The Implications of Aging on Vascular Health
Abstract
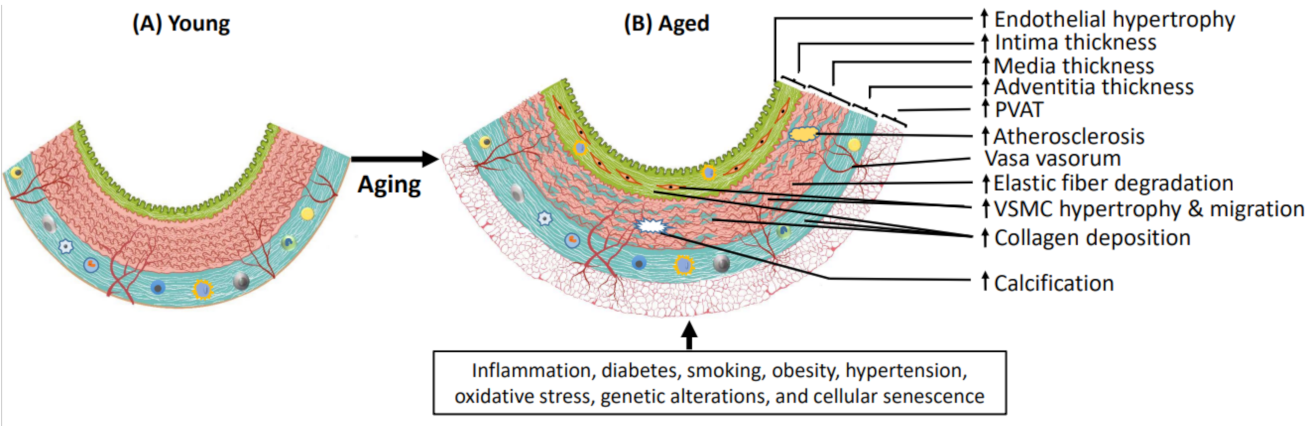
1. Introduction
2. Molecular and Cellular Changes in Aging Arteries
2.1. Endothelial Dysfunction
2.2. Vascular Smooth Muscle Cell (VSMC) Dysfunction
2.3. Cellular Senescence
2.4. Calcification
2.5. Adventitial Layer Remodeling
2.6. Perivascular Adipose Tissue Deposition
3. Structural Changes in Aging Arteries
3.1. Arterial Wall Thickness
3.2. Arterial Diameter
3.3. Arterial Stiffness
3.4. Rarefaction of Collaterals
4. Therapeutics and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber, T.; Mayer, C.C. “Man Is as Old as His Arteries” Taken Literally: In Search of the Best Metric. Hypertension 2020, 76, 1425–1427. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar]
- Mocumbi, A.O. Cardiovascular Health Care in Low- and Middle-Income Countries. Circulation 2024, 149, 557–559. [Google Scholar]
- Zhao, D.; Wang, Y.; Wong, N.D.; Wang, J. Impact of Aging on Cardiovascular Diseases: From Chronological Observation to Biological Insights: JACC Family Series. JACC Asia 2024, 4, 345–358. [Google Scholar] [CrossRef]
- Rich, M.W.; Chyun, D.A.; Skolnick, A.H.; Alexander, K.P.; Forman, D.E.; Kitzman, D.W.; Maurer, M.S.; McClurken, J.B.; Resnick, B.M.; Shen, W.K.; et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population. Circulation 2016, 133, 2103–2122. [Google Scholar]
- Tirziu, D.; Moodie, K.L.; Zhuang, Z.W.; Singer, K.; Helisch, A.; Dunn, J.F.; Li, W.; Singh, J.; Simons, M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation 2005, 112, 2501–2509. [Google Scholar]
- Ambrose, C.T. Pro-Angiogenesis Therapy and Aging: A Mini-Review. Gerontology 2017, 63, 393–400. [Google Scholar] [CrossRef]
- Harris, J.R.; Korolchuk, V.I. (Eds.) Biochemistry and Cell Biology of Ageing: Part IV, Clinical Science; Springer: Berlin/Heidelberg, Germany, 2023; Volume 103. [Google Scholar]
- Zhang, C.; Tao, J. Cardiovascular Group, Society of Geriatrics, Chinese Medical Association. Expert consensus on clinical assessment and intervention of vascular aging in China (2018). Aging Med. 2018, 1, 228–237. [Google Scholar]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging Causes Collateral Rarefaction and Increased Severity of Ischemic Injury in Multiple Tissues. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef]
- Sadoun, E.; Reed, M.J. Impaired Angiogenesis in Aging Is Associated with Alterations in Vessel Density, Matrix Composition, Inflammatory Response, and Growth Factor Expression. J. Histochem. Cytochem. 2003, 51, 1119–1130. [Google Scholar]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar]
- Lakatta, E.G.; Levy, D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises. Circulation 2003, 107, 139–146. [Google Scholar]
- Kohn, J.C.; Zhou, D.W.; Bordeleau, F.; Zhou, A.L.; Mason, B.N.; Mitchell, M.J.; King, M.R.; Reinhart-King, C.A. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 2015, 108, 471–478. [Google Scholar]
- Luttrell, M.; Kim, H.; Shin, S.Y.; Holly, D.; Massett, M.P.; Woodman, C.R. Heterogeneous effect of aging on vasorelaxation responses in large and small arteries. Physiol. Rep. 2020, 8, e14341. [Google Scholar]
- Barton, M.; Cosentino, F.; Brandes, R.P.; Moreau, P.; Shaw, S.; Lüscher, T.F. Anatomic Heterogeneity of Vascular Aging. Hypertension 1997, 30, 817–824. [Google Scholar]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Califano, J.P.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011, 3, 112ra122. [Google Scholar] [CrossRef]
- Cavallaro, U.; Castelli, V.; Del Monte, U.; Soria, M.R. Phenotypic alterations in senescent large-vessel and microvascular endothelial cells. Mol. Cell Biol. Res. Commun. 2000, 4, 117–121. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Kaun, C.; Buchberger, E.; Ebenbauer, B.; Demyanets, S.; Huk, I.; Eppel, W.; Maurer, G.; Huber, K.; Wojta, J. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochim. Biophys. Acta 2016, 1863, 360–367. [Google Scholar] [CrossRef]
- Bloom, S.I.; Tucker, J.R.; Lim, J.; Thomas, T.G.; Stoddard, G.J.; Lesniewski, L.A.; Donato, A.J. Aging results in DNA damage and telomere dysfunction that is greater in endothelial versus vascular smooth muscle cells and is exacerbated in atheroprone regions. GeroScience 2022, 44, 2741–2755. [Google Scholar] [CrossRef]
- Wang, M.; Lakatta, E.G. Central arterial aging: Humans to molecules. In Handbook of Hypertension: Arterial Stiffness in Hypertension; Elsevier: New York, NY, USA, 2006; pp. 137–160. [Google Scholar]
- Asai, K.; Kudej, R.K.; Shen, Y.T.; Yang, G.P.; Takagi, G.; Kudej, A.B.; Geng, Y.J.; Sato, N.; Nazareno, J.B.; Vatner, D.E.; et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1493–1499. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Henson, G.D.; Thorburn, A.; Sindler, A.L.; Pierce, G.L.; Seals, D.R. Translational evidence that impaired autophagy contributes to arterial ageing. J. Physiol. 2012, 590, 3305–3316. [Google Scholar] [CrossRef]
- Vion, A.C.; Kheloufi, M.; Hammoutene, A.; Poisson, J.; Lasselin, J.; Devue, C.; Pic, I.; Dupont, N.; Busse, J.; Stark, K.; et al. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc. Natl. Acad. Sci. USA 2017, 114, E8675–E8684. [Google Scholar] [CrossRef]
