The Ambivalent Role of miRNA-21 in Trauma and Acute Organ Injury
Abstract
1. Introduction
2. miR
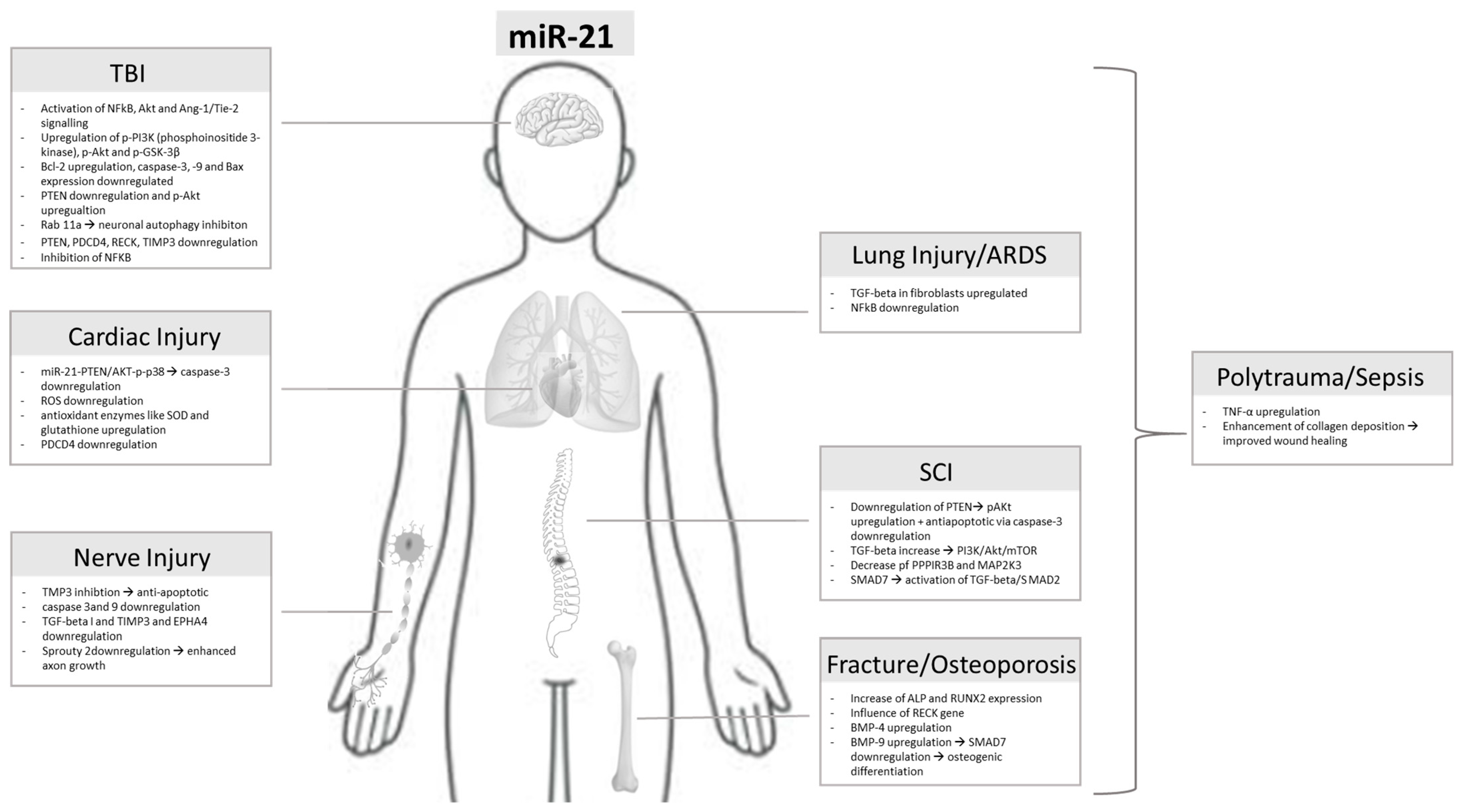
2.1. MiR-21 in Traumatic Brain Injury
2.2. In Vitro Studies
2.3. In Vivo Studies
2.4. Patient Studies
3. miRNA-21 in Spinal Cord Injury
3.1. In Vitro Studies
3.2. In Vivo Studies
3.3. Patient Studies
| miR-21 in SCI |
| [42,43,46,47,54] |
| Contusion SCI in rats |
| [45,46] |
| Mouse model of SCI, treatment (neuroprotection) of Gypenoside XVII (GP-17) |
| [53] |
| SCI rat model, injection of MSC EVs via tail vein |
| [49] |
| Mouse model of SCI In vitro spinal fibroblast culture |
| [56] |
| Administration of miR-21 inhibitor in activated microglia cells of rat |
| [57] |
4. Peripheral Nerve Injury/Nerve Trauma
4.1. In Vitro Studies
4.2. In Vivo Studies
4.3. Patient Studies
| Rat dorsal root ganglion (DRG) neurons |
| [62] |
| Spinal nerve ligation, dorsal root transection, ventral root transection in rats |
| [64,65,67,68,69] |
5. Bone Injuries
5.1. In Vitro Studies
5.2. In Vivo Studies
5.3. Patient Studies
6. MiR-21 in Cardiac Damage
6.1. In Vitro Studies
6.2. In Vivo Studies
6.3. Patient Studies
7. Lung Injuries
7.1. In Vitro Studies
7.2. In Vivo Studies
7.3. Patient Studies
8. miRNA-21 in Polytrauma
Patient Studies
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lobera, E.S.; Varela, M.A.; Jimenez, R.L.; Moreno, R.B. miRNA as biomarker in lung cancer. Mol. Biol. Rep. 2023, 50, 9521–9527. [Google Scholar] [CrossRef]
- Petri, B.J.; Klinge, C.M. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev. 2020, 39, 837–886. [Google Scholar] [CrossRef]
- Weber, B.; Franz, N.; Marzi, I.; Henrich, D.; Leppik, L. Extracellular vesicles as mediators and markers of acute organ injury: Current concepts. Eur. J. Trauma Emerg. Surg. 2021, 48, 1525–1544. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of Novel Genes Coding for Small Expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Fan, M.; Pfeffer, L.M. IFN Induces miR-21 through a Signal Transducer and Activator of Transcription 3–Dependent Pathway as a Suppressive Negative Feedback on IFN-Induced Apoptosis. Cancer Res. 2010, 70, 8108–8116. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Q.; Hu, Y.; Li, J.; Li, X.; Zhou, L.; Huang, Y. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol. Rep. 2012, 27, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-G.; Wang, J.-J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.-H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.; Teuben, M.; Andruszkow, H.; Barkatali, B.M.; Pape, H.-C. Mortality Patterns in Patients with Multiple Trauma: A Systematic Review of Autopsy Studies. PLoS ONE 2016, 11, e0148844. [Google Scholar] [CrossRef]
- van Breugel, J.M.M.; Niemeyer, M.J.S.; Houwert, R.M.; Groenwold, R.H.H.; Leenen, L.P.H.; van Wessem, K.J.P. Global changes in mortality rates in polytrauma patients admitted to the ICU—A systematic review. World J. Emerg. Surg. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Unterberg, A.; Marzi, I.; Steudel, W.-I.; Uhl, E.; Lemcke, J.; Berg, F.; Woschek, M.; Friedrich, M.; Clusmann, H.; et al. Development and first results of a national databank on care and treatment outcome after traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2023, 49, 1171–1181. [Google Scholar] [CrossRef]
- Ge, X.; Huang, S.; Gao, H.; Han, Z.; Chen, F.; Zhang, S.; Wang, Z.; Kang, C.; Jiang, R.; Yue, S.; et al. miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 2016, 1650, 31–40. [Google Scholar] [CrossRef]
- Ge, X.; Han, Z.; Chen, F.; Wang, H.; Zhang, B.; Jiang, R.; Lei, P.; Zhang, J. miR-21 alleviates secondary blood–brain barrier damage after traumatic brain injury in rats. Brain Res. 2015, 1603, 150–157. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Z.; Wang, F.; Han, Z.; Wang, Y.; Huang, S.; Hu, T.; Guo, M.; Lei, P. Hydrogen exerts neuroprotection by activation of the miR-21/PI3K/AKT/GSK-3β pathway in an in vitro model of traumatic brain injury. J. Cell. Mol. Med. 2020, 24, 4061–4071. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chen, F.; Ge, X.; Tan, J.; Lei, P.; Zhang, J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014, 1582, 12–20. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun. 2019, 83, 270–282. [Google Scholar] [CrossRef]
