Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum
Abstract
1. Introduction
2. Results
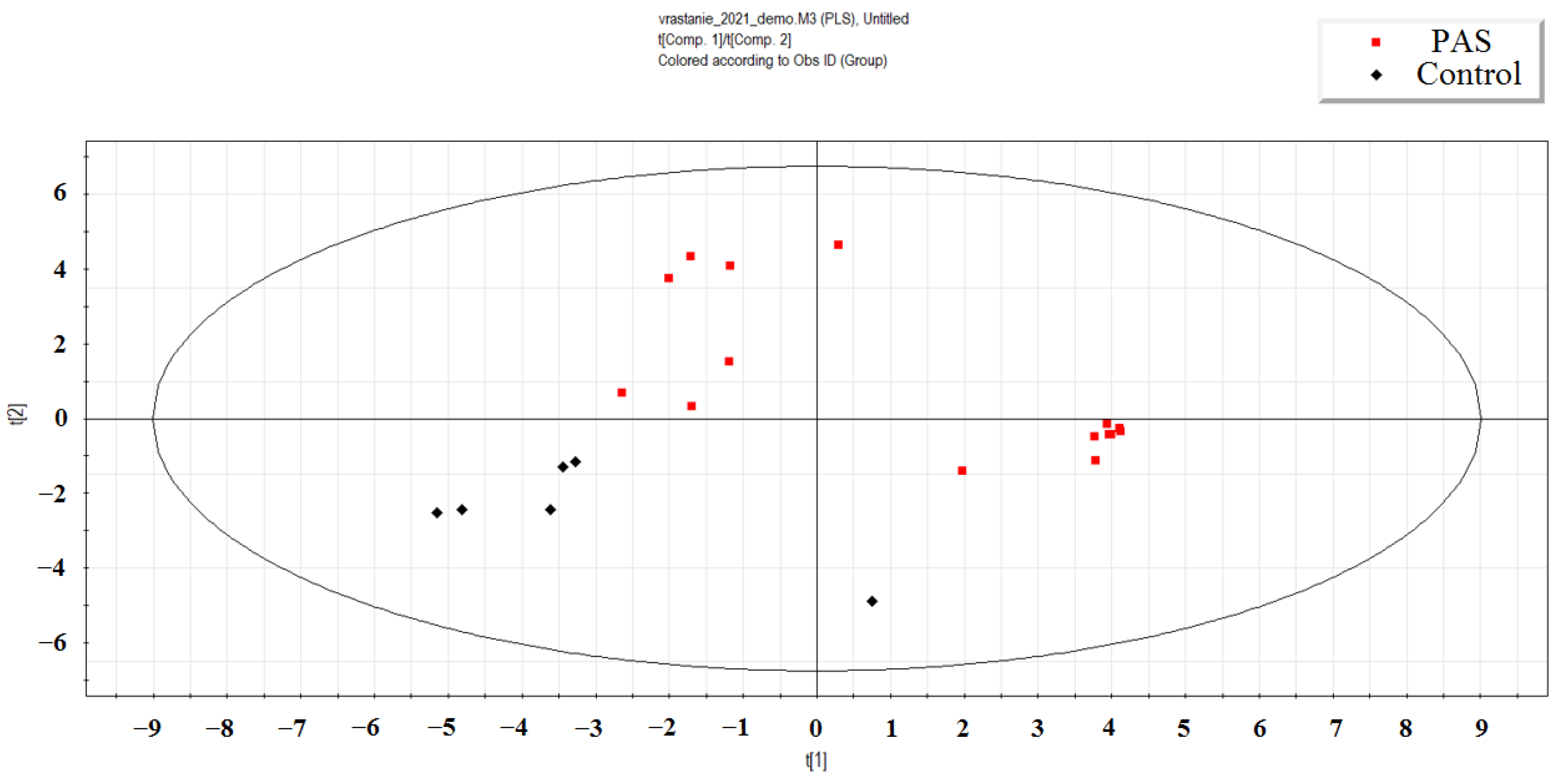
2.1. Deep Sequencing of Neonatal Blood Plasma miRNA
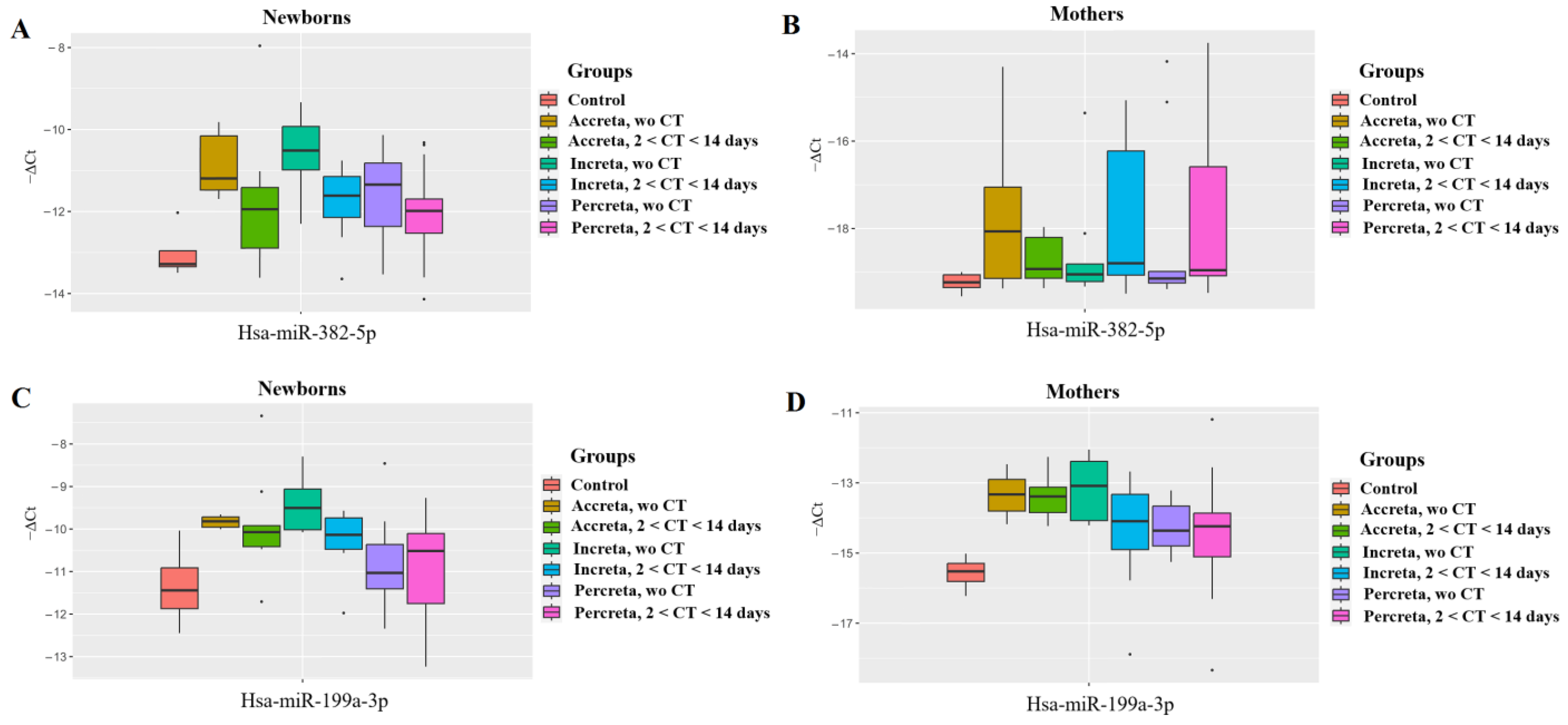
2.2. Validation of miRNAs Sequencing Data by Quantitative Real-Time PCR
- A direct correlation between the levels of hsa-miR-382-5p and hsa-miR-199a-3p in the blood plasma of newborns (r = 0.49; p < 0.001);