- Signori, D.; Magliocca, A.; Hayashida, K.; Graw, J.A.; Malhotra, R.; Bellani, G.; Berra, L.; Rezoagli, E. Inhaled nitric oxide: Role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med. Exp. 2022, 10, 28. [Google Scholar] [CrossRef]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Wang, M.; Khazan, B.; Lakatta, E.G. Central Arterial Aging and Angiotensin II Signaling. Curr. Hypertens. Rev. 2010, 6, 266–281. [Google Scholar] [CrossRef]
- Edelberg, J.M.; Tang, L.; Hattori, K.; Lyden, D.; Rafii, S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ. Res. 2002, 90, E89–E93. [Google Scholar] [CrossRef]
- Zhu, G.; Song, M.; Wang, H.; Zhao, G.; Yu, Z.; Yin, Y.; Zhao, X.; Huang, L. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: Involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann. Vasc. Surg. 2009, 23, 519–534. [Google Scholar] [CrossRef]
- Giallauria, F.; Vigorito, C.; Ferrara, N.; Ferrucci, L. Cardiovascular Calcifications in Old Age: Mechanisms and Clinical Implications. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 255–267. [Google Scholar] [CrossRef][Green Version]
- Monk, B.A.; George, S.J. The Effect of Ageing on Vascular Smooth Muscle Cell Behaviour—A Mini-Review. Gerontology 2014, 61, 416–426. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Jiang, L.Q.; Spinetti, G.; Pintus, G.; Monticone, R.; Kolodgie, F.D.; Virmani, R.; Lakatta, E.G. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007, 50, 219–227. [Google Scholar] [CrossRef]
- Ribeiro-Silva, J.C.; Nolasco, P.; Krieger, J.E.; Miyakawa, A.A. Dynamic Crosstalk between Vascular Smooth Muscle Cells and the Aged Extracellular Matrix. Int. J. Mol. Sci. 2021, 22, 10175. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular Stiffness in Aging and Disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef]
- Zha, Y.; Zhuang, W.; Yang, Y.; Zhou, Y.; Li, H.; Liang, J. Senescence in Vascular Smooth Muscle Cells and Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 910580. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Monticone, R.E.; Telljohann, R.; Wu, J.; Wang, M.; Lakatta, E.G. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 2012, 60, 1192–1199. [Google Scholar] [CrossRef]
- Lino Cardenas, C.L.; Jiang, W.; Kajuluri, L.P.; Singh, K.; Ostrom, K.; Li, R.; Cherbonneau, F.; Boerboom, S.; Birchenough, C.; Roh, K.; et al. Treatment of calcific arterial disease via enhancement of autophagy using GSK343. iScience 2023, 26, 108360. [Google Scholar] [CrossRef]
- Osonoi, Y.; Mita, T.; Azuma, K.; Nakajima, K.; Masuyama, A.; Goto, H.; Nishida, Y.; Miyatsuka, T.; Fujitani, Y.; Koike, M.; et al. Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis. Autophagy 2018, 14, 1991–2006. [Google Scholar]
- Abdellatif, M.; Rainer, P.P.; Sedej, S.; Kroemer, G. Hallmarks of cardiovascular ageing. Nat. Rev. Cardiol. 2023, 20, 754–777. [Google Scholar]
- Jiang, L.; Wang, M.; Zhang, J.; Monticone, R.E.; Telljohann, R.; Spinetti, G.; Pintus, G.; Lakatta, E.G. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE 2008, 3, e2231. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: Basic biology and clinical relevance. Nat. Rev. Cardiol. 2022, 19, 250–264. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar]
- de Magalhães, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar]
- Kumar, M.; Yan, P.; Kuchel, G.A.; Xu, M. Cellular Senescence as a Targetable Risk Factor for Cardiovascular Diseases: Therapeutic Implications: JACC Family Series. JACC Basic Transl. Sci. 2024, 9, 522–534. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Han, X.; Liang, F.; Zhang, Q.; Huang, X.; Shi, X.; Huo, H.; Han, M.; Liu, X.; et al. Dynamics of Endothelial Cell Generation and Turnover in Arteries During Homeostasis and Diseases. Circulation 2024, 149, 135–154. [Google Scholar]
- Foreman, K.E.; Tang, J. Molecular mechanisms of replicative senescence in endothelial cells. Exp. Gerontol. 2003, 38, 1251–1257. [Google Scholar] [CrossRef]
- Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar]
- Hwang, H.V.; Lin, Y.; Rebuffatti, M.N.; Tran, D.T.; Lee, L.; Gomes, A.V.; Li, C.S.; Knowlton, A.A. Impaired proteostasis in senescent vascular endothelial cells: A perspective on estrogen and oxidative stress in the aging vasculature. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H421–H429. [Google Scholar] [CrossRef]
- Gates, P.E.; Strain, W.D.; Shore, A.C. Human endothelial function and microvascular ageing. Exp. Physiol. 2009, 94, 311–316. [Google Scholar]
- Sato, I.; Morita, I.; Kaji, K.; Ikeda, M.; Nagao, M.; Murota, S. Reduction of Nitric Oxide Producing Activity Associated with in Vitro Aging in Cultured Human Umbilical Vein Endothelial Cell. Biochem. Biophys. Res. Commun. 1993, 195, 1070–1076. [Google Scholar]
- Holdt, L.M.; Sass, K.; Gäbel, G.; Bergert, H.; Thiery, J.; Teupser, D. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis 2011, 214, 264–270. [Google Scholar] [CrossRef]
- Graves, S.I.; Baker, D.J. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin. Pharmacol. Toxicol. 2020, 127, 102–110. [Google Scholar]