- Ziu, M.; Fletcher, L.; Rana, S.; Jimenez, D.F.; Digicaylioglu, M. Temporal Differences in MicroRNA Expression Patterns in Astrocytes and Neurons after Ischemic Injury. PLoS ONE 2011, 6, e14724. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, S.; Zhu, J.; Hu, T.; Han, Z.; Zhang, S.; Zhao, J.; Chen, F.; Lei, P. Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med. Sci. Monit. 2019, 25, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Zhao, J.; Dash, P.K. Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. J. Neurosci. Res. 2010, 89, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Gregory, E.; Berman, N.E. Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochem. Int. 2014, 78, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Cartagena, C.M.; Tortella, F.C.; Dave, J.R.; Schmid, K.E.; Boutté, A.M. Acute and subacute microRNA dysregulation is associated with cytokine responses in the rodent model of penetrating ballistic-like brain injury. J. Trauma Acute Care Surg. 2017, 83, S145–S149. [Google Scholar] [CrossRef]
- Harmon, J.L.; Gibbs, W.S.; Whitaker, R.M.; Schnellmann, R.G.; Adkins, D.L. Striatal Mitochondrial Disruption following Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 487–494. [Google Scholar] [CrossRef]
- Meissner, L.; Gallozzi, M.; Balbi, M.; Schwarzmaier, S.M.; Tiedt, S.; Terpolilli, N.A.; Plesnila, N. Temporal Profile of MicroRNA Expression in Contused Cortex after Traumatic Brain Injury in Mice. J. Neurotrauma 2016, 33, 713–720. [Google Scholar] [CrossRef]
- Lei, P.; Li, Y.; Chen, X.; Yang, S.; Zhang, J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009, 1284, 191–201. [Google Scholar] [CrossRef]
- Miao, W.; Bao, T.; Han, J.; Yin, M.; Yan, Y.; Wang, W.; Zhu, Y. Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz. J. Med. Biol. Res. 2015, 48, 433–439. [Google Scholar] [CrossRef]
- Bao, T.-H.; Miao, W.; Han, J.-H.; Yin, M.; Yan, Y.; Wang, W.-W.; Zhu, Y.-H. Spontaneous Running Wheel Improves Cognitive Functions of Mouse Associated with miRNA Expressional Alteration in Hippocampus Following Traumatic Brain Injury. J. Mol. Neurosci. 2014, 54, 622–629. [Google Scholar] [CrossRef]
- Gao, X.; Xiong, Y.; Li, Q.; Han, M.; Shan, D.; Yang, G.; Zhang, S.; Xin, D.; Zhao, R.; Wang, Z.; et al. Extracellular vesicle-mediated transfer of miR-21-5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-T.; Lei, P.; Wang, H.-C.; Zhang, A.-L.; Han, Z.L.; Chen, X.; Li, S.-H.; Jiang, R.-C.; Kang, C.-S.; Zhang, J.-N. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci. Rep. 2015, 4, 6718. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, W.; Huang, S.; Yin, Z.; Yang, M.; Han, Z.; Han, Z.; Chen, F.; Wang, H.; Lei, P.; et al. Increased miR-21-3p in Injured Brain Microvascular Endothelial Cells after Traumatic Brain Injury Aggravates Blood–Brain Barrier Damage by Promoting Cellular Apoptosis and Inflammation through Targeting MAT2B. J. Neurotrauma 2019, 36, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Morris-Blanco, K.C.; Ly, N.; Maves, C.; Dempsey, R.J.; Vemuganti, R. MicroRNA miR-21 Decreases Post-stroke Brain Damage in Rodents. Transl. Stroke Res. 2021, 13, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Pinchi, E.; Frati, A.; Cipolloni, L.; Aromatario, M.; Gatto, V.; La Russa, R.; Pesce, A.; Santurro, A.; Fraschetti, F.; Frati, P.; et al. Clinical-pathological study on β-APP, IL-1β, GFAP, NFL, Spectrin II, 8OHdG, TUNEL, miR-21, miR-16, miR-92 expressions to verify DAI-diagnosis, grade and prognosis. Sci. Rep. 2018, 8, 2387. [Google Scholar] [CrossRef]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef]