- an inverse correlation between the level of hsa-miR-199a-3p in the blood plasma of mothers and their newborns with the depth of trophoblast invasion (r = −0.46; p < 0.001 for mothers and r = −0.29; p = 0.028 for newborns);
- an inverse relationship between hsa-miR-382-5p levels in newborns of women with PAS and their weight (r = −0.39; p = 0.002);
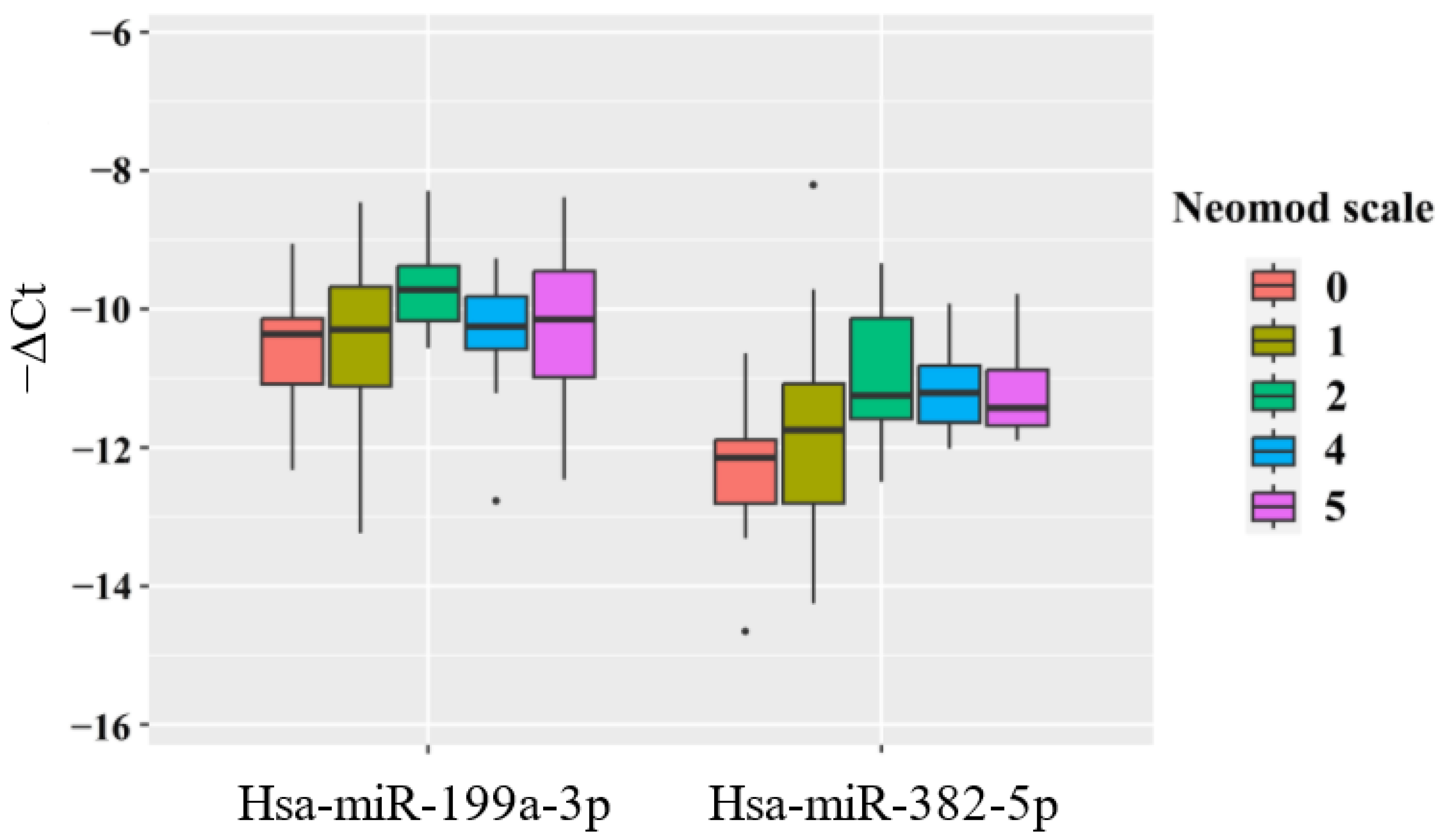
- a direct relationship between the level of hsa-miR-382-5p in the blood plasma of the newborn and the required fraction of oxygen in the NICU (r = 0.41; p = 0.001), duration of stay in the NICU (r = 0.31; p = 0.019), and the severity of the newborn’s condition according to the NEOMOD scale (r = 0.36; p = 0.005).
