Changes in Cx43 and AQP4 Proteins, and the Capture of 3 kDa Dextran in Subpial Astrocytes of the Rat Medial Prefrontal Cortex after Both Sham Surgery and Sciatic Nerve Injury
Abstract
1. Introduction
2. Results
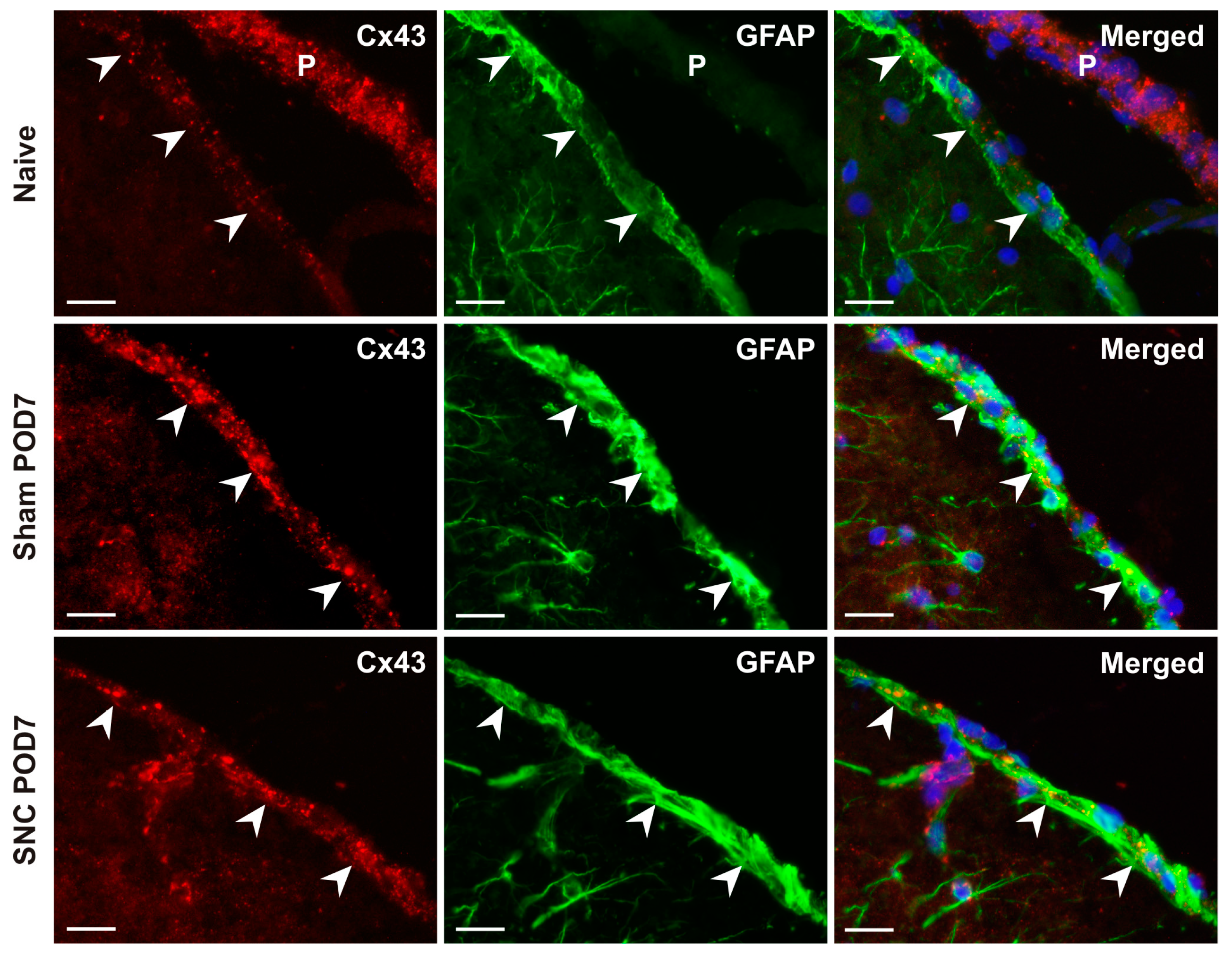
2.1. Cx43 Immunostaining in Subpial Astrocytes
2.2. AQP4 Immunostaining in Subpial Astrocytes
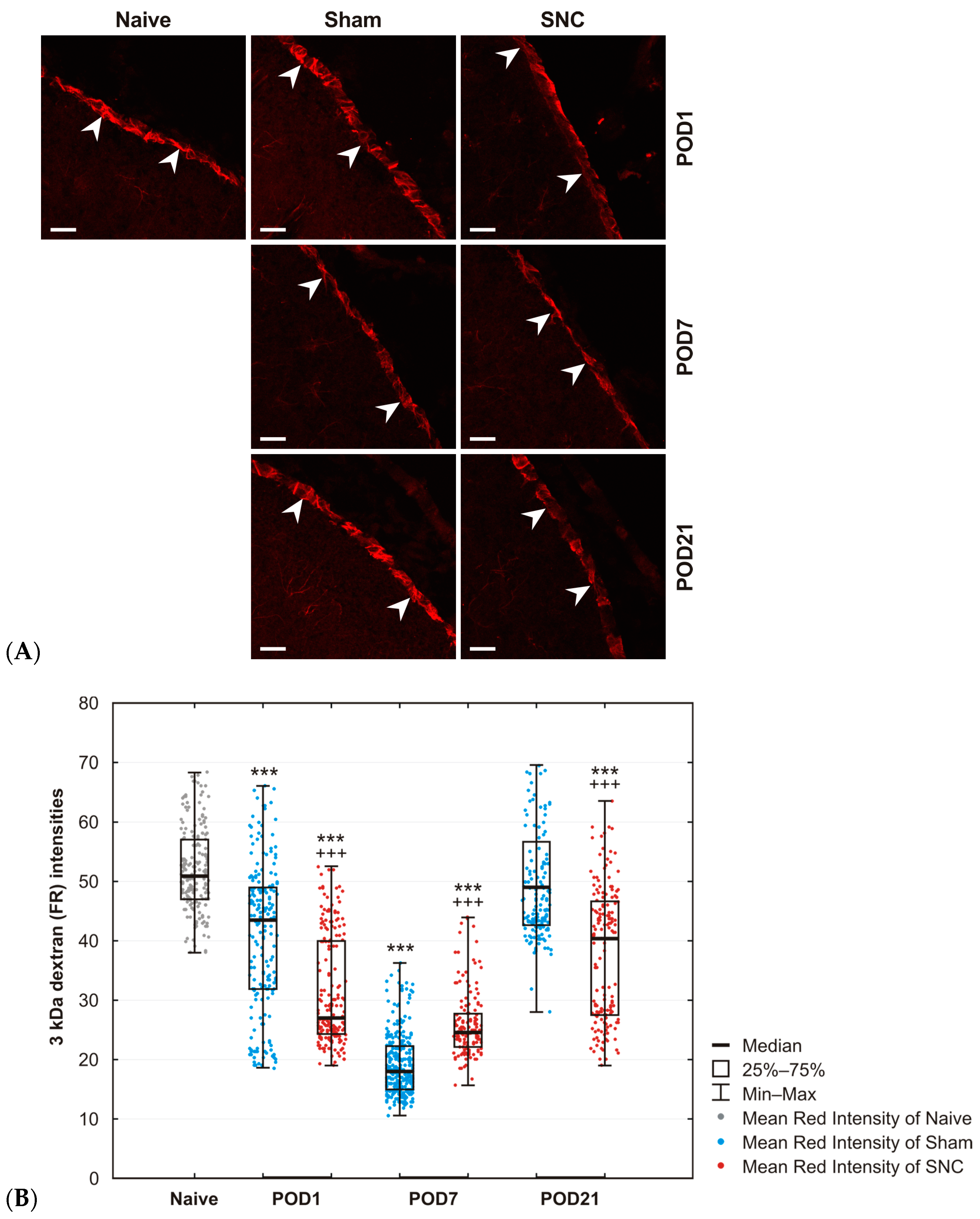
2.3. Results of 3 kDa Dextran Uptake in Subpial Astrocytes
3. Discussion
3.1. Changes in Cx43 in Subpial Astrocytes
3.2. Changes in AQP4 in Subpial Astrocytes
3.3. Changes in Cx43 and AQP4 in Subpial Astrocytes after Both Sham and SNC Operations
4. Materials and Methods
4.1. Animals and Surgical Procedures
4.2. Immunohistochemical Staining
4.3. Double Immunohistochemical Staining
4.4. Uptake of 3 kDa Dextran by Subpial Astrocytes
4.5. Microscopy and Image Analysis
4.5.1. Semiquantitative Analysis of Cx43
4.5.2. Semiquantitative Analysis of AQP4
4.5.3. Analysis of the 3 kDa Dextran Taken up by Subpial Astrocytes
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of Reactive Astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell. Neurosci. 2018, 12, 401. [Google Scholar] [CrossRef]
- Rakic, P. Elusive Radial Glial Cells: Historical and Evolutionary Perspective. Glia 2003, 43, 19–32. [Google Scholar] [CrossRef]
- Engelhardt, B.; Coisne, C. Fluids and Barriers of the CNS Establish Immune Privilege by Confining Immune Surveillance to a Two-Walled Castle Moat Surrounding the CNS Castle. Fluids Barriers CNS 2011, 8, 4. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Guo, W.; Burnstock, G.; He, C.; Xiang, Z. The Superficial Glia Limitans of Mouse and Monkey Brain and Spinal Cord: Glia Limitans of Brain and Spinal Cord. Anat. Rec. 2013, 296, 995–1007. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.M. Chapter 3—Astroglial Physiology. In Neuroglia; Verkhratsky, A., Butt, A.M., Eds.; Academic Press, Ltd.: London, UK, 2023; pp. 89–197. ISBN 978-0-12-821565-4. [Google Scholar]
- Weller, R.O.; Sharp, M.M.; Christodoulides, M.; Carare, R.O.; Møllgård, K. The Meninges as Barriers and Facilitators for the Movement of Fluid, Cells and Pathogens Related to the Rodent and Human CNS. Acta Neuropathol. 2018, 135, 363–385. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The Role of Brain Barriers in Fluid Movement in the CNS: Is There a ‘Glymphatic’ System? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Eto, K.; Kim, S.K.; Takeda, I.; Nabekura, J. The Roles of Cortical Astrocytes in Chronic Pain and Other Brain Pathologies. Neurosci. Res. 2018, 126, 3–8. [Google Scholar] [CrossRef]