- Seršić, L.V.; Alić, V.K.; Biberić, M.; Zrna, S.; Jagoić, T.; Tarčuković, J.; Grabušić, K. Real-Time PCR Quantification of 87 miRNAs from Cerebrospinal Fluid: miRNA Dynamics and Association with Extracellular Vesicles after Severe Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 4751. [Google Scholar] [CrossRef]
- Bache, S.; Rasmussen, R.; Rossing, M.; Laigaard, F.P.; Nielsen, F.C.; Møller, K. MicroRNA Changes in Cerebrospinal Fluid After Subarachnoid Hemorrhage. Stroke 2017, 48, 2391–2398. [Google Scholar] [CrossRef]
- Eli, I.; Lerner, D.P.; Ghogawala, Z. Acute Traumatic Spinal Cord Injury. Neurol. Clin. 2021, 39, 471–488. [Google Scholar] [CrossRef]
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord. 2006, 44, 523–529. [Google Scholar] [CrossRef]
- Krueger, H.; Noonan, V.; Trenaman, L.; Joshi, P.; Rivers, C. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis. Can. 2013, 33, 113–122. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Rastegar-Moghaddam, S.H.; Ebrahimzadeh-Bideskan, S.; Lombardi, G.; Ebrahimzadeh-Bideskan, A.; Mohammadipour, A. Targeting miR-21 in spinal cord injuries: A game-changer? Mol. Med. 2022, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, Y.; Han, X.; Qu, P.; Wang, W. Long noncoding RNA PTENP1 affects the recovery of spinal cord injury by regulating the expression of miR-19b and miR-21. J. Cell. Physiol. 2019, 235, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Song, P.; Wu, Z.; Liu, Y.; Ying, W.; Shen, C. Inflammation Modifies miR-21 Expression Within Neuronal Extracellular Vesicles to Regulate Remyelination Following Spinal Cord Injury. Stem Cell Rev. Rep. 2023, 19, 2024–2037. [Google Scholar] [CrossRef]
- Huang, J.-H.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.-B. Anti-Apoptotic Effect of MicroRNA-21 after Contusion Spinal Cord Injury in Rats. J. Neurotrauma 2013, 30, 1349–1360. [Google Scholar] [CrossRef]
- Xu, G.; Ao, R.; Zhi, Z.; Jia, J.; Yu, B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J. Cell. Physiol. 2018, 234, 10205–10217. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Zhi, Z.; Wang, S.; Xu, G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019, 26, 491–503. [Google Scholar] [CrossRef]
- He, F.; Ren, Y.; Shi, E.; Liu, K.; Yan, L.; Jiang, X. Overexpression of microRNA-21 protects spinal cords against transient ischemia. J. Thorac. Cardiovasc. Surg. 2016, 152, 1602–1608. [Google Scholar] [CrossRef]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. microRNA-21 Regulates Astrocytic Response Following Spinal Cord Injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.; Wang, S.; Xie, W.; Li, H.; Ning, B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging 2018, 10, 1474–1488. [Google Scholar] [CrossRef]
- Huang, J.-H.; Cao, Y.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.-B.; Hu, J.-Z. Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res. 2016, 1648, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, B.; Wang, Z.; Li, X.; Chen, F. Sevoflurane pretreatment regulates abnormal expression of MicroRNAs associated with spinal cord ischemia/reperfusion injury in rats. Ann. Transl. Med. 2021, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Duan, L.; Li, J.; Guo, H.; Xiong, M. Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA-21-mediated PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 2021, 48, 146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Yip, P.K.; Adams, L.; Davies, M.; Lee, J.W.; Michael, G.J.; Priestley, J.V.; Michael-Titus, A.T. A Single Bolus of Docosahexaenoic Acid Promotes Neuroplastic Changes in the Innervation of Spinal Cord Interneurons and Motor Neurons and Improves Functional Recovery after Spinal Cord Injury. J. Neurosci. 2015, 35, 12733–12752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chu, X.; Yuan, H.; Qiu, J.; Zhao, C.; Xin, D.; Li, T.; Ma, W.; Wang, H.; Wang, Z.; et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed. Pharmacother. 