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation of RNA from Peripheral Blood Plasma Samples
4.3. Deep Sequencing of miRNA
4.4. Reverse Transcription and Quantitative Real-Time PCR
4.5. Statistical Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tantbirojn, P.; Crum, C.P.; Parast, M.M. Pathophysiology of Placenta Creta: The Role of Decidua and Extravillous Trophoblast. Placenta 2008, 29, 639–645. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists, and Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstet. Gynecol. 2018, 132, e259–e275. [Google Scholar] [CrossRef]
- Jauniaux, E.; Bunce, C.; Grønbeck, L.; Langhoff-Roos, J. Prevalence and Main Outcomes of Placenta Accreta Spectrum: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2019, 221, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hull, A.D.; Moore, T.R. Multiple Repeat Cesareans and the Threat of Placenta Accreta: Incidence, Diagnosis, Management. Clin. Perinatol. 2011, 38, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.J.; Patterson, J.A.; Nippita, T.A.; Torvaldsen, S.; Ibiebele, I.; Simpson, J.M.; Ford, J.B. Antecedents of Abnormally Invasive Placenta in Primiparous Women: Risk Associated with Gynecologic Procedures. Obstet. Gynecol. 2018, 131, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kocherginsky, M.; Hibbard, J.U. Abnormal Placentation: Twenty-Year Analysis. Am. J. Obstet. Gynecol. 2005, 192, 1458–1461. [Google Scholar] [CrossRef]
- Spillane, N.T.; Zamudio, S.; Alvarez-Perez, J.; Andrews, T.; Nyirenda, T.; Alvarez, M.; Al-Khan, A. Increased Incidence of Respiratory Distress Syndrome in Neonates of Mothers with Abnormally Invasive Placentation. PLoS ONE 2018, 13, e0201266. [Google Scholar] [CrossRef]
- Munoz, J.L.; Kimura, A.M.; Julia, J.; Tunnell, C.; Hernandez, B.; Curbelo, J.; Ramsey, P.S.; Ireland, K.E. Impact of Placenta Accreta Spectrum (PAS) Pathology on Neonatal Respiratory Outcomes in Cesarean Hysterectomies. J. Matern.-Fetal Neonatal Med. 2022, 35, 10692–10697. [Google Scholar] [CrossRef]
- Haram, K.; Mortensen, J.H.S.; Wollen, A.-L. Preterm Delivery: An Overview. Acta Obstet. Gynecol. Scand. 2003, 82, 687–704. [Google Scholar] [CrossRef]
- Patel, R.M. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am. J. Perinatol. 2016, 33, 318–328. [Google Scholar] [CrossRef]
- Doyle, L.W. Outcome at 5 Years of Age of Children 23 to 27 Weeks’ Gestation: Refining the Prognosis. Pediatrics 2001, 108, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An Overview of Mortality and Sequelae of Preterm Birth from Infancy to Adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Balashova, E.N.; Ionov, O.V.; Kirtbaya, A.R.; Nikonets, A.D.; Mikheeva, A.A.; Vasilchenko, O.N.; Zubkov, V.V.; Shmakov, R.G.; Degtyarev, D.N. The Features of Respiratory and Cardiovascular Disorders in Preterm Infants Born to Mothers with Abnormally Invasive Placenta. Obstet. Gynegology 2021, 5, 85–93. [Google Scholar] [CrossRef]
- Jobe, A.H.; Soll, R.F. Choice and Dose of Corticosteroid for Antenatal Treatments. Am. J. Obstet. Gynecol. 2004, 190, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi-Bannerman, C.; Thom, E.A.; Blackwell, S.C.; Tita, A.T.N.; Reddy, U.M.; Saade, G.R.; Rouse, D.J.; McKenna, D.S.; Clark, E.A.S.; Thorp, J.M.J.; et al. Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. N. Engl. J. Med. 2016, 374, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Ballard, P.L.; Ballard, R.A. Scientific Basis and Therapeutic Regimens for Use of Antenatal Glucocorticoids. Am. J. Obstet. Gynecol. 1995, 173, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Ramson, J.A.; Brownfoot, F.C. Different Corticosteroids and Regimens for Accelerating Fetal Lung Maturation for Babies at Risk of Preterm Birth. Cochrane Database Syst. Rev. 2022, 8, CD006764. [Google Scholar] [CrossRef]