- Boulay, A.-C.; Gilbert, A.; Moreira, V.O.; Blugeon, C.; Perrin, S.; Pouch, J.; Le Crom, S.; Ducos, B.; Cohen-Salmon, M. Connexin 43 Controls the Astrocyte Immunoregulatory Phenotype. Brain Sci. 2018, 8, 50. [Google Scholar] [CrossRef]
- Nagy, J.I.; Rash, J.E. Cx36, Cx43 and Cx45 in Mouse and Rat Cerebellar Cortex: Species-Specific Expression, Compensation in Cx36 Null Mice and Co-Localization in Neurons vs. Glia. Eur. J. Neurosci. 2017, 46, 1790–1804. [Google Scholar] [CrossRef]
- Kielian, T. Glial Connexins and Gap Junctions in CNS Inflammation and Disease. J. Neurochem. 2008, 106, 1000–1016. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-Dependent Glymphatic Solute Transport in the Rodent Brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes–Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; Slaoui, L.; Mazaré, N.; Gilbert, A.; Oudart, M.; Alvear-Perez, R.; Elorza-Vidal, X.; Chever, O.; Boulay, A.-C. Astrocytes in the Regulation of Cerebrovascular Functions. Glia 2021, 69, 817–841. [Google Scholar] [CrossRef]
- Beggs, S.; Liu, X.J.; Kwan, C.; Salter, M.W. Peripheral Nerve Injury and TRPV1-Expressing Primary Afferent C-Fibers Cause Opening of the Blood-Brain Barrier. Mol. Pain 2010, 6, 74. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Chen, S.-X.; Liu, J.-Y.; Yao, P.-W.; Duan, Y.-W.; Li, Y.-Y.; Zang, Y. Neuroinflammation in the Anterior Cingulate Cortex: The Potential Supraspinal Mechanism Underlying the Mirror-Image Pain Following Motor Fiber Injury. J. Neuroinflamm. 2022, 19, 162. [Google Scholar] [CrossRef]
- Austin, P.J.; Fiore, N.T. Supraspinal Neuroimmune Crosstalk in Chronic Pain States. Curr. Opin. Physiol. 2019, 11, 7–15. [Google Scholar] [CrossRef]
- Joukal, M.; Klusáková, I.; Solár, P.; Kuklová, A.; Dubový, P. Cellular Reactions of the Choroid Plexus Induced by Peripheral Nerve Injury. Neurosci. Lett. 2016, 628, 73–77. [Google Scholar] [CrossRef]
- Dellarole, A.; Morton, P.; Brambilla, R.; Walters, W.; Summers, S.; Bernardes, D.; Grilli, M.; Bethea, J.R. Neuropathic Pain-Induced Depressive-like Behavior and Hippocampal Neurogenesis and Plasticity Are Dependent on TNFR1 Signaling. Brain. Behav. Immun. 2014, 41, 65–81. [Google Scholar] [CrossRef]
- Zhuo, M. Neural Mechanisms Underlying Anxiety–Chronic Pain Interactions. Trends Neurosci. 2016, 39, 136–145. [Google Scholar] [CrossRef]
- Sang, K.; Bao, C.; Xin, Y.; Hu, S.; Gao, X.; Wang, Y.; Bodner, M.; Zhou, Y.-D.; Dong, X.-W. Plastic Change of Prefrontal Cortex Mediates Anxiety-like Behaviors Associated with Chronic Pain in Neuropathic Rats. Mol. Pain 2018, 14, 1744806918783931. [Google Scholar] [CrossRef]
- Bretová, K.; Svobodová, V.; Dubový, P. Astrocyte Reactivity in the Glia Limitans Superficialis of the Rat Medial Prefrontal Cortex Following Sciatic Nerve Injury. Histochem. Cell Biol. 2023, 159, 185–198. [Google Scholar] [CrossRef]