- Stojanović, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-induced inflammation: An important player and key therapeutic target in atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular Smooth Muscle Cells Undergo Telomere-Based Senescence in Human Atherosclerosis. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef]
- Ragnauth, C.D.; Warren, D.T.; Liu, Y.; McNair, R.; Tajsic, T.; Figg, N.; Shroff, R.; Skepper, J.; Shanahan, C.M. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 2010, 121, 2200–2210. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Hoffmann, J.; Shmeleva, E.; Zglinicki, T.v.; Richardson, G.; Draganova, L.; Redgrave, R.; Collerton, J.; Arthur, H.; Keavney, B.; et al. CMV-independent increase in CD27−CD28+ CD8+ EMRA T cells is inversely related to mortality in octogenarians. NPJ Aging Mech. Dis. 2020, 6, 3. [Google Scholar]
- Nakajima, T.; Schulte, S.; Warrington, K.J.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. T-Cell–Mediated Lysis of Endothelial Cells in Acute Coronary Syndromes. Circulation 2002, 105, 570–575. [Google Scholar]
- Shimizu, I.; Minamino, T. Cellular senescence in cardiac diseases. J. Cardiol. 2019, 74, 313–319. [Google Scholar] [CrossRef]
- McClelland, R.L.; Chung, H.; Detrano, R.; Post, W.; Kronmal, R.A. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006, 113, 30–37. [Google Scholar] [CrossRef]
- Newman, A.B.; Naydeck, B.L.; Sutton-Tyrrell, K.; Feldman, A.; Edmundowicz, D.; Kuller, L.H. Coronary Artery Calcification in Older Adults to Age 99. Circulation 2001, 104, 2679–2684. [Google Scholar] [CrossRef]
- Guo, J.; Fujiyoshi, A.; Willcox, B.; Choo, J.; Vishnu, A.; Hisamatsu, T.; Ahuja, V.; Takashima, N.; Barinas-Mitchell, E.; Kadota, A.; et al. Increased Aortic Calcification Is Associated with Arterial Stiffness Progression in Multiethnic Middle-Aged Men. Hypertension 2017, 69, 102–108. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification-New Insights Into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Malhotra, R.; Mauer, A.C.; Lino Cardenas, C.L.; Guo, X.; Yao, J.; Zhang, X.; Wunderer, F.; Smith, A.V.; Wong, Q.; Pechlivanis, S.; et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat. Genet. 2019, 51, 1580–1587. [Google Scholar]
- de Vries, P.S.; Conomos, M.P.; Singh, K.; Nicholson, C.J.; Jain, D.; Hasbani, N.R.; Jiang, W.; Lee, S.; Lino Cardenas, C.L.; Lutz, S.M.; et al. Whole-genome sequencing uncovers two loci for coronary artery calcification and identifies ARSE as a regulator of vascular calcification. Nat. Cardiovasc. Res. 2023, 2, 1159–1172. [Google Scholar]
- Derwall, M.; Malhotra, R.; Lai, C.S.; Beppu, Y.; Aikawa, E.; Seehra, J.S.; Zapol, W.M.; Bloch, K.D.; Yu, P.B. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 613–622. [Google Scholar]
- Malhotra, R.; Burke, M.F.; Martyn, T.; Shakartzi, H.R.; Thayer, T.E.; O’Rourke, C.; Li, P.; Derwall, M.; Spagnolli, E.; Kolodziej, S.A.; et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS ONE 2015, 10, e0117098. [Google Scholar]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar]
- Liu, Y.; Drozdov, I.; Shroff, R.; Beltran, L.E.; Shanahan, C.M. Prelamin A Accelerates Vascular Calcification via Activation of the DNA Damage Response and Senescence-Associated Secretory Phenotype in Vascular Smooth Muscle Cells. Circ. Res. 2013, 112, e99–e109. [Google Scholar]
- Sutton, N.R.; Malhotra, R.; St Hilaire, C.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef]
- Singh, A.; Tandon, S.; Tandon, C. An update on vascular calcification and potential therapeutics. Mol. Biol. Rep. 2021, 48, 887–896. [Google Scholar] [CrossRef]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Shroff, R.C.; McNair, R.; Figg, N.; Skepper, J.N.; Schurgers, L.; Gupta, A.; Hiorns, M.; Donald, A.E.; Deanfield, J.; Rees, L.; et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008, 118, 1748–1757. [Google Scholar] [CrossRef]
- Kim, S.H.; Monticone, R.E.; McGraw, K.R.; Wang, M. Age-associated proinflammatory elastic fiber remodeling in large arteries. Mech. Ageing Dev. 2021, 196, 111490. [Google Scholar] [CrossRef]
- Owens, D.S.; Katz, R.; Takasu, J.; Kronmal, R.; Budoff, M.J.; O’Brien, K.D. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am. J. Cardiol. 2010, 105, 701–708. [Google Scholar]
- Leopold, J.A. Cellular Mechanisms of Aortic Valve Calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, X.Y.; Wang, L.J.; Wong, K.S. Intracranial Artery Calcification and Its Clinical Significance. J. Clin. Neurol. 2016, 12, 253–261. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Rumberger, J.A.; Severson, A.; Edwards, W.D.; Gregoire, J.; Fitzpatrick, L.A.; Schwartz, R.S. Arterial Calcification and Not Lumen Stenosis Is Highly Correlated with Atherosclerotic Plaque Burden in Humans: A Histologic Study of 723 Coronary Artery Segments Using Nondecalcifying Methodology. J. Am. Coll. Cardiol. 1998, 31, 126–133. [Google Scholar] [CrossRef]
- Tesauro, M.; Mauriello, A.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Cardillo, C.; Melino, G.; Di Daniele, N. Arterial ageing: From endothelial dysfunction to vascular calcification. J. Intern. Med. 2017, 281, 471–482. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar]