2019, 115, 108818. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Su, Y.; Li, H.; Xie, W.; Ning, B. MicroRNA-21-5p mediates TGF-β-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int. J. Biol. Sci. 2018, 14, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.-L.; Zhu, H.; Shao, J.; Liu, Y.-C.; Lan, J.; Miao, J. MiR-21 inhibitor improves locomotor function recovery by inhibiting IL-6R/JAK-STAT pathway-mediated inflammation after spinal cord injury in model of rat. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 433–440. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, M.; Zhi, J.; Wu, S.; Wang, Y.; Pei, F. Research Hotspots and Trends of Peripheral Nerve Injuries Based on Web of Science From 2017 to 2021: A Bibliometric Analysis. Front. Neurol. 2022, 13, 872261. [Google Scholar] [CrossRef]
- Babaei-Ghazani, A.; Eftekharsadat, B.; Samadirad, B.; Mamaghany, V.; Abdollahian, S. Traumatic lower extremity and lumbosacral peripheral nerve injuries in adults: Electrodiagnostic studies and patients symptoms. J. Forensic Leg. Med. 2017, 52, 89–92. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, S.; Wang, Y.; Yi, S.; Zhao, L.; Tang, X.; Yu, B.; Gu, X.; Ding, F. miR-21 and miR-222 inhibit apoptosis of adult dorsal root ganglion neurons by repressing TIMP3 following sciatic nerve injury. Neurosci. Lett. 2015, 586, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Wang, H.-C.; Chunag, Y.-T.; Chou, C.-W.; Lin, I.-L.; Lai, C.-S.; Chang, L.-L.; Cheng, K.-I. miRNA Expression Change in Dorsal Root Ganglia After Peripheral Nerve Injury. J. Mol. Neurosci. 2016, 61, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhou, S.; Qian, T.; Wang, Y.; Ding, F.; Gu, X. Altered microRNA expression following sciatic nerve resection in dorsal root ganglia of rats. Acta Biochim. Biophys. Sin. 2011, 43, 909–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ning, X.-J.; Lu, X.-H.; Luo, J.-C.; Chen, C.; Gao, Q.; Li, Z.-Y.; Wang, H. Molecular mechanism of microRNA-21 promoting Schwann cell proliferation and axon regeneration during injured nerve repair. RNA Biol. 2020, 17, 1508–1519. [Google Scholar] [CrossRef]
- Strickland, I.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.-F. Axotomy-Induced miR-21 Promotes Axon Growth in Adult Dorsal Root Ganglion Neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef]
- Ikuma, Y.; Sakai, A.; Sakamoto, A.; Suzuki, H. Increased extracellular release of microRNAs from dorsal root ganglion cells in a rat model of neuropathic pain caused by peripheral nerve injury. PLoS ONE 2023, 18, e0280425. [Google Scholar] [CrossRef]
- Sakai, A.; Suzuki, H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem. Biophys. Res. Commun. 2013, 435, 176–181. [Google Scholar] [CrossRef]
- Banerjee, M.; Bouillon, B.; Shafizadeh, S.; Paffrath, T.; Lefering, R.; Wafaisade, A. Epidemiology of extremity injuries in multiple trauma patients. Injury 2013, 44, 1015–1021. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Dong, Y.; Wu, C.; Ma, Y.; Jin, Y.; Ji, Y. MiR-21 overexpression improves osteoporosis by targeting RECK. Mol. Cell. Biochem. 2015, 405, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, G.; Hu, C.; Shi, Y.; Liao, L.; Shi, S.; Cai, Y.; Cheng, S.; Wang, X.; Liu, Y.; et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency–induced osteoporosis. J. Bone Miner. Res. 2012, 28, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Vijakumaran, U.; Shanmuganantha, L.; Law, J.-X.; Alias, E.; Ng, M.-H. The Role and Mechanism of MicroRNA 21 in Osteogenesis: An Update. Int. J. Mol. Sci. 2023, 24, 11330. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, B.; Song, C.; Jia, J.; Wang, Y.; Pang, K.; Wang, Y.; Chen, C. Exosome MiR-21-5p Upregulated by HIF-1α Induces Adipose Stem Cell Differentiation to Promote Ectopic Bone Formation. Chem. Biodivers. 2024, 21, e202301972. [Google Scholar] [CrossRef]
