Tibbett, M.; Carter, D.O. Research in Forensic Taphonomy: A Soil-Based Perspective. In Criminal and Environmental Soil Forensics; Ritz, K., Dawson, L., Miller, D., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 317–331. ISBN 978-1-4020-9203-9. [Google Scholar]





| Ref. | The Study Addresses the Problem. | Acceptable Cohort Recruitment | Precisely Measured Exposure | Precisely Measured Results | Important Confounding Factors Identified | Critical Confounding Factors Are Taken into Account | Accurate Results | Credible Results | The Results Agree with Other Available Data | Overall Quality Score |
| [20] | ✓ | 🗴 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [13] | ✓ | 🗴 | ✓ | 🗴 | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [21] | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [22] | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | ✓ | ✓ | ✓ | Moderate |
| [23] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [24] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | ✓ | Moderate |
| [25] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [26] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [27] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | 🗴 | Moderate |
| [28] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [29] | ✓ | 🗴 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [30] | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | ✓ | ✓ | Good |
| [31] | ✓ | 🗴 | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | ✓ | Poor |
| [32] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [33] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [14] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [34] | ✓ | 🗴 | 🗴 | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [35] | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | Poor |
| [36] | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | ✓ | ✓ | ✓ | Moderate |
| [37] | ✓ | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | 🗴 | ✓ | Poor |
| [38] | ✓ | 🗴 | 🗴 | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [39] | ✓ | 🗴 | ✓ | 🗴 | 🗴 | 🗴 | ✓ | ✓ | ✓ | Moderate |
| [40] | ✓ | 🗴 | ✓ | ✓ | 🗴 | 🗴 | ✓ | ✓ | ✓ | Good |
| [41] | ✓ | 🗴 | ✓ | 🗴 | 🗴 | 🗴 | ✓ | ✓ | ✓ | Moderate |
| Biological Matrix | Lipid or Acid Detected | Laboratory Method | References |
|---|---|---|---|
| Muscle tissue | Sterols (cholesterol, 5α-cholestanol, and cholestanone) SFA (C18:0 and C16:0) and UFA (C18:1 and C18:2) | GC–MS/MS | [23] |
| Choline phosphate | LC-MS | [26] | |
| PG, PC, PtdE, PlsCh, PlsEtn, and VLCFA | HR-MS | [27] | |
| Sterols (Cholesterol sulfate and DHEA sulfate), PlsE, PlsCh, PG, and FA (VLCFA and UFA) | Shotgun lipidomic analysis | [35] | |
| Bone | PC | HR-MS | [21] |
| LysoPC, PI, and PC | LC–MS | [13] | |
| Textile in contact with decomposing remains | SFA, UFA, and bile acids | GC–MS/MS | [20] |
| Textile in contact with corpse | SFA (C14:0, C16:0, and C18:0) and UFA (C16:1, C18:1, and C18:2) | GC–MS | [29] |
| Soil from burial sites | Sterols (coprostanol and epicoprostanol) | ICP-OES-/ICP-MS | [25] |
| SFA (C16:0 and C18:0) and UFA (C18:1) | GC-FID | [28] | |
| Sterols (cholesterol) | GC–MS/MS | [32] | |
| FA (C14:0, C16:0, C18:0, C18:1, C18:2, and 10-OHC18:0) | GC–MS and GC-FID | [33] | |
| SFA (C16:0 and C18:0) | GC-FID | [31] | |
| VFA (C3:0, isobutyric acid, n-butyric acid, isovaleric acid, and n-valeric acid) | GC–MS | [34] | |
| Sterols (Cholesterol, β-sitosterol, and coprostanol) | GC–MS | [36] | |
| SFA (C14:0, C15:0, C16:0, C17:0, and C18:0), UFA (C18:1, C18:2, and C16:1), and hydroxy fatty acids (10-OH-C18:0) | LC–MS | [37] | |
| FA (TG, SFA, UFA, salts of acids, and hydroxy fatty acids) | FTIR-ATR | [38] | |