- Wagner, H.-J.; Barthel, J.; Pilgrim, C. Permeability of the External Glial Limiting Membrane of Rat Parietal Cortex. Anat. Embryol. 1983, 166, 427–437. [Google Scholar] [CrossRef]
- Rite, I.; Machado, A.; Cano, J.; Venero, J.L. Intracerebral VEGF Injection Highly Upregulates AQP4 mRNA and Protein in the Perivascular Space and Glia Limitans Extema. Neurochem. Int. 2008, 52, 897–903. [Google Scholar] [CrossRef]
- Hoddevik, E.H.; Khan, F.H.; Rahmani, S.; Ottersen, O.P.; Boldt, H.B.; Amiry-Moghaddam, M. Factors Determining the Density of AQP4 Water Channel Molecules at the Brain–Blood Interface. Brain Struct. Funct. 2017, 222, 1753–1766. [Google Scholar] [CrossRef]
- Markou, A.; Kitchen, P.; Aldabbagh, A.; Repici, M.; Salman, M.M.; Bill, R.M.; Balklava, Z. Mechanisms of Aquaporin-4 Vesicular Trafficking in Mammalian Cells. J. Neurochem. 2024, 168, 100–114. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The Anatomy and Immunology of Vasculature in the Central Nervous System. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Pannasch, U.; Rouach, N. Emerging Role for Astroglial Networks in Information Processing: From Synapse to Behavior. Trends Neurosci. 2013, 36, 405–417. [Google Scholar] [CrossRef]
- Dong, A.; Liu, S.; Li, Y. Gap Junctions in the Nervous System: Probing Functional Connections Using New Imaging Approaches. Front. Cell. Neurosci. 2018, 12, 320. [Google Scholar] [CrossRef]
- Weber, P.A.; Chang, H.-C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The Permeability of Gap Junction Channels to Probes of Different Size Is Dependent on Connexin Composition and Permeant-Pore Affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- Metz, A.E.; Yau, H.-J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and Functional Reorganization of Rat Medial Prefrontal Cortex in Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef]
- Fiore, N.T.; Austin, P.J. Peripheral Nerve Injury Triggers Neuroinflammation in the Medial Prefrontal Cortex and Ventral Hippocampus in a Subgroup of Rats with Coincident Affective Behavioural Changes. Neuroscience 2019, 416, 147–167. [Google Scholar] [CrossRef]
- Kummer, K.K.; Mitrić, M.; Kalpachidou, T.; Kress, M. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. Int. J. Mol. Sci. 2020, 21, 3440. [Google Scholar] [CrossRef]
- Chen, G.; Park, C.-K.; Xie, R.-G.; Berta, T.; Nedergaard, M.; Ji, R.-R. Connexin-43 Induces Chemokine Release from Spinal Cord Astrocytes to Maintain Late-Phase Neuropathic Pain in Mice. Brain 2014, 137, 2193–2209. [Google Scholar] [CrossRef]