- Song, Q.; Zhong, L.; Chen, C.; Tang, Z.; Liu, H.; Zhou, Y.; Tang, M.; Zhou, L.; Zuo, G.; Luo, J.; et al. miR-21 synergizes with BMP9 in osteogenic differentiation by activating the BMP9/Smad signaling pathway in murine multilineage cells. Int. J. Mol. Med. 2015, 36, 1497–1506. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Huang, S.; Hou, Y.; Liu, Y.; Chan, K.-M.; Pan, X.-H.; Li, G. mir-21 Overexpressing Mesenchymal Stem Cells Accelerate Fracture Healing in a Rat Closed Femur Fracture Model. BioMed Res. Int. 2015, 2015, 412327. [Google Scholar] [CrossRef]
- Silva, A.M.; Almeida, M.I.; Teixeira, J.H.; Ivan, C.; Oliveira, J.; Vasconcelos, D.; Neves, N.; Ribeiro-Machado, C.; Cunha, C.; Barbosa, M.A.; et al. Profiling the circulating miRnome reveals a temporal regulation of the bone injury response. Theranostics 2018, 8, 3902–3917. [Google Scholar] [CrossRef]
- Oka, S.; Li, X.; Zhang, F.; Tewari, N.; Ma, R.; Zhong, L.; Makishima, M.; Liu, Y.; Bhawal, U.K. MicroRNA-21 facilitates osteoblast activity. Biochem. Biophys. Rep. 2021, 25, 100894. [Google Scholar] [CrossRef]
- Zarecki, P.; Hackl, M.; Grillari, J.; Debono, M.; Eastell, R. Serum microRNAs as novel biomarkers for osteoporotic vertebral fractures. Bone 2019, 130, 115105. [Google Scholar] [CrossRef]
- Seeliger, C.; Karpinski, K.; Haug, A.T.; Vester, H.; Schmitt, A.; Bauer, J.S.; van Griensven, M. Five Freely Circulating miRNAs and Bone Tissue miRNAs Are Associated with Osteoporotic Fractures. J. Bone Miner. Res. 2014, 29, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawaf, H.A.; Gabr, S.A.; Iqbal, A.; Alghadir, A.H. MicroRNAs as potential biopredictors for premenopausal osteoporosis: A biochemical and molecular study. BMC Women’s Health 2023, 23, 481. [Google Scholar] [CrossRef]
- Suarjana, I.N.; Isbagio, H.; Soewondo, P.; Rachman, I.A.; Sadikin, M.; Prihartono, J.; Malik, S.G.; Soeroso, J. The Role of Serum Expression Levels of Microrna-21 on Bone Mineral Density in Hypostrogenic Postmenopausal Women with Osteoporosis: Study on Level of RANKL, OPG, TGFβ-1, Sclerostin, RANKL/OPG Ratio, and Physical Activity. Acta Med. Indones 2019, 51, 245–252. [Google Scholar] [PubMed]
- Panach, L.; Mifsut, D.; Tarín, J.J.; Cano, A.; García-Pérez, M. Serum Circulating MicroRNAs as Biomarkers of Osteoporotic Fracture. Calcif. Tissue Int. 2015, 97, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kelch, S.; Balmayor, E.R.; Seeliger, C.; Vester, H.; Kirschke, J.S.; van Griensven, M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017, 7, 15861. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Anastasilakis, A.D.; Makras, P.; Tsalikakis, D.G.; Grammatiki, M.; Yovos, J.G. Expression of microRNAs that regulate bone turnover in the serum of postmenopausal women with low bone mass and vertebral fractures. Eur. J. Endocrinol. 2017, 176, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Malhotra, R.; Mittal, S.; Kumar, A.; Mehta, N.; Malik, G.; Gupta, M.; Trikha, V. Differential miRNA Expression in Osteoporotic Elderly Patients with Hip Fractures Compared to Young Patients. Indian. J. Orthop. 2021, 56, 399–411. [Google Scholar] [CrossRef]
- Hanschen, M.; Kanz, K.-G.; Kirchhoff, C.; Khalil, P.N.; Wierer, M.; van Griensven, M.; Laugwitz, K.-L.; Biberthaler, P.; Lefering, R.; Huber-Wagner, S.; et al. Blunt Cardiac Injury in the Severely Injured—A Retrospective Multicentre Study. PLoS ONE 2015, 10, e0131362. [Google Scholar] [CrossRef]
- Huber, S.; Biberthaler, P.; Delhey, P.; Trentzsch, H.; Winter, H.; van Griensven, M.; Lefering, R.; Huber-Wagner, S. Predictors of poor outcomes after significant chest trauma in multiply injured patients: A retrospective analysis from the German Trauma Registry (Trauma Register DGU®). Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 52. [Google Scholar] [CrossRef]
- Elie, M.C. Blunt cardiac injury. Mt. Sinai J. Med. 2006, 73, 542–552. [Google Scholar]