| FA (TG, SFA, and UFA) | GC–MS and FTIR | [39] | |
| SFA (C14:0, C16:0, and C18:0), UFA (C18:1), and hydroxy fatty acids (10-OH-C18:0) | GC–MS | [40] | |
| SFA (C14:0, C16:0, and C18:0), UFA (C16:1 and C18:1), and hydroxy fatty acids (10-OH-C18:0) | GC–MS | [41] | |
| SFA (C14:0, C16:0, and C18:0), hydroxy fatty acids, and fatty acid salts | DRIFTS | [24] | |
| Adipose tissue | FA | FTIR-ATR | [30] |
| Animal blood | SFA (C16:0 and C18:0) and UFA (C18:1) | GC–MS | [14] |
| Soft tissue | SFA (C18:0 and C16:0) and UFA (C18:1, C18:2, and C16:1) | GC–MS | [2] |
| Ref. | Sample | Human/Animal | Cadaver Sample Data | Postmortem Sampling (Days) | Category | Lipid Type | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | PMI of the Samples | Decomposition Environment | Age | Weight (kg) | Cause of Death | |||||||
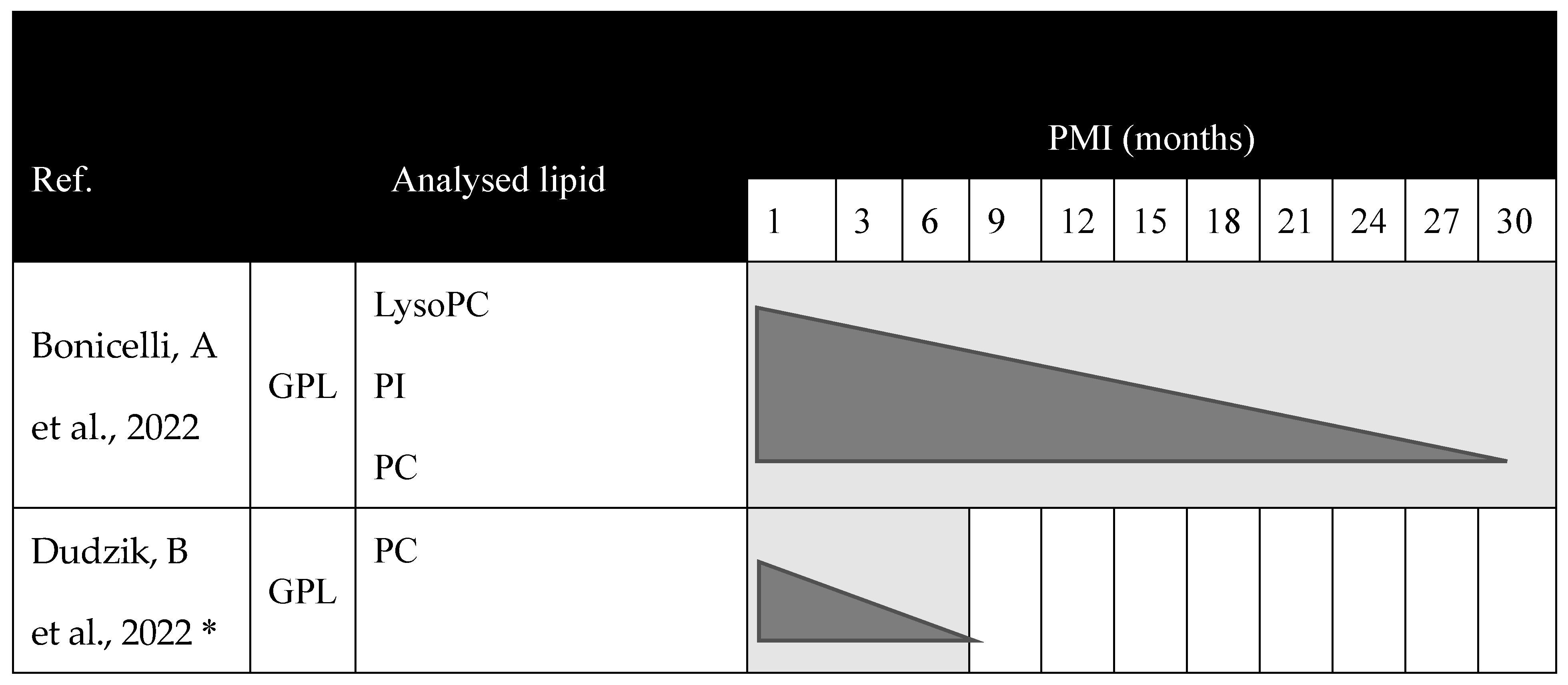
| [21] | The medial calcaneus, proximal tibia, and vertebral body (fourth lumbar) | Human | - | Between less than 1 year and 30 years | External environment | - | - | - | Sampling every 6 months for 24 months | GPL | PC (34:1) PC (34:2) PC (36:1) PC (36:2) PC (36:4) | Decrease with PMI between the first and sixth month since the beginning of this study. |
| [13] | Anterior midshaft tibia | Human | 4 W | 2, 3, and 10 days | Two subjects in shallow open pits | Between 61 and 91 years old | - | - | Days 0, 219, 790, and 872 | GPL | LysoPC PI PC | Drastic reduction in intensity of markers between the fresh state and the state after decomposition. |
| Two subjects buried in pits | ||||||||||||
| [26] | Biceps femoris muscle tissue. | Human | 1 W | - | Refrigerated | 69 | - | Suicide | Days 11, 12, 13, 14, and 15 | GPL | Choline phosphate | Shows an increasing pattern with PMI from day 7 to 19 postmortem. |
| 5 M | - | Refrigerated | 60 | - | Hemorrhagic stroke | Days 7, 8, 9, and 10 | ||||||
| Refrigerated | 62 | - | Pulmonary embolism | Days 11, 12, 13, 14, and 15 | ||||||||
| Refrigerated | 69 | - | Metastatic nonsmall cell lung cancer | Days 12, 13, 14, 15, and 16 | ||||||||
| External environment | 59 | - | Acute respiratory distress syndrome | Days 3, 4, and 5 | ||||||||
| External environment | 60 | - | Cardiovascular disease | Days 18 and 19 | ||||||||
| [27] | Vastus lateralis muscle | Human | - | - | External environment | - | - | - | Daily samples up to 2000 ADD or until the muscle is no longer available | GPL | PG (34:0) PC (36:2) PtdE (36:4) | Decrease with PMI. Regression models were developed with PG 34:0 and PtdE 36:4. |
| Plasmalogen | PlsCh (34:2) PlsEtn (36:4) | Decrease with PMI. | ||||||||||
| VLCFA | - | Increase with PMI. | ||||||||||
| [35] | Skeletal muscle | Human | - | - | - | - | - | - | Days 1, 9, and 24 | Sterol sulfate | Cholesterol sulfate and DHEA sulfate | Increase with PMI. |
| Plasmalogen | PlsE (36:1) and (40:6) PlsCh (34:1) and (36:4) | Decrease with PMI. | ||||||||||
| GPL | PG | Decrease with PMI. | ||||||||||
| 5FA | VLCFA | Decrease with PMI. | ||||||||||
| PUFA | Increase with PMI. | |||||||||||
| [26] | Biceps femoris muscle tissue | Animal (rat) | 8 M | 0 days | Two of them immediately dissected | Adult | - | Euthanasia | Day 0, 1, 2, and 3 | GPL | Choline phosphate | There is an increase during the investigation period (3 days). |
| Six remaining were dissected for 3 days. | ||||||||||||
| Ref. | Sample | Human/Animal | Cadaver Sample Data | Postmortem Sampling * | Marker Category | Lipid Type | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Decomposition Environment | Age | Weight | BMI | Cause of Death | |||||||