- Nikonets, A.D.; Balashova, E.N.; Ionov, O.V.; Kirtbaya, A.R.; Zubkov, V.V.; Shmakov, R.G.; Degtyarev, D.N. Prevention of Respiratory Disorders in Late Preterm Neonates Born to Mothers with Abnormally Invasive Placenta. Obstet. Gynegology 2024, 1, 90–100. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Fedorov, I.S.; Suhova, Y.V.; Tarasova, A.M.; Ezhova, L.S.; Zabelina, T.M.; Vasilchenko, O.N.; Ivanets, T.Y.; Sukhikh, G.T. Diagnostic Role of Cell-Free MiRNAs in Identifying Placenta Accreta Spectrum during First-Trimester Screening. Int. J. Mol. Sci. 2024, 25, 871. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Fedorov, I.S.; Pirogova, M.M.; Vasilchenko, O.N.; Chagovets, V.V.; Ezhova, L.S.; Zabelina, T.M.; Shmakov, R.G.; Sukhikh, G.T. Clusterin and Its Potential Regulatory MicroRNAs as a Part of Secretome for the Diagnosis of Abnormally Invasive Placenta: Accreta, Increta, and Percreta Cases. Life 2021, 11, 270. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.-P.; et al. An Estimate of the Total Number of True Human MiRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. Anatomy of Four Human Argonaute Proteins. Nucleic Acids Res. 2022, 50, 6618–6638. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H.A. The Diverse Functions of MicroRNAs in Animal Development and Disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Ospina-Prieto, S.; Chaiwangyen, W.; Schoenleben, M.; Markert, U.R. Pregnancy-Associated MiRNA-Clusters. J. Reprod. Immunol. 2013, 97, 51–61. [Google Scholar] [CrossRef]
- Reza, A.M.M.T.; Choi, Y.-J.; Han, S.G.; Song, H.; Park, C.; Hong, K.; Kim, J.-H. Roles of MicroRNAs in Mammalian Reproduction: From the Commitment of Germ Cells to Peri-Implantation Embryos. Biol. Rev. Camb. Philos. Soc. 2019, 94, 415–438. [Google Scholar] [CrossRef]
- Hayder, H.; O’Brien, J.; Nadeem, U.; Peng, C. MicroRNAs: Crucial Regulators of Placental Development. Reproduction 2018, 155, R259–R271. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Fedorov, I.S.; Shamina, M.A.; Chagovets, V.V.; Makarova, N.P.; Kalinina, E.A.; Nazarenko, T.A.; Sukhikh, G.T. Clinical Relevance of Secreted Small Noncoding RNAs in an Embryo Implantation Potential Prediction at Morula and Blastocyst Development Stages. Life 2021, 11, 1328. [Google Scholar] [CrossRef]
- Zhao, Z.; Moley, K.H.; Gronowski, A.M. Diagnostic Potential for MiRNAs as Biomarkers for Pregnancy-Specific Diseases. Clin. Biochem. 2013, 46, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Fedorov, I.S.; Sukhova, Y.V.; Ivanets, T.Y.; Sukhikh, G.T. Prediction of Early- and Late-Onset Pre-Eclampsia in the Preclinical Stage via Placenta-Specific Extracellular MiRNA Profiling. Int. J. Mol. Sci. 2023, 24, 8006. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Fedorov, I.