- Kalbitz, M.; Pressmar, J.; Stecher, J.; Weber, B.; Weiss, M.; Schwarz, S.; Miltner, E.; Gebhard, F.; Huber-Lang, M. The Role of Troponin in Blunt Cardiac Injury After Multiple Trauma in Humans. World J. Surg. 2016, 41, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.Y.; Edwards, J.D.; Nightingale, P. Early cardiorespiratory findings after severe blunt thoracic trauma and their relation to outcome. Br. J. Surg. 1992, 79, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Pan, H.; Zheng, Z.; Guo, Y.; Peng, J.; Yang, J.; Luo, Y.; He, J.; Yan, S.; Wang, P.; et al. Prostaglandin E1 protects cardiomyocytes against hypoxia-reperfusion induced injury via the miR-21-5p/FASLG axis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wei, H.; Lan, M.; Jia, N.; Liu, J.; Zhang, M. MicroRNA-21 mediates the protective effects of salidroside against hypoxia/reoxygenation-induced myocardial oxidative stress and inflammatory response. Exp. Ther. Med. 2020, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xue, J.; Wu, H.; Wang, S.; Liu, Y.; Zheng, S.; Zhang, C.; Yang, C. Ginsenoside-Rb1 Protects Hypoxic- and Ischemic-Damaged Cardiomyocytes by Regulating Expression of miRNAs. Evid.-Based Complement. Altern. Med. 2015, 2015, 171306. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, J.; Xu, B.; Yu, J.; Zhang, A.; Qin, L.; Liu, C.; Yang, Y. miR-21-5p inhibits inflammation injuries in LPS-treated H9c2 cells by regulating PDCD4. Am. J. Transl. Res. 2021, 13, 11450–11460. [Google Scholar] [PubMed]
- Huang, W.; Tian, S.-S.; Hang, P.-Z.; Sun, C.; Guo, J.; Du, Z.-M. Combination of microRNA-21 and microRNA-146a Attenuates Cardiac Dysfunction and Apoptosis During Acute Myocardial Infarction in Mice. Mol. Ther.-Nucleic Acids 2016, 5, e296. [Google Scholar] [CrossRef]
- Gryshkova, V.; Fleming, A.; McGhan, P.; De Ron, P.; Fleurance, R.; Valentin, J.-P.; da Costa, A.N. miR-21-5p as a potential biomarker of inflammatory infiltration in the heart upon acute drug-induced cardiac injury in rats. Toxicol. Lett. 2018, 286, 31–38. [Google Scholar] [CrossRef]
- Viczenczova, C.; Kura, B.; Benova, T.E.; Yin, C.; Kukreja, R.C.; Slezak, J.; Tribulova, N.; Bacova, B.S. Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin. Int. J. Mol. Sci. 2018, 19, 1128. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, P.; Zhou, Y.; Xing, C.; Zhao, L.; Li, Z.; Yuan, L. Ultrasound targeted microbubble destruction assisted exosomal delivery of miR-21 protects the heart from chemotherapy associated cardiotoxicity. Biochem. Biophys. Res. Commun. 2020, 532, 60–67. [Google Scholar] [CrossRef]
- Permenter, M.G.; McDyre, B.C.; Ippolito, D.L.; Stallings, J.D. Alterations in tissue microRNA after heat stress in the conscious rat: Potential biomarkers of organ-specific injury. BMC Genom. 2019, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Lötterle, L.; Han, J.; Kalbitz, M.; Henrich, D.; Marzi, I.; Leppik, L.; Weber, B. Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. J. Clin. Med. 2024, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A. The mir-21 Inhibition Enhanced HUVEC Cellular Viability during Hypoxia-Reoxygenation Injury by Regulating PDCD4. Mediat. Inflamm. 2022, 2022, 9661940. [Google Scholar] [CrossRef]
- Sadat-Ebrahimi, S.-R.; Rezabakhsh, A.; Aslanabadi, N.; Asadi, M.; Zafari, V.; Shanebandi, D.; Zarredar, H.; Enamzadeh, E.; Taghizadeh, H.; Badalzadeh, R. Novel diagnostic potential of miR-1 in patients with acute heart failure. PLoS ONE 2022, 17, e0275019. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, D.J.; Moore, E.E.; Johnson, J.L.; Cothren, C.C.; Banerjee, A.; Burch, J.M.; Sauaia, A. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery 2006, 140, 640–648. [Google Scholar] [CrossRef] [PubMed]
- van Wessem, K.J.P.; Leenen, L.P.H. Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg. Acute Care Open 2018, 3, e000232. [Google Scholar] [CrossRef]