| [23] | The tissue of the upper arm, lower abdomen/torso region, and the buttocks/upper thigh (right side) | Human | 2 M | External environment (summer). One donor was previously frozen, while the other was not. | 68 and 77 years old | 104 and 90 kg | 32.5 and 30.8 (kg/cm2) | - | Days 0, 2, 4, 6, 8, 8, 10, 10, 12, 14, 14, 17, 20, 24, 48, and 69 | Sterols | Cholesterol, 5a-cholestanol, and cholestanone | They were not suitable biomarkers for the estimation of PMI since there is no specificity among donors in the long term. |
| SFA | C18:0 C16:0 | Good biomarkers for PMI estimation. | ||||||||||
| MUFA and PUFA | C18:1 C18:2 | |||||||||||
| [24] | Adipose tissue. | Animal (rat) | 462 M | Controlled environment at 5 °C and humidity 50 ± 5%. | - | 24–26 g | - | Cervical dislocation | From day 0 to 10 and day 14 | FA | - | An increase in free fatty acids and a decrease in fatty acid-glycerol bonds with PMI are detected. Regression models are obtained for the estimation of the PMI. |
| Controlled environment at 15 °C and humidity 50 ± 5%. | From day 0 to 10 and day 14 | |||||||||||
| Controlled environment at 25 °C and humidity 50 ± 5%. | From day 0 to 10 and day 14 | |||||||||||
| Controlled environment at 35 °C and humidity 50 ± 5%. | Days 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 | |||||||||||
| [30] | Blood. | Animal (rat) | 42 M | In plastic bags, in an environment with constant temperature and humidity at 12 ± 2 °C and 50% ± 10%, respectively. | - | - | - | Suffocated | Hour 0, 3, 6, 12, 12, 24, 48, and 72. Minimum one day, maximum two days. | SFA and MUFA | C16:0 C18:0 C18:1 | An increase in compounds is determined as the PMI progresses. Regression models are obtained for the estimation of the PMI. |
| 42 F | ||||||||||||
| [14] | The soft tissue of the upper and lower torso. | Animal (Sus scrofa domesticus) | - | External environment with presence and absence of insects. | Adults | 20–30 kg | - | Head bolt | Maximum 111 days | SFA | C18:0 C16:0 | An increase in compounds is determined as the PMI progresses. |
| MUFA and PUFA | C18:1 C18:2 C16:1 | A decrease in compounds is observed as the PMI progresses. | ||||||||||
| [20] | Textile in contact with decomposing corpse. Control samples are taken. | Human | 2 M | One donor is deposited in summer, and the other one in winter. | 84 and 86 years old | 63 and 100 kg | 20.8 and 30.2 (kg/cm2) | Alzheimer Metastatic bladder cancer | Days 0 and 105 | SFA, UFA, and bile acids | C16:1, C18:2, C16:0, and C18:1, Deoxycholic and lithocholic acids | In summer, a decrease in UFA and an increase in SFA analogs is detected. An increase in bile acids is also detected. In winter, there is a minimal presence of SFAs and UFAs. |
| [31] | The soil in contact with the corpse (soil in contact with large muscle mass). | Human | M | External environment. | Adult | - | - | - | * ADD for the 90 days prior to the discovery of the corpse are taken. | VFA | C3:0 Isobutyric acid n-butyric acid Isovaleric acid n-valeric acid | These fatty acids are detected in soil samples. |
| [39] | Soil from grave exhumations (area under the trunk region of the remains). | Human | - | Buried in graves. | - | - | - | - | Graves of different PMI (6 and 12 years) | SFA and MUFA | C14:0 C16:0 C18:0 C18:1 10-OH-C18:0 | A lower concentration of fatty acids is detected in 12-year-old samples and higher in 6-year-old samples. |
| [25] | Soils of burial sites. Abdominal fat is buried with muscle and skin. | Animal (Sus scrofa) | - | Burial in a vessel with animal and soil sample (acid pH = 3.74). | - | - | - | - | Days 0, 3, 5, 7, 15, 15, 17, 21, and 28 | SFA and MUFA | C16:0 C18:0 C18:1 | An increase in the concentration of these FAs is detected in the early stages of decomposition and a decrease towards the end of the test period. Highest concentration of C16:0, followed by C18:0 and C18:1. |