- Arad, Y.; Goodman, K.J.; Roth, M.; Newstein, D.; Guerci, A.D. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: The St. Francis Heart Study. J. Am. Coll. Cardiol. 2005, 46, 158–165. [Google Scholar] [CrossRef]
- Tota-Maharaj, R.; Blaha, M.J.; Rivera, J.J.; Henry, T.S.; Choi, E.K.; Chang, S.A.; Yoon, Y.E.; Chun, E.J.; Choi, S.I.; Blumenthal, R.S.; et al. Differences in coronary plaque composition with aging measured by coronary computed tomography angiography. Int. J. Cardiol. 2012, 158, 240–245. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Allison, M.A.; Criqui, M.H.; Wright, C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 331–336. [Google Scholar] [CrossRef]
- Kwee, R.M. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J. Vasc. Surg. 2010, 51, 1015–1025. [Google Scholar] [CrossRef]
- Wendorff, C.; Wendorff, H.; Pelisek, J.; Tsantilas, P.; Zimmermann, A.; Zernecke, A.; Kuehnl, A.; Eckstein, H.H. Carotid Plaque Morphology Is Significantly Associated With Sex, Age, and History of Neurological Symptoms. Stroke 2015, 46, 3213–3219. [Google Scholar]
- Vos, A.; Kockelkoren, R.; de Vis, J.B.; van der Schouw, Y.T.; van der Schaaf, I.C.; Velthuis, B.K.; Mali, W.P.T.M.; de Jong, P.A.; Majoie, C.B.; Roos, Y.B.; et al. Risk factors for atherosclerotic and medial arterial calcification of the intracranial internal carotid artery. Atherosclerosis 2018, 276, 44–49. [Google Scholar] [CrossRef]
- Blaha, M.J.; Budoff, M.J.; Rivera, J.J.; Katz, R.; O’Leary, D.H.; Polak, J.F.; Takasu, J.; Blumenthal, R.S.; Nasir, K. Relationship of carotid distensibility and thoracic aorta calcification: Multi-ethnic study of atherosclerosis. Hypertension 2009, 54, 1408–1415. [Google Scholar]
- Holzapfel, G.A.; Ogden, R.W. Biomechanical relevance of the microstructure in artery walls with a focus on passive and active components. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H540–H549. [Google Scholar]
- Stenmark, K.R.; Yeager, M.E.; Kasmi, K.C.E.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The Adventitia: Essential Regulator of Vascular Wall Structure and Function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Fleenor, B.S.; Marshall, K.D.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: Reversal by aerobic exercise. J. Physiol. 2010, 588 Pt 20, 3971–3982. [Google Scholar]
- Rademakers, T.; Douma, K.; Hackeng, T.M.; Post, M.J.; Sluimer, J.C.; Daemen, M.J.A.P.; Biessen, E.A.L.; Heeneman, S.; Zandvoort, M.A.M.J.v. Plaque-Associated Vasa Vasorum in Aged Apolipoprotein E–Deficient Mice Exhibit Proatherogenic Functional Features In Vivo. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 249–256. [Google Scholar]
- Urabe, G.; Hoshina, K.; Shimanuki, T.; Nishimori, Y.; Miyata, T.; Deguchi, J. Structural analysis of adventitial collagen to feature aging and aneurysm formation in human aorta. J. Vasc. Surg. 2016, 63, 1341–1350. [Google Scholar] [CrossRef]
- Gräbner, R.; Lötzer, K.; Döpping, S.; Hildner, M.; Radke, D.; Beer, M.; Spanbroek, R.; Lippert, B.; Reardon, C.A.; Getz, G.S.; et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J. Exp. Med. 2009, 206, 233–248. [Google Scholar]
- Moos, M.P.; John, N.; Gräbner, R.; Nossmann, S.; Günther, B.; Vollandt, R.; Funk, C.D.; Kaiser, B.; Habenicht, A.J. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2386–2391. [Google Scholar] [CrossRef]
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar]
- Gollasch, M. Adipose-Vascular Coupling and Potential Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 417–436. [Google Scholar]
- Saxton, S.N.; Ryding, K.E.; Aldous, R.G.; Withers, S.B.; Ohanian, J.; Heagerty, A.M. Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 880–891. [Google Scholar]
- Costa, R.M.; Neves, K.B.; Tostes, R.C.; Lobato, N.S. Perivascular Adipose Tissue as a Relevant Fat Depot for Cardiovascular Risk in Obesity. Front. Physiol. 2018, 9, 253. [Google Scholar]
- Ahmadieh, S.; Kim, H.W.; Weintraub, N.L. Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin. Sci. 2020, 13, 3–13. [Google Scholar]
- Fleenor, B.S.; Carlini, N.A.; Ouyang, A.; Du, B.; Harber, M.P. Greater aortic perivascular adipose tissue density is associated with aging, aortic stiffness, and central blood pressure in humans. J. Appl. Physiol. 2023, 134, 703–709. [Google Scholar]
- Fernández-Alfonso, M.S.; Somoza, B.; Tsvetkov, D.; Kuczmanski, A.; Dashwood, M.; Gil-Ortega, M. Role of Perivascular Adipose Tissue in Health and Disease. Compr. Physiol. 2011, 8, 23–59. [Google Scholar]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar]
- Britton, K.A.; Wang, N.; Palmisano, J.; Corsini, E.; Schlett, C.L.; Hoffmann, U.; Larson, M.G.; Vasan, R.S.; Vita, J.A.; Mitchell, G.F.; et al. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity 2013, 21, 1496–1503. [Google Scholar]
- Lehman, S.J.; Massaro, J.M.; Schlett, C.L.; O’Donnell, C.J.; Hoffmann, U.; Fox, C.S. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The Framingham Heart Study. Atherosclerosis 2010, 210, 656–661. [Google Scholar]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar]