- Chen, F.-L.; Dong, Y.-L.; Zhang, Z.-J.; Cao, D.-L.; Xu, J.; Hui, J.; Zhu, L.; Gao, Y.-J. Activation of Astrocytes in the Anterior Cingulate Cortex Contributes to the Affective Component of Pain in an Inflammatory Pain Model. Brain Res. Bull. 2012, 87, 60–66. [Google Scholar] [CrossRef]
- Doyen, P.J.; Vergouts, M.; Pochet, A.; Desmet, N.; van Neerven, S.; Brook, G.; Hermans, E. Inflammation-Associated Regulation of RGS in Astrocytes and Putative Implication in Neuropathic Pain. J. Neuroinflamm. 2017, 14, 209. [Google Scholar] [CrossRef]
- Morioka, N.; Nakamura, Y.; Zhang, F.F.; Hisaoka-Nakashima, K.; Nakata, Y. Role of Connexins in Chronic Pain and Their Potential as Therapeutic Targets for Next-Generation Analgesics. Biol. Pharm. Bull. 2019, 42, 857–866. [Google Scholar] [CrossRef]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Ye, Z.-C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional Hemichannels in Astrocytes: A Novel Mechanism of Glutamate Release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef]
- Bennett, M.V.L.; Contreras, J.E.; Bukauskas, F.F.; Sáez, J.C. New Roles for Astrocytes: Gap Junction Hemichannels Have Something to Communicate. Trends Neurosci. 2003, 26, 610–617. [Google Scholar] [CrossRef]
- Giaume, C.; Leybaert, L.; Naus, C.C.; Saez, J.C. Connexin and Pannexin Hernichannels in Brain Glial Cells: Properties, Pharmacology, and Roles. Front. Pharmacol. 2013, 4, 88. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Wang, Z.-Z.; Chen, N.-H. Connexin 43: An Interface Connecting Neuroinflammation to Depression. Molecules 2023, 28, 1820. [Google Scholar] [CrossRef]
- Dubový, P. Wallerian Degeneration and Peripheral Nerve Conditions for Both Axonal Regeneration and Neuropathic Pain Induction. Ann. Anat.-Anat. Anz. 2011, 193, 267–275. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian Degeneration: Gaining Perspective on Inflammatory Events after Peripheral Nerve Injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporin Water Channels and Endothelial Cell Function. J. Anat. 2002, 200, 617–627. [Google Scholar] [CrossRef]
- Manley, G.T.; Binder, D.K.; Papadopoulos, M.C.; Verkman, A.S. New Insights into Water Transport and Edema in the Central Nervous System from Phenotype Analysis of Aquaporin-4 Null Mice. Neuroscience 2004, 129, 983–991. [Google Scholar] [CrossRef]
- Vindedal, G.F.; Thoren, A.E.; Jensen, V.; Klungland, A.; Zhang, Y.; Holtzman, M.J.; Ottersen, O.P.; Nagelhus, E.A. Removal of Aquaporin-4 from Glial and Ependymal Membranes Causes Brain Water Accumulation. Mol. Cell. Neurosci. 2016, 77, 47–52. [Google Scholar] [CrossRef]