- Park, P.K.; Cannon, J.W.; Ye, W.; Blackbourne, L.H.; Holcomb, J.B.; Beninati, W.; Napolitano, L.M. Incidence, risk factors, and mortality associated with acute respiratory distress syndrome in combat casualty care. J. Trauma Acute Care Surg. 2016, 81, S150–S156. [Google Scholar] [CrossRef]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, J.; Zhang, M.; Zhu, T.; Zhang, Y.; Sun, K. MicroRNA-21 inhibits lipopolysaccharide-induced acute lung injury by targeting nuclear factor-B. Exp. Ther. Med. 2018, 16, 4616–4622. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; He, Y.; Ran, Y.; Lu, B.; Gao, J.; Shu, C.; Li, J.; Zhao, Y.; Zhang, X.; et al. Protective effect of melatonin entrapped PLGA nanoparticles on radiation-induced lung injury through the miR-21/TGF-β1/Smad3 pathway. Int. J. Pharm. 2021, 602, 120584. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Li, H.; Cai, X.-H.; Gu, J.-Q.; Meng, J.; Xie, H.-Q.; Zhang, J.-L.; Chen, J.; Jin, X.-G.; Tang, Q.; et al. Lipoxin A4 activates alveolar epithelial sodium channel gamma via the microRNA-21/PTEN/AKT pathway in lipopolysaccharide-induced inflammatory lung injury. Mod. Pathol. 2015, 95, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, G.; Wang, Q.; Gong, F. Prognostic value of pulmonary ultrasound score combined with plasma miR-21-3p expression in patients with acute lung injury. J. Clin. Lab. Anal. 2022, 36, e24275. [Google Scholar] [CrossRef] [PubMed]
- Lansink, K.W.W.; Gunning, A.C.; Spijkers, A.T.E.; Leenen, L.P.H. Evaluation of Trauma Care in a Mature Level I Trauma Center in The Netherlands: Outcomes in a Dutch Mature Level I Trauma Center. World J. Surg. 2013, 37, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.-C. Patterns of mortality and causes of death in polytrauma patients—Has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.A.; Hagebusch, P.; Lefering, R.; Faul, P.; Hoffmann, R.; Schweigkofler, U.; Dgu, T. Changes in injury patterns, injury severity and hospital mortality in motorized vehicle accidents: A retrospective, cross-sectional, multicenter study with 19,225 cases derived from the TraumaRegister DGU®. Eur. J. Trauma Emerg. Surg. 2023, 49, 1917–1925. [Google Scholar] [CrossRef]
- Hardy, B.M.; Varghese, A.; Adams, M.J.; Enninghorst, N.; Balogh, Z.J. The outcomes of the most severe polytrauma patients: A systematic review of the use of high ISS cutoffs for performance measurement. Eur. J. Trauma Emerg. Surg. 2023, 50, 1305–1312. [Google Scholar] [CrossRef]
- Meakes, S.; Enninghorst, N.; Weaver, N.; Hardy, B.M.; Balogh, Z.J. Long-term functional outcomes in polytrauma: A fundamentally new approach is needed in prediction. Eur. J. Trauma Emerg. Surg. 2024, 50, 1439–1452. [Google Scholar] [CrossRef]
- Pfeifer, R.; Schick, S.; Holzmann, C.; Graw, M.; Teuben, M.; Pape, H. Analysis of Injury and Mortality Patterns in Deceased Patients with Road Traffic Injuries: An Autopsy Study. World J. Surg. 2017, 41, 3111–3119. [Google Scholar] [CrossRef]
- Pape, H.-C.; Moore, E.; McKinley, T.; Sauaia, A. Pathophysiology in patients with polytrauma. Injury 2022, 53, 2400–2412. [Google Scholar] [CrossRef]
- Billiar, T.R.; Vodovotz, Y. Time for trauma immunology. PLoS Med. 2017, 14, e1002342. [Google Scholar] [CrossRef]
- Vicente, D.A.; Schobel, S.A.; Anfossi, S.; Hensman, H.; Lisboa, F.; Robertson, H.; Khatri, V.P.; Bradley, M.J.; Shimizu, M.B.; Buchman, T.G.; et al. Cellular microRNAs correlate with clinical parameters in multiple injury patients. J. Trauma Acute Care Surg. 2022, 93, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, G.; Wang, X.; Zhang, B.; Zhao, T.; Wu, L. Correlation analysis of serum miR-21 and miR-210 with hs-CRP, TNF-α, IL-6, and ICAM-1 in patients with sepsis after burns. Burns 2021, 48, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Henrich, D.; Marzi, I.; Leppik, L. Decrease of exosomal miR-21-5p and the increase of CD62p+ exosomes are associated with the development of sepsis in polytraumatized patients. Mol. Cell. Probes 2024, 74, 101954. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, Y.; Sun, H.; Zhang, L.; Hao, L.; Shi, C.; Wang, J.; Li, R.; Ran, X.; Su, Y.; et al. miR-21 Regulates Skin Wound Healing by Targeting Multiple Aspects of the Healing Process. Am. J. Pathol. 2012, 181, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, M.; Jochems, D.; Houwert, R.; van Es, M.; Leenen, L.; van Wessem, K. Mortality in polytrauma patients with moderate to severe TBI on par with isolated TBI patients: TBI as last frontier in polytrauma patients. Injury 2022, 53, 1443–1448. [Google Scholar] [CrossRef]