- Shi, K.L.; Qi, L.; Mao, D.B.; Chen, Y.; Qian, J.Y.; Sun, Y.B.; Guo, X.G. Impact of age on epicardial and pericoronary adipose tissue volume. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3257–3265. [Google Scholar]
- Waddell, H.M.M.; Moore, M.K.; Herbert-Olsen, M.A.; Stiles, M.K.; Tse, R.D.; Coffey, S.; Lamberts, R.R.; Aitken-Buck, H.M. Identifying sex differences in predictors of epicardial fat cell morphology. Adipocyte 2022, 11, 325–334. [Google Scholar]
- Ojha, S.; Fainberg, H.P.; Wilson, V.; Pelella, G.; Castellanos, M.; May, S.T.; Lotto, A.A.; Sacks, H.; Symonds, M.E.; Budge, H. Gene pathway development in human epicardial adipose tissue during early life. JCI Insight 2016, 1, e87460. [Google Scholar]
- Schütz, E.; Gogiraju, R.; Pavlaki, M.; Drosos, I.; Georgiadis, G.S.; Argyriou, C.; Rim Ben Hallou, A.; Konstantinou, F.; Mikroulis, D.; Schüler, R.; et al. Age-Dependent and -Independent Effects of Perivascular Adipose Tissue and Its Paracrine Activities during Neointima Formation. Int. J. Mol. Sci. 2019, 21, 282. [Google Scholar] [CrossRef]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar]
- Fleenor, B.S.; Carlini, N.A.; Ouyang, A.; Harber, M.P. Perivascular adipose tissue-mediated arterial stiffening in aging and disease: An emerging translational therapeutic target? Pharmacol. Res. 2022, 178, 106150. [Google Scholar]
- Ouyang, A.; Garner, T.B.; Fleenor, B.S. Hesperidin reverses perivascular adipose-mediated aortic stiffness with aging. Exp. Gerontol. 2017, 97, 68–72. [Google Scholar]
- Fleenor, B.S.; Eng, J.S.; Sindler, A.L.; Pham, B.T.; Kloor, J.D.; Seals, D.R. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 2014, 13, 576–578. [Google Scholar]
- Bailey-Downs, L.C.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging Exacerbates Obesity-Induced Oxidative Stress and Inflammation in Perivascular Adipose Tissue in Mice: A Paracrine Mechanism Contributing to Vascular Redox Dysregulation and Inflammation. J. Gerontol. Ser. A 2012, 68, 780–792. [Google Scholar]
- Pan, X.-X.; Ruan, C.-C.; Liu, X.-Y.; Kong, L.-R.; Ma, Y.; Wu, Q.-H.; Li, H.-Q.; Sun, Y.-J.; Chen, A.-Q.; Zhao, Q.; et al. Perivascular adipose tissue-derived stromal cells contribute to vascular remodeling during aging. Aging Cell 2019, 18, e12969. [Google Scholar]
- Lefranc, C.; Friederich-Persson, M.; Braud, L.; Palacios-Ramirez, R.; Karlsson, S.; Boujardine, N.; Motterlini, R.; Jaisser, F.; Cat, A.N.D. MR (Mineralocorticoid Receptor) Induces Adipose Tissue Senescence and Mitochondrial Dysfunction Leading to Vascular Dysfunction in Obesity. Hypertension 2019, 73, 458–468. [Google Scholar]
- Kong, L.-R.; Zhou, Y.-P.; Chen, D.-R.; Ruan, C.-C.; Gao, P.-J. Decrease of Perivascular Adipose Tissue Browning Is Associated with Vascular Dysfunction in Spontaneous Hypertensive Rats During Aging. Front. Physiol. 2018, 9, 400. [Google Scholar]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar]
- Barandier, C.; Montani, J.-P.; Yang, Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: Effects of aging and obesity. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H1807–H1813. [Google Scholar]
- Virmani, R.; Avolio, A.P.; Mergner, W.J.; Robinowitz, M.; Herderick, E.E.; Cornhill, J.F.; Guo, S.Y.; Liu, T.H.; Ou, D.Y.; O’Rourke, M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am. J. Pathol. 1991, 139, 1119–1129. [Google Scholar]
- Miura, K. Tunica intima compensation for reduced stiffness of the tunica media in aging renal arteries as measured with scanning acoustic microscopy. PLoS ONE 2020, 15, e0234759. [Google Scholar]
- Thijssen, D.H.; Carter, S.E.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar]
- Benetos, A.; Laurent, S.; Hoeks, A.P.; Boutouyrie, P.H.; Safar, M.E. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arterioscler. Thromb. 1993, 13, 90–97. [Google Scholar]
- van der Heijden-Spek, J.J.; Staessen, J.A.; Fagard, R.H.; Hoeks, A.P.; Boudier, H.A.; van Bortel, L.M. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: A population study. Hypertension 2000, 35, 637–642. [Google Scholar]
- Nagasawa, S.; Handa, H.; Okumura, A.; Naruo, Y.; Moritake, K.; Hayashi, K. Mechanical properties of human cerebral arteries. Part 1: Effects of age and vascular smooth muscle activation. Surg. Neurol. 1979, 12, 297–304. [Google Scholar]
- Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S. Reference intervals for common carotid intima-media thickness measured with echotracking: Relation with risk factors. Eur. Heart J. 2013, 34, 2368–2380. [Google Scholar]
- Homma, S.; Hirose, N.; Ishida, H.; Ishii, T.; Araki, G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke 2001, 32, 830–835. [Google Scholar]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; DeSouza, C.A.; Seals, D.R. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 82–87. [Google Scholar]
- van den Munckhof, I.; Scholten, R.; Cable, N.T.; Hopman, M.T.; Green, D.J.; Thijssen, D.H. Impact of age and sex on carotid and peripheral arterial wall thickness in humans. Acta Physiol. 2012, 206, 220–228. [Google Scholar]