- Nesic, O.; Lee, J.; Ye, Z.; Unabia, G.C.; Rafati, D.; Hulsebosch, C.E.; Perez-Polo, J.R. Acute and Chronic Changes in Aquaporin 4 Expression after Spinal Cord Injury. Neuroscience 2006, 143, 779–792. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Sessle, B.J.; Dostrovsky, J.O. Role of Astrocytes in Pain. Neurochem. Res. 2012, 37, 2419–2431. [Google Scholar] [CrossRef]
- Bojarskaite, L.; Nafari, S.; Ravnanger, A.K.; Frey, M.M.; Skauli, N.; Åbjørsbråten, K.S.; Roth, L.C.; Amiry-Moghaddam, M.; Nagelhus, E.A.; Ottersen, O.P.; et al. Role of Aquaporin-4 Polarization in Extracellular Solute Clearance. Fluids Barriers CNS 2024, 21, 28. [Google Scholar] [CrossRef]
- In ’T Veen, J.P.M.; Van Den Berg, M.P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W.H.M. Uptake of Fluorescein Isothiocyanate-Labelled Dextran into the CSF after Intranasal and Intravenous Administration to Rats. Eur. J. Pharma. Biopharma. 2005, 61, 27–31. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Functional Analysis of Tight Junctions. Methods 2003, 30, 228–234. [Google Scholar] [CrossRef]
- Lippoldt, A.; Liebner, S.; Andbjer, B.; Kalbacher, H.; Wolburg, H.; Haller, H.; Fuxe, K. Organization of Choroid Plexus Epithelial and Endothelial Cell Tight Junctions and Regulation of Claudin-1, -2 and -5 Expression by Protein Kinase C. NeuroReport 2000, 11, 1427. [Google Scholar] [CrossRef]
- Kratzer, I.; Ek, J.; Stolp, H. The Molecular Anatomy and Functions of the Choroid Plexus in Healthy and Diseased Brain. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183430. [Google Scholar] [CrossRef]
- Katoozi, S.; Skauli, N.; Zahl, S.; Deshpande, T.; Ezan, P.; Palazzo, C.; Steinhäuser, C.; Frigeri, A.; Cohen-Salmon, M.; Ottersen, O.P.; et al. Uncoupling of the Astrocyte Syncytium Differentially Affects AQP4 Isoforms. Cells 2020, 9, 382. [Google Scholar] [CrossRef]
- Rao, S.B.; Skauli, N.; Jovanovic, N.; Katoozi, S.; Frigeri, A.; Froehner, S.C.; Adams, M.E.; Ottersen, O.P.; Amiry-Moghaddam, M. Orchestrating Aquaporin-4 and Connexin-43 Expression in Brain: Differential Roles of A1- and Β1-Syntrophin. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183616. [Google Scholar] [CrossRef]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical Astrocytes Rewire Somatosensory Cortical Circuits for Peripheral Neuropathic Pain. J. Clin. Investig. 2016, 126, 1983. [Google Scholar] [CrossRef]
- Kurabe, M.; Sasaki, M.; Furutani, K.; Furue, H.; Kamiya, Y.; Baba, H. Structural and Functional Properties of Spinal Dorsal Horn Neurons after Peripheral Nerve Injury Change Overtime via Astrocyte Activation. iScience 2022, 25, 105555. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of Neuropathology-Associated Reactive Astrocytes: A Systematic Review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Jean-Toussaint, R.; Tian, Y.; Chaudhuri, A.D.; Haughey, N.J.; Sacan, A.; Ajit, S.K. Proteome Characterization of Small Extracellular Vesicles from Spared Nerve Injury Model of Neuropathic Pain. J. Proteom. 2020, 211, 103540. [Google Scholar] [CrossRef]