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V.V.; Volochaeva, M.V.; Starodubtseva, N.L.; Frankevich, V.E.; Nikolaev, E.N.; Shmakov, R.G.; et al. MiRNAs and Their Gene Targets-A Clue to Differentiate Pregnancies with Small for Gestational Age Newborns, Intrauterine Growth Restriction, and Preeclampsia. Diagnostics 2021, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA Profiling: Approaches and Considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Gantier, M.P.; McCoy, C.E.; Rusinova, I.; Saulep, D.; Wang, D.; Xu, D.; Irving, A.T.; Behlke, M.A.; Hertzog, P.J.; Mackay, F.; et al. Analysis of MicroRNA Turnover in Mammalian Cells Following Dicer1 Ablation. Nucleic Acids Res. 2011, 39, 5692–5703. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal Corticosteroids for Accelerating Fetal Lung Maturation for Women at Risk of Preterm Birth. Cochrane Database Syst. Rev. 2020, 12, CD004454. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V. Antenatal Corticosteroids for Maturity of Term or near Term Fetuses: Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMJ 2016, 355, i5044. [Google Scholar] [CrossRef]
- Pan, J.; Ho, M. Role of Glypican-1 in Regulating Multiple Cellular Signaling Pathways. Am. J. Physiol. Cell Physiol. 2021, 321, C846–C858. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted Role of MTOR (Mammalian Target of Rapamycin) Signaling Pathway in Human Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Van Acker, T.; Tavernier, J.; Peelman, F. The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity. Int. J. Mol. Sci. 2019, 20, 2209. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK Pathway: Metabolism and Growth Control in Tumour Suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Toussia-Cohen, S.; Castel, E.; Friedrich, L.; Mor, N.; Ohayon, A.; Levin, G.; Meyer, R. Neonatal Outcomes in Pregnancies Complicated by Placenta Accreta- a Matched Cohort Study. Arch. Gynecol. Obstet. 2024, 310, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Perepelitsa, S.A. Acute Respiratory Distress Syndrome in Preterm Newborns (Morphological Study). Gen. Reanimatol. 2020, 16, 35–44. [Google Scholar] [CrossRef]
- Heidemann, S.M.; Nair, A.; Bulut, Y.; Sapru, A. Pathophysiology and Management of Acute Respiratory Distress Syndrome in Children. Pediatr. Clin. N. Am. 2017, 64, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Ragaller, M.; Richter, T. Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Emerg. Trauma. Shock 2010, 3, 43–51. [Google Scholar] [CrossRef]
- Huang, Q.; Le, Y.; Li, S.; Bian, Y. Signaling Pathways and Potential Therapeutic Targets in Acute Respiratory Distress Syndrome (ARDS). Respir. Res. 2024, 25, 30. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, S.; Meng, X.; Shi, M.; Huang, S.; Li, J.; Zhang, J.; Liang, Y.; Ji, M.; Zhao, Y.; et al. MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2022, 23, 5545. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Pattarayan, D.; Rajaguru, P.; Sudhakar Gandhi, P.S.; Thimmulappa, R.K. MicroRNA Regulation of Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Cell. Physiol. 2016, 231, 2097–2106. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, H.; Zhang, J.-L.; Zheng, Z.; Wang, H.-T.; Tao, K.; Han, S.-C.; Su, L.-L.; Hu, D. Acute Downregulation of MiR-199a Attenuates Sepsis-Induced Acute Lung Injury by Targeting SIRT1. Am. J. Physiol. Cell Physiol. 2018, 314, C449–C455. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Dong, X.; Yang, Y.; Liu, L. MiR-199a-3p-Regulated Alveolar Macrophage-Derived Secretory Autophagosomes Exacerbate Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome. Front. Cell. Infect. Microbiol. 