- Barnett, R.E.; Conklin, D.J.; Ryan, L.; Keskey, R.C.; Ramjee, V.; A Sepulveda, E.; Srivastava, S.; Bhatnagar, A.; Cheadle, W.G. Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J. Leukoc. Biol. 2015, 99, 361–371. [Google Scholar] [CrossRef]

| Scratch injury model on cultured brain microvascular endothelial cells (BMVECs), PC12 cells |
| [16,17,18] |
| Scratch injury model on cultured neurons Transfection of miR-21 agomir/antagomir |
| [19] |
| Coculture of PC12 (neuron) and BV2 (microglia) cells |
| [20] |
| Cortical primary neuronal and astrocytic cells in oxygen- and glucose-deprived (OGD) medium |
| [21] |
| Fluid percussion injury rat model | Upregulation of miR-21 was linked to the following:
| [17,32] |
| TBI mimic model: cultured HT-22 neurons treated with mouse TBI brain extract |
| [22] |
| Controlled cortical impact injury in adult and aged mice, miRNA analysis of injured cortex |
| [24] |
| TBI models in rats and mice |
| [23,25,26,27,28,29,30] |
| Rat model of subarachnoid hemorrhage (SAH), transfer of MSC-derived EVs |
| [31] |
| Brain microvascular endothelial cells (BMVECs), cortical impact on mouse brain |
| [33] |
| TBI patients |
| [35,36] |
| Rat bone marrow-derived mesenchymal stem cells (rBMSCs) and repair capacity in a rat closed femur fracture model with internal fixation |
| [77] |
| Levels of circulating miRNAs, analysis of bone tissue samples in patients with fractures |
| [81,82,83] |
| Analysis of miRNA from serum samples, bone tissue analysis |
| [80,84,85,86] |
| Rat H9C2 cells and isolated primary cardiomyocytes were cultured under hypoxic conditions and treated with Prostaglandin-E1 (PGE1) |
| [93] |
| Serum samples of polytrauma patients with and without increased troponin levels |
| [102] |
| Patients with coronary artery disease (CAD) In vitro hypoxia–reoxygenation (HR)-exposed HUVECs |
| [103] |
| Mouse model of cardiac infarction with co-injection of agomiR-21 and agomiR-146a |
| [97] |
| Plasma samples from patients with acute heart failure (HF) compared to healthy controls |
| [104] |
| Acute lung injury in rats |
| [109] |
| miRNA serum levels in patients |
| [111,112] |
| miRNA analysis in plasma samples of polytraumatized patients |
| [121,123] |
| miRNA analysis in plasma samples of patients after burn with and without sepsis |
| [122] |
| Peritonitis in cecal ligation mouse model |
| [126] |
| Skin wounds were treated with miR-21 antagomir |
| [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, A.; Han, J.; Bianconi, S.; Henrich, D.; Marzi, I.; Leppik, L.; Weber, B. The Ambivalent Role of miRNA-21 in Trauma and Acute Organ Injury. Int. J. Mol. Sci. 2024, 25, 11282. https://doi.org/10.3390/ijms252011282
Ritter A, Han J, Bianconi S, Henrich D, Marzi I, Leppik L, Weber B. The Ambivalent Role of miRNA-21 in Trauma and Acute Organ Injury. International Journal of Molecular Sciences. 2024; 25(20):11282. https://doi.org/10.3390/ijms252011282
Chicago/Turabian StyleRitter, Aileen, Jiaoyan Han, Santiago Bianconi, Dirk Henrich, Ingo Marzi, Liudmila Leppik, and Birte Weber. 2024. "The Ambivalent Role of miRNA-21 in Trauma and Acute Organ Injury" International Journal of Molecular Sciences 25, no. 20: 11282. https://doi.org/10.3390/ijms252011282
APA StyleRitter, A., Han, J., Bianconi, S., Henrich, D., Marzi, I., Leppik, L., & Weber, B. (2024). The Ambivalent Role of miRNA-21 in Trauma and Acute Organ Injury. International Journal of Molecular Sciences, 25(20), 11282. https://doi.org/10.3390/ijms252011282