- Luo, X.; Jean-Toussaint, R.; Tian, Y.; Balashov, S.V.; Sacan, A.; Ajit, S.K. Small Extracellular Vesicles From Spared Nerve Injury Model and Sham Control Mice Differentially Regulate Gene Expression in Primary Microglia. J. Pain 2023, 24, 1570–1581. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, J.; Liu, C.; Gan, L.; Chen, H.; Sun, Y.-L.; Liu, J.; Tao, Y.-X.; Zhu, T.; Chen, C. Schwann Cell-Derived Extracellular Vesicles Promote Memory Impairment Associated with Chronic Neuropathic Pain. J. Neuroinflamm. 2024, 21, 99. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Szalad, A.; Liu, X.S.; Zhang, Y.; Wang, X.; Golembieski, W.A.; Powell, B.; Mccann, M.; Lu, M.; et al. Schwann Cell-Derived Exosomes Ameliorate Peripheral Neuropathy Induced by Ablation of Dicer in Schwann Cells. Front. Cell. Neurosci. 2024, 18, 1462228. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, R.; Zhou, R.; Chen, H.; Liu, C.; Zhu, T.; Chen, C. The Emerging Power and Promise of Non-Coding RNAs in Chronic Pain. Front. Mol. Neurosci. 2022, 15, 1037929. [Google Scholar] [CrossRef]
- He, X.; Yang, H.; Zheng, Y.; Zhao, X.; Wang, T. The Role of Non-Coding RNAs in Neuropathic Pain. Pflugers Arch.-Eur. J. Physiol. 2024, 476, 1625–1643. [Google Scholar] [CrossRef]
- Jullienne, A.; Fukuda, A.M.; Ichkova, A.; Nishiyama, N.; Aussudre, J.; Obenaus, A.; Badaut, J. Modulating the Water Channel AQP4 Alters miRNA Expression, Astrocyte Connectivity and Water Diffusion in the Rodent Brain. Sci. Rep. 2018, 8, 4186. [Google Scholar] [CrossRef]
- Xian, S.; Ding, R.; Li, M.; Chen, F. LncRNA NEAT1/miR-128-3p/AQP4 Axis Regulating Spinal Cord Injury-Induced Neuropathic Pain Progression. J. Neuroimmunol. 2021, 351, 577457. [Google Scholar] [CrossRef]
- Neumann, E.; Hermanns, H.; Barthel, F.; Werdehausen, R.; Brandenburger, T. Expression Changes of MicroRNA-1 and Its Targets Connexin 43 and Brain-Derived Neurotrophic Factor in the Peripheral Nervous System of Chronic Neuropathic Rats. Mol. Pain 2015, 11, 39. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, S.; Dai, J.; Zheng, H.; Peng, X.; Cheng, L.; Chen, H.; Hu, Y. MiR-23b-3p Improves Brain Damage after Status Epilepticus by Reducing the Formation of Pathological High-Frequency Oscillations via Inhibition of Cx43 in Rat Hippocampus. ACS Chem. Neurosci. 2024, 15, 2633–2642. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Zamboni, L.; Demartin, C. Buffered Picric Acid-Formaldehyde—A New Rapid Fixative for Electron Microscopy. J. Cell Biol. 1967, 35, A148. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates. J. Anat. 1997, 191, 315–317. [Google Scholar] [CrossRef]