2022, 12, 1061790. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.; Zaragosi, L.-E.; Truchi, M.; Becavin, C.; Ruiz García, S.; Arguel, M.-J.; Plaisant, M.; Magnone, V.; Lebrigand, K.; Abelanet, S.; et al. A Single-Cell Atlas of the Human Healthy Airways. Am. J. Respir. Crit. Care Med. 2020, 202, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Jardine, L.; Haniffa, M. Human Lung Macrophages: Roll up for the MISTRG Tour. Immunity 2021, 54, 194–196. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Dong, X.; Qiu, H.; Yang, Y.; Liu, L. Secretory Autophagosomes from Alveolar Macrophages Exacerbate Acute Respiratory Distress Syndrome by Releasing IL-1β. J. Inflamm. Res. 2022, 15, 127–140. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, Y.; Li, C.; Zhao, Y.; Wang, Q.; Zhang, X. PAK4 Suppresses TNF-Induced Release of Endothelial Microparticles in HUVECs Cells. Aging 2020, 12, 12740–12749. [Google Scholar] [CrossRef]
- Mishra, R.; Benlhabib, H.; Guo, W.; Lerma Cervantes, C.B.; Mendelson, C.R. Developmental Decline in the MicroRNA 199a (MiR-199a)/MiR-214 Cluster in Human Fetal Lung Promotes Type II Cell Differentiation by Upregulating Key Transcription Factors. Mol. Cell. Biol. 2018, 38, e00037-18. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory Functions of Surfactant Proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef]
- Lawson, P.R.; Reid, K.B. The Roles of Surfactant Proteins A and D in Innate Immunity. Immunol. Rev. 2000, 173, 66–78. [Google Scholar] [CrossRef]
- Condon, J.C.; Jeyasuria, P.; Faust, J.M.; Mendelson, C.R. Surfactant Protein Secreted by the Maturing Mouse Fetal Lung Acts as a Hormone That Signals the Initiation of Parturition. Proc. Natl. Acad. Sci. USA 2004, 101, 4978–4983. [Google Scholar] [CrossRef]
- Montalbano, A.P.; Hawgood, S.; Mendelson, C.R. Mice Deficient in Surfactant Protein A (SP-A) and SP-D or in TLR2 Manifest Delayed Parturition and Decreased Expression of Inflammatory and Contractile Genes. Endocrinology 2013, 154, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Rabbitt, E.H.; Condon, J.C.; Renthal, N.E.; Johnston, J.M.; Mitsche, M.A.; Chambon, P.; Xu, J.; O’Malley, B.W.; Mendelson, C.R. Steroid Receptor Coactivators 1 and 2 Mediate Fetal-to-Maternal Signaling That Initiates Parturition. J. Clin. Investig. 2015, 125, 2808–2824. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.D.; Flecknoe, S.J.; Tan, K.H.; Olsson, P.F.; Antony, N.; Mantamadiotis, T.; Mollard, R.; Hooper, S.B.; Cole, T.J. CAMP Response Element Binding Protein Is Required for Differentiation of Respiratory Epithelium during Murine Development. PLoS ONE 2011, 6, e17843. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.B.; Berg, T.; Barton, J.L.; Didon, L.; Nord, M. Airway Epithelial Cell Differentiation during Lung Organogenesis Requires C/EBPα and C/EBPβ. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2012, 241, 911–923. [Google Scholar] [CrossRef]
- Garg, M. Targeting MicroRNAs in Epithelial-to-Mesenchymal Transition-Induced Cancer Stem Cells: Therapeutic Approaches in Cancer. Expert Opin. Ther. Targets 2015, 19, 285–297. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Mathias, R.A.; Liu, L.; Sheng, J.; Zhu, H.-J.; Simpson, R.J. Emerging Roles of Exosomes during Epithelial-Mesenchymal Transition and Cancer Progression. Semin. Cell Dev. Biol. 2015, 40, 60–71. [Google Scholar] [CrossRef]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef]