| Burial in a vessel with animal and soil sample (slightly alkaline pH = 7.45). | An increase in the concentration of these FAs is detected later in the decomposition. Highest concentration of C18:1, followed by C18:0 and C16:0. | |||||||||||
| [29] | Textile in contact with decomposing corpse. | Animal (Sus scrofa) | - | Burial of the animal with the textile remains in summer. | - | - | - | - | Minimum day three, maximum day 499 | SFA and UFA | C14:0 C16:0 C16:1 C18:0 C18:1 C18:2 | Increase in the percentage of SFA in the first stages (up to day 31) and then maintain it until the end of the trial. |
| Burial of the animal with the textile remains in winter. | There is a decrease in the percentage of SFA at the beginning of the decomposition until day 65; then, there is an increase in the percentage of SFA until the end of the trial. | |||||||||||
| [33] | Sandy soil | Animal (Sus scrofa) | - | Pig fatty flesh is buried in a vial with sandy soil. | - | - | - | - | Days 0, 3, 5, 10, 20, 50, and 90 | SFA | C16:0 C18:0 | It increases in concentration, reaching a maximum on day 20. Subsequently, it decreases until the end of the trial (day 90). |
| Ref. | Sample | Human/Animal | PMI | Lipid Markers | Type | Results | Use |
|---|---|---|---|---|---|---|---|
| [22] | Soil of a grave at different depths | Human | Unknown | Sterols | Coprostanol Epicoprostanol | The presence of these markers is detected in the grave samples and is absent in the control samples. | Determination of burial sites. |
| [28] | Soil in the burial area (under the corpse), at different distances from the grave. | Human | 14 days | Sterols | Cholesterol | The presence of cholesterol is detected in the different samples taken from the grave but not in the control samples. | Determination of burial sites. |
| [32] | Sampling of the soil under the thorax and abdomen of the corpse at different depths and taking control samples. | Human | Estimated 11–18 days. Left in the ground until 358 days later | FA | C14:0, C16:0, C18:0, C18:1, C18:2, and 10-OH-C18:0 | With a PMI of 18 days, these FAs are detected, but in slightly higher concentrations in the thorax compared to the abdominal area (5–330 mg/g and 5–52 mg/g). The concentration of these compounds decreases with increasing depth. In upper samples, C16:0 and 10-OH-C18:0 predominate. With a PMI of 358 days, SFA dominates over UFA and 10-OH-C18:0. | Determination of burial sites. |
| [31] | The soil in contact with the corpse (soil in contact with large muscle mass). | Human | Unknown | VFA | Propionic acid Isobutyric acid N-butyric acid Isovaleric acid N-valeric acid | The presence of these compounds is detected in samples taken from soil under decomposing remains. | Determination of burial sites and estimation of PMI. |
| [36] | Cemetery soil in areas adjacent to graves with skeletal corpses. Soil samples from burial areas with adipocere. | Human | Unknown | FA | C14:0, C15:0, C16:0, C17:0, C18:0, C18:1, C18:2, C16:1, and 10-OH-C18:0 | The presence of the markers was detected in all the samples taken in the cemetery and the adipocere samples. | Determination of burial sites. The presence of 10-OH-C18:0 is detected in soil samples. |
| [39] | Soils from grave exhumations (area under the trunk region of human remains) and control sampling. | Human | Between 6 and 12 years old | FA | C14:0, C16:0, C18:0, C18:1, and 10-OH-C18:0 | These compounds are detected in the exhumation soil samples, not the control samples. | Determination of burial sites with adipocere and estimation of PMI. |