- Bonithon-Kopp, C.; Touboul, P.J.; Berr, C.; Magne, C.; Ducimetière, P. Factors of Carotid Arterial Enlargement in a Population Aged 59 to 71 Years. Stroke 1996, 27, 654–660. [Google Scholar]
- Schmidt-Trucksäss, A.; Grathwohl, D.; Schmid, A.; Boragk, R.; Upmeier, C.; Keul, J.; Huonker, M. Structural, functional, and hemodynamic changes of the common carotid artery with age in male subjects. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1091–1097. [Google Scholar]
- Magnussen, C.G. Carotid artery intima-media thickness and hypertensive heart disease: A short review. Clin. Hypertens. 2017, 23, 7. [Google Scholar]
- Nagai, Y.; Metter, E.J.; Earley, C.J.; Kemper, M.K.; Becker, L.C.; Lakatta, E.G.; Fleg, J.L. Increased Carotid Artery Intimal-Medial Thickness in Asymptomatic Older Subjects with Exercise-Induced Myocardial Ischemia. Circulation 1998, 98, 1504–1509. [Google Scholar]
- Andreotti, L.; Bussotti, A.; Cammelli, D.; di Giovine, F.; Sampognaro, S.; Sterrantino, G.; Varcasia, G.; Arcangeli, P. Aortic Connective Tissue in Ageing—A Biochemical Study. Angiology 1985, 36, 872–879. [Google Scholar]
- Sheppard, M.N. The Normal Aorta and Changes with Age. In Surgical Management of Aortic Pathology: Current Fundamentals for the Clinical Management of Aortic Disease; Stanger, O.H., Pepper, J.R., Svensson, L.G., Eds.; Springer: Vienna, Austria, 2019; pp. 77–83. [Google Scholar]
- Farkas, E.; Luiten, P.G.M. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar]
- Lakatta, E.G.; Wang, M.; Najjar, S.S. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med. Clin. N. Am. 2009, 93, 583–604. [Google Scholar]
- Sugawara, J.; Otsuki, T.; Maeda, S.; Tanabe, T.; Kuno, S.; Ajisaka, R.; Matsuda, M. Effect of Arterial Lumen Enlargement on Carotid Arterial Compliance in Normotensive Postmenopausal Women. Hypertens. Res. 2005, 28, 323–329. [Google Scholar]
- Green, D.J.; Swart, A.; Exterkate, A.; Naylor, L.H.; Black, M.A.; Cable, N.T.; Thijssen, D.H. Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis 2010, 210, 525–530. [Google Scholar]
- Chang, H.W.; Kim, S.H.; Hakim, A.R.; Chung, S.; Kim, D.J.; Lee, J.H.; Kim, J.S.; Lim, C.; Park, K.H. Diameter and growth rate of the thoracic aorta-analysis based on serial computed tomography scans. J. Thorac. Dis. 2020, 12, 4002–4013. [Google Scholar]
- Li, A.; Yan, J.; Zhao, Y.; Yu, Z.; Tian, S.; Khan, A.H.; Zhu, Y.; Wu, A.; Zhang, C.; Tian, X.L. Vascular Aging: Assessment and Intervention. Clin. Interv. Aging 2023, 18, 1373–1395. [Google Scholar]
- O’Rourke, M.F.; Nichols, W.W. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005, 45, 652–658. [Google Scholar]
- O’Rourke, M.F. Arterial aging: Pathophysiological principles. Vasc. Med. 2007, 12, 329–341. [Google Scholar]
- James, M.A.; Watt, P.A.C.; Potter, J.F.; Thurston, H.; Swales, J.D. Pulse Pressure and Resistance Artery Structure in the Elderly. Hypertension 1995, 26, 301–306. [Google Scholar] [CrossRef]
- Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic reference values for aortic root size: The Framingham Heart Study. J. Am. Soc. Echocardiogr. 1995, 8, 793–800. [Google Scholar]
- Wang, X.; Ren, X.S.; An, Y.Q.; Hou, Z.H.; Yu, Y.T.; Lu, B.; Wang, F. A Specific Assessment of the Normal Anatomy of the Aortic Root in Relation to Age and Gender. Int. J. Gen. Med. 2021, 14, 2827–2837. [Google Scholar]
- Devereux, R.B.; de Simone, G.; Arnett, D.K.; Best, L.G.; Boerwinkle, E.; Howard, B.V.; Kitzman, D.; Lee, E.T.; Mosley, T.H., Jr.; Weder, A.; et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012, 110, 1189–1194. [Google Scholar]
- Campens, L.; Demulier, L.; De Groote, K.; Vandekerckhove, K.; De Wolf, D.; Roman, M.J.; Devereux, R.B.; De Paepe, A.; De Backer, J. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am. J. Cardiol. 2014, 114, 914–920. [Google Scholar]
- Hansen, F.; Mangell, P.; Sonesson, B.; Länne, T. Diameter and compliance in the human common carotid artery—Variations with age and sex. Ultrasound Med. Biol. 1995, 21, 1–9. [Google Scholar]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar]
- Duprez, D.A.; Cohn, J.N. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr. Atheroscler. Rep. 2007, 9, 139–144. [Google Scholar]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson Simon, G.; Benjamin Emelia, J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar]
- Mitchell, G.F.; Guo, C.-Y.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D. Cross-Sectional Correlates of Increased Aortic Stiffness in the Community. Circulation 2007, 115, 2628–2636. [Google Scholar]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial Dysfunction, Arterial Stiffness, and Heart Failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar]
- Wang, M.; Lakatta, E.G. Altered Regulation of Matrix Metalloproteinase-2 in Aortic Remodeling during Aging. Hypertension 2002, 39, 865–873. [Google Scholar]
- Greenwald, S. Ageing of the conduit arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar]
- Cattell, M.A.; Anderson, J.C.; Hasleton, P.S. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin. Chim. Acta 1996, 245, 73–84. [Google Scholar]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar]