- Hylden, J.L.K.; Wilcox, G.L. Intrathecal Morphine in Mice: A New Technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Dubový, P.; Svízenská, I.; Klusáková, I. Computer-Assisted Quantitative Analysis of Immunofluorescence Staining of the Extracellular Matrix in Rat Dorsal and Ventral Spinal Roots. Acta Histochem. 2002, 104, 371–374. [Google Scholar] [CrossRef]



| Protein | Antibody | Source | Product | City, Country | Cat. No | Dilution |
|---|---|---|---|---|---|---|
| Cx43 | mAb * | mouse | Millipore | Burlington, USA | MAV3067 | 1:100 |
| AQP4 | pAb * | rabbit | Cell Signaling | Danvers, USA | 59678 | 1:800 |
| GFAP | pAb * | rabbit | Dako | Santa Clara, USA | Z 0334 | 1:250 |
| GFAP | pAb * | chicken | Abcam | Cambridge, UK | ab4674 | 1:500 |
| Secondary antibody | ||||||
| Anti-mouse-A647 | pAb * | goat | Jackson | Ely, UK | 115-605-146 | 1:100 |
| Anti-rabbit-A549 | pAb * | goat | Abcam | Cambridge, UK | ab96900 | 1:100 |
| Anti-rabbit-FITC | pAb * | goat | Jackson | Ely, UK | 111-095-144 | 1:100 |
| Anti-chicken-A488 | pAb * | goat | Abcam | Cambridge, UK | ab150173 | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bretová, K.; Svobodová, V.; Dubový, P. Changes in Cx43 and AQP4 Proteins, and the Capture of 3 kDa Dextran in Subpial Astrocytes of the Rat Medial Prefrontal Cortex after Both Sham Surgery and Sciatic Nerve Injury. Int. J. Mol. Sci. 2024, 25, 10989. https://doi.org/10.3390/ijms252010989
Bretová K, Svobodová V, Dubový P. Changes in Cx43 and AQP4 Proteins, and the Capture of 3 kDa Dextran in Subpial Astrocytes of the Rat Medial Prefrontal Cortex after Both Sham Surgery and Sciatic Nerve Injury. International Journal of Molecular Sciences. 2024; 25(20):10989. https://doi.org/10.3390/ijms252010989
Chicago/Turabian StyleBretová, Karolína, Viktorie Svobodová, and Petr Dubový. 2024. "Changes in Cx43 and AQP4 Proteins, and the Capture of 3 kDa Dextran in Subpial Astrocytes of the Rat Medial Prefrontal Cortex after Both Sham Surgery and Sciatic Nerve Injury" International Journal of Molecular Sciences 25, no. 20: 10989. https://doi.org/10.3390/ijms252010989
APA StyleBretová, K., Svobodová, V., & Dubový, P. (2024). Changes in Cx43 and AQP4 Proteins, and the Capture of 3 kDa Dextran in Subpial Astrocytes of the Rat Medial Prefrontal Cortex after Both Sham Surgery and Sciatic Nerve Injury. International Journal of Molecular Sciences, 25(20), 10989. https://doi.org/10.3390/ijms252010989