| [40] | Grave soils with adipocere after exhumation of corpses and forensic burials, with differences in burial depth (1.2–1.8 m) and humid and dry environments. | Human | Exhumations between 5.5 and 50 years. Forensic burials: unknown | FA | C14:0, C16:0, C18:0, C18:1, C16:1, and 10-OH-C18:0 | The presence of the markers is detected in the grave samples, but there is a total absence of the markers in the control samples. The 10-OH-C18:0 is only detected in the 13-, 22- and 26-year-old PMI samples and humid environments. Difference in wet and dry environments is observed. Higher C16:0 and lower C18:0 concentrations are detected in soils with humid environments. | Determination of burial sites with adipocere. |
| [41] | Soils with adipocere of exhumations in cemeteries and control sampling. | Human | Three samples aged 13, 22, and 23 years 27 months | FA | C14:0, C16:0, and C18:0 Hydroxy acids Fatty acid salts | Fatty acids are present in all samples, with the primary fatty acid being C16:0. | Determination of burial sites with adipocere. |
| [34] | Soils of burial areas (depth of 40 cm) of four pigs. | Animal (S. s. domesticus) | 3 and 6 months after burial | Sterols | Cholesterol β-sitosterol Coprostanol | There is an increase in the presence of cholesterol and β-sitosterol relative to control samples at three months of burial. Coprostanol can also be detected in some samples, but it is absent in control samples. At six months, a decrease in cholesterol and β-sitosterol concentration is detected. | Determination of burial sites with adipocere. |
| [37] | Adipocere samples from burial areas of animal adipose tissue. Different environments: silty soil and coffin simulation. | Animal (S. s. domesticus) | 12 months of burial | FA | Triglycerides Saturated and unsaturated fatty acids Fatty acid salts Hydroxy acids | A difference is detected between the profile obtained by analyzing adipose tissue and adipocytes. In adipose tissue, triglycerides are mainly present. In adipocere samples, triglycerides disappear, and FA, fatty acid salts, and hydroxy acids appear. | Determination of burial sites with adipocere. |
| [38] | Soil below the decomposition remains. Adipocere samples directly from the cadaver (abdominal area and lower thorax) and control sampling. | Animal (pig) | Different decomposition times (5, 6, 8, 8, 13, and 14 months) | FA | Triglycerides Saturated (C16:0 and C18:0) and unsaturated fatty acids | It can determine the adipocere decomposition profile. Triglycerides are degraded, with decreasing concentration and increasing SFA and UFA. Subsequently, there is a decrease in UFA with an increase in C16:0. This is followed by a further decrease in UFA with a consequent increase in C18:0. | Determination of burial sites and adipocere formation process |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-Moreno, L.; Luna, A.; Legaz, I. Lipidomes in Cadaveric Decomposition and Determination of the Postmortem Interval: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 984. https://doi.org/10.3390/ijms25020984
Caballero-Moreno L, Luna A, Legaz I. Lipidomes in Cadaveric Decomposition and Determination of the Postmortem Interval: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(2):984. https://doi.org/10.3390/ijms25020984
Chicago/Turabian StyleCaballero-Moreno, Leticia, Aurelio Luna, and Isabel Legaz. 2024. "Lipidomes in Cadaveric Decomposition and Determination of the Postmortem Interval: A Systematic Review" International Journal of Molecular Sciences 25, no. 2: 984. https://doi.org/10.3390/ijms25020984