- Willis, A.I.; Pierre-Paul, D.; Sumpio, B.E.; Gahtan, V. Vascular Smooth Muscle Cell Migration: Current Research and Clinical Implications. Vasc. Endovasc. Surg. 2004, 38, 11–23. [Google Scholar]
- Fonck, E.; Feigl, G.G.; Fasel, J.; Sage, D.; Unser, M.; Rüfenacht, D.A.; Stergiopulos, N. Effect of Aging on Elastin Functionality in Human Cerebral Arteries. Stroke 2009, 40, 2552–2556. [Google Scholar]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar]
- Maurel, E.; Shuttleworth, C.A.; Bouissou, H. Interstitial collagens and ageing in human aorta. Virchows. Arch. A Pathol. Anat. Histopathol. 1987, 410, 383–390. [Google Scholar]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix Metalloproteinases Promote Arterial Remodeling in Aging, Hypertension, and Atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Kawecki, C.; Guillot, A.; Martiny, L.; Debelle, L.; et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar]
- Santhanam, L.; Tuday, E.C.; Webb, A.K.; Dowzicky, P.; Kim, J.H.; Oh, Y.J.; Sikka, G.; Kuo, M.; Halushka, M.K.; Macgregor, A.M.; et al. Decreased S-Nitrosylation of Tissue Transglutaminase Contributes to Age-Related Increases in Vascular Stiffness. Circ. Res. 2010, 107, 117–125. [Google Scholar]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Kurotobi, T.; Sato, H.; Kinjo, K.; Nakatani, D.; Mizuno, H.; Shimizu, M.; Imai, K.; Hirayama, A.; Kodama, K.; Hori, M. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2004, 44, 28–34. [Google Scholar] [CrossRef]
- Tracy, E.; Rowe, G.; LeBlanc, A.J. Cardiac tissue remodeling in healthy aging: The road to pathology. Am. J. Physiol. Cell Physiol. 2020, 319, C166–C182. [Google Scholar] [CrossRef]
- Robich, M.P.; Chu, L.M.; Oyamada, S.; Sodha, N.R.; Sellke, F.W. Myocardial therapeutic angiogenesis: A review of the state of development and future obstacles. Expert Rev. Cardiovasc. Ther. 2011, 9, 1469–1479. [Google Scholar] [CrossRef]
- Riddle, D.R.; Sonntag, W.E.; Lichtenwalner, R.J. Microvascular plasticity in aging. Ageing Res. Rev. 2003, 2, 149–168. [Google Scholar] [CrossRef]
- Che Azemin, M.Z.; Ab Hamid, F.; Aminuddin, A.; Wang, J.J.; Kawasaki, R.; Kumar, D.K. Age-related rarefaction in retinal vasculature is not linear. Exp. Eye Res. 2013, 116, 355–358. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Meier, P.; Gloekler, S.; Zbinden, R.; Beckh, S.; de Marchi, S.F.; Zbinden, S.; Wustmann, K.; Billinger, M.; Vogel, R.; Cook, S.; et al. Beneficial effect of recruitable collaterals: A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 2007, 116, 975–983. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Lurbe, E.; Laurent, S. The early life origins of vascular ageing and cardiovascular risk: The EVA syndrome. J. Hypertens. 2008, 26, 1049–1057. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Narula, J.; Jones, M.K.; Deng, X.; Tarnawski, A.S. Impaired angiogenesis in aging myocardial microvascular endothelial cells is associated with reduced importin alpha and decreased nuclear transport of HIF1 alpha: Mechanistic implications. J. Physiol. Pharmacol. 2010, 61, 133–139. [Google Scholar]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Warrington, J.P.; Ashpole, N.; Csiszar, A.; Lee, Y.W.; Ungvari, Z.; Sonntag, W.E. Whole Brain Radiation-Induced Vascular Cognitive Impairment: Mechanisms and Implications. J. Vasc. Res. 2013, 50, 445–457. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Hertelendy, P.; Valcarcel-Ares, M.N.; Fülöp, G.A.; Logan, S.; Kiss, T.; Farkas, E.; Csiszar, A.; Yabluchanskiy, A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience 2017, 39, 33–42. [Google Scholar] [CrossRef]
- Ashpole, N.M.; Warrington, J.P.; Mitschelen, M.C.; Yan, H.; Sosnowska, D.; Gautam, T.; Farley, J.A.; Csiszar, A.; Ungvari, Z.; Sonntag, W.E. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: A role for endothelial progenitor cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H858–H868. [Google Scholar] [CrossRef]
- Berthiaume, A.-A.; Schmid, F.; Stamenkovic, S.; Coelho-Santos, V.; Nielson, C.D.; Weber, B.; Majesky, M.W.; Shih, A.Y. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat. Commun. 2022, 13, 5912. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Arcuri, G.; Ferlosio, A.; Orlandi, A. Ageing and microvasculature. Vasc. Cell 2014, 6, 19. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Mohler, E.R.; Lederman, R.J.; Mendelsohn, F.O.; Saucedo, J.F.; Goldman, C.K.; Blebea, J.; Macko, J.; Kessler, P.D.; Rasmussen, H.S.; et al. Regional Angiogenesis with Vascular Endothelial Growth Factor in Peripheral Arterial Disease. Circulation 2003, 108, 1933–1938. [Google Scholar] [CrossRef]
- Bosch-Marce, M.; Okuyama, H.; Wesley, J.B.; Sarkar, K.; Kimura, H.; Liu, Y.V.; Zhang, H.; Strazza, M.; Rey, S.; Savino, L. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007, 101, 1310–1318. [Google Scholar] [CrossRef]
- Yu, J.; Lei, L.; Liang, Y.; Hinh, L.; Hickey, R.P.; Huang, Y.; Liu, D.; Yeh, J.L.; Rebar, E.; Case, C. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J. 2006, 20, 479–481. [Google Scholar] [CrossRef]
- Cho, H.; Kozasa, T.; Bondjers, C.; Betsholtz, C.; Kehrl, J.H. Pericyte-specific expression of Rgs5: Implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 2003, 17, 1–17. [Google Scholar] [CrossRef]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and Age Differences in Growth Factor Concentrations From Platelet-Rich Plasma in Adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef]
- Bach, M.H.; Sadoun, E.; Reed, M.J. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech. Ageing Dev. 2005, 126, 467–473. [Google Scholar] [CrossRef]
- Dai, X.; Faber, J.E. Endothelial Nitric Oxide Synthase Deficiency Causes Collateral Vessel Rarefaction and Impairs Activation of a Cell Cycle Gene Network During Arteriogenesis. Circ. Res. 2010, 106, 1870–1881. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117. [Google Scholar] [CrossRef]
- Nowak, K.L.; Rossman, M.J.; Chonchol, M.; Seals, D.R. Strategies for Achieving Healthy Vascular Aging. Hypertension 2018, 71, 389–402. [Google Scholar] [CrossRef]
- Gomez-Sanchez, M.; Gomez-Sanchez, L.; Patino-Alonso, M.C.; Cunha, P.G.; Recio-Rodriguez, J.I.; Alonso-Dominguez, R.; Sanchez-Aguadero, N.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Garcia-Ortiz, L.; et al. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: Early Vascular Ageing Study. J. Hypertens. 2020, 38, 1110–1122. [Google Scholar] [CrossRef]
- DeSouza, C.A.; Shapiro, L.F.; Clevenger, C.M.; Dinenno, F.A.; Monahan, K.D.; Tanaka, H.; Seals, D.R. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000, 102, 1351–1357. [Google Scholar] [CrossRef]
- Vaitkevicius, P.V.; Fleg, J.L.; Engel, J.H.; O’Connor, F.C.; Wright, J.G.; Lakatta, L.E.; Yin, F.C.; Lakatta, E.G. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993, 88 Pt 1, 1456–1462. [Google Scholar] [CrossRef]
- Sugawara, J.; Tomoto, T.; Noda, N.; Matsukura, S.; Tsukagoshi, K.; Hayashi, K.; Hieda, M.; Maeda, S. Effects of endothelin-related gene polymorphisms and aerobic exercise habit on age-related arterial stiffening: A 10-yr longitudinal study. J. Appl. Physiol. 2018, 124, 312–320. [Google Scholar]
- Somani, S.M.; Husain, K. Exercise training alters kinetics of antioxidant enzymes in rat tissues. Biochem. Mol. Biol. Int. 1996, 38, 587–595. [Google Scholar]
- Hoetzer, G.L.; Van Guilder, G.P.; Irmiger, H.M.; Keith, R.S.; Stauffer, B.L.; DeSouza, C.A. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J. Appl. Physiol. 2007, 102, 847–852. [Google Scholar]
- Alfaras, I.; Di Germanio, C.; Bernier, M.; Csiszar, A.; Ungvari, Z.; Lakatta, E.G.; de Cabo, R. Pharmacological Strategies to Retard Cardiovascular Aging. Circ. Res. 2016, 118, 1626–1642. [Google Scholar]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H292–H306. [Google Scholar]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014, 5, 3557. [Google Scholar]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013, 12, 772–783. [Google Scholar]
- Guo, Z.; Mitchell-Raymundo, F.; Yang, H.; Ikeno, Y.; Nelson, J.; Diaz, V.; Richardson, A.; Reddick, R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech. Ageing Dev. 2002, 123, 1121–1131. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 1062–1073. [Google Scholar]
- Pignolo, R.J.; Passos, J.F.; Khosla, S.; Tchkonia, T.; Kirkland, J.L. Reducing Senescent Cell Burden in Aging and Disease. Trends Mol. Med. 2020, 26, 630–638. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Ahmed, B.; Farb, M.G.; Gokce, N. Cardiometabolic implications of adipose tissue aging. Obes. Rev. 2024, 25, e13806. [Google Scholar] [CrossRef]
- Cao, F.; Wu, K.; Zhu, Y.Z.; Bao, Z.W. Roles and Mechanisms of Dipeptidyl Peptidase 4 Inhibitors in Vascular Aging. Front. Endocrinol. 2021, 12, 731273. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, R.; Sun, Z.F.; Long, J.W.; Gong, Y.Q. Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases. Aging Dis. 2022, 13, 468–490. [Google Scholar] [CrossRef]
- Ahmed, B.; Farb, M.G.; Karki, S.; D’Alessandro, S.; Edwards, N.M.; Gokce, N. Pericardial Adipose Tissue Thrombospondin-1 Associates with Antiangiogenesis in Ischemic Heart Disease. Am. J. Cardiol. 2024, 210, 201–207. [Google Scholar] [CrossRef]
- Sabatini, D.M. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Zigler, M.C.; Durrant, J.R.; Donato, A.J.; Seals, D.R. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech. Ageing Dev. 2012, 133, 368–371. [Google Scholar] [CrossRef]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The Implications of Aging on Vascular Health. Int. J. Mol. Sci. 2024, 25, 11188. https://doi.org/10.3390/ijms252011188
Ahmed B, Rahman AA, Lee S, Malhotra R. The Implications of Aging on Vascular Health. International Journal of Molecular Sciences. 2024; 25(20):11188. https://doi.org/10.3390/ijms252011188
Chicago/Turabian StyleAhmed, Bulbul, Ahmed A. Rahman, Sujin Lee, and Rajeev Malhotra. 2024. "The Implications of Aging on Vascular Health" International Journal of Molecular Sciences 25, no. 20: 11188. https://doi.org/10.3390/ijms252011188
APA StyleAhmed, B., Rahman, A. A., Lee, S., & Malhotra, R. (2024). The Implications of Aging on Vascular Health. International Journal of Molecular Sciences, 25(20), 11188. https://doi.org/10.3390/ijms252011188