- Das, V.; Bhattacharya, S.; Chikkaputtaiah, C.; Hazra, S.; Pal, M. The Basics of Epithelial–Mesenchymal Transition (EMT): A Study from a Structure, Dynamics, and Functional Perspective. J. Cell. Physiol. 2019, 234, 14535–14555. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Nigam, D.; Mahdi, A.A.; Das, V.; Agarwal, A.; Pandey, A.; Gautam, A. Aberrant Akt Activation During Implantation Window in Infertile Women With Intramural Uterine Fibroids. Reprod. Sci. 2018, 25, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tao, T.; Yin, Y.; Zhao, L.; Yang, L.; Hu, L. MiR-144 May Regulate the Proliferation, Migration and Invasion of Trophoblastic Cells through Targeting PTEN in Preeclampsia. Biomed. Pharmacother. 2017, 94, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.-X.; Zhou, X.-T.; Tian, Q.-C.; Xie, H.-Q.; Zhang, J.-Y. Low Expression of MicroRNA-21 Inhibits Trophoblast Cell Infiltration Through Targeting PTEN. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6181–6189. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN Induces Lung Fibrosis via Alveolar Epithelial Cell Senescence Depending on NF-ΚB Activation. Aging Cell 2019, 18, e12858. [Google Scholar] [CrossRef]
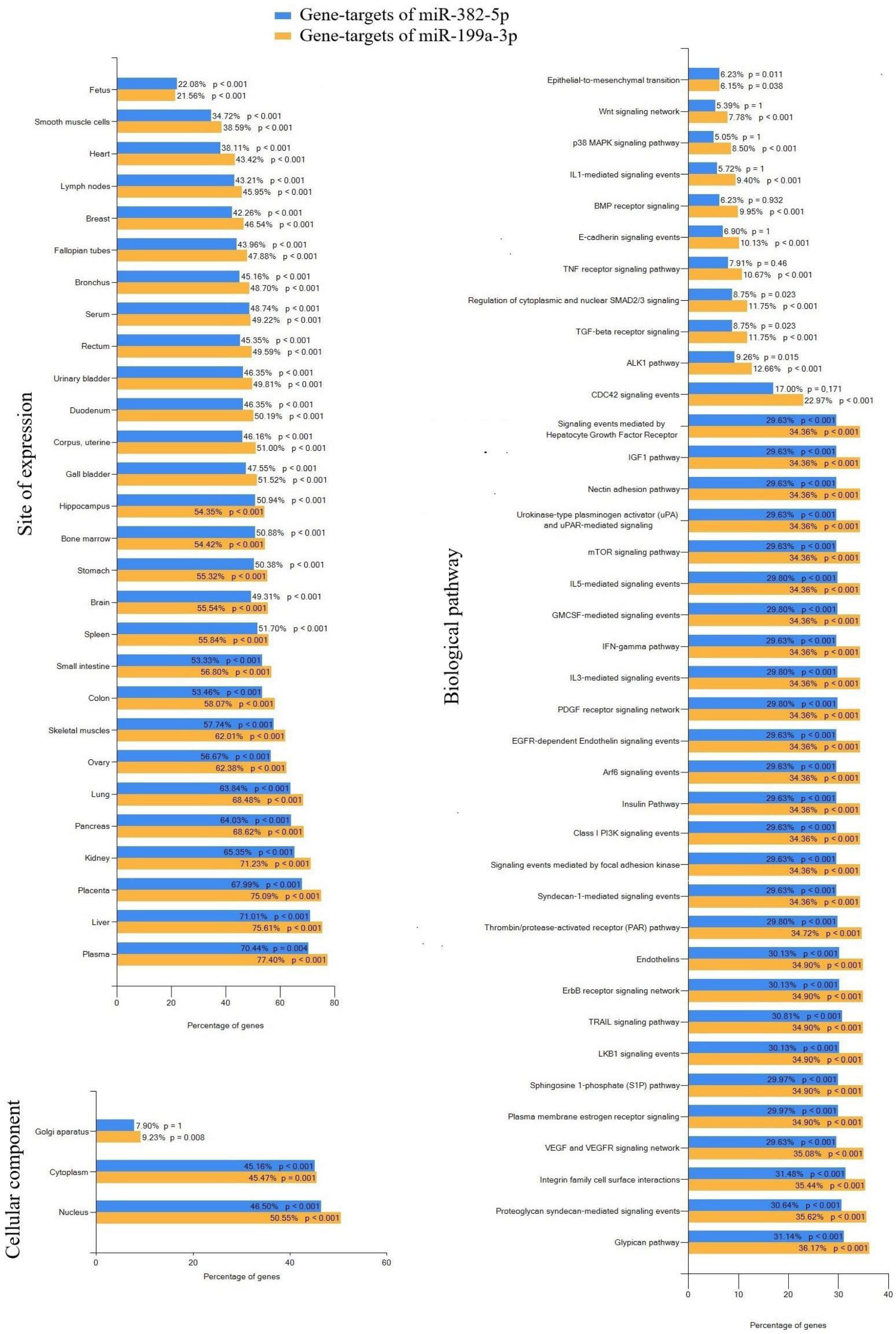
- Murrieta-Coxca, J.M.; Barth, E.; Fuentes-Zacarias, P.; Gutiérrez-Samudio, R.N.; Groten, T.; Gellhaus, A.; Köninger, A.; Marz, M.; Markert, U.R.; Morales-Prieto, D.M. Identification of Altered MiRNAs and Their Targets in Placenta Accreta. Front. Endocrinol. 2023, 14, 1021640. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zheng, M.; Diao, Z.; Shen, L.; Liu, M.; Gong, P.; Sun, H.; Hu, Y. MiR-155* Mediates Suppressive Effect of PTEN 3’-Untranslated Region on AP-1/NF-ΚB Pathway in HTR-8/SVneo Cells. Placenta 2013, 34, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; Daels, P.; Loux, S.C.; Esteller-Vico, A.; Carossino, M.; Scoggin, K.E.; Ball, B.A. Kinetics of the Chromosome 14 MicroRNA Cluster Ortholog and Its Potential Role during Placental Development in the Pregnant Mare. BMC Genom. 2018, 19, 954. [Google Scholar] [CrossRef]
- Dini, P.; El-Sheikh Ali, H.; Carossino, M.; Loux, S.C.; Esteller-Vico, A.; E. Scoggin, K.; Daels, P.; A. Ball, B. Expression Profile of the Chromosome 14 MicroRNA Cluster (C14MC) Ortholog in Equine Maternal Circulation throughout Pregnancy and Its Potential Implications. Int. J. Mol. Sci. 2019, 20, 6285. [Google Scholar] [CrossRef]
- Xing, Y.; Fu, J.; Yang, H.; Yao, L.; Qiao, L.; Du, Y.; Xue, X. MicroRNA Expression Profiles and Target Prediction in Neonatal Wistar Rat Lungs During the Development of Bronchopulmonary Dysplasia. Int. J. Mol. Med. 2015, 36, 1253–1263. [Google Scholar] [CrossRef][Green Version]
- Lv, Y.; Li, Y.; Wang, J.; Li, M.; Zhang, W.; Zhang, H.; Shen, Y.; Li, C.; Du, Y.; Jiang, L. MiR-382-5p Suppresses M1 Macrophage Polarization and Inflammatory Response in Response to Bronchopulmonary Dysplasia through Targeting CDK8: Involving Inhibition of STAT1 Pathway. Genes Cells 2021, 26, 772–781. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, H.; Chen, B. Identification of Macrophage-Related Genes in Sepsis-Induced ARDS Using Bioinformatics and Machine Learning. Sci. Rep. 2023, 13, 9876. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Tao, Y.; Xu, X.; Zhao, H.; Zou, L.; Li, Y. The Role of Lung Macrophages in Acute Respiratory Distress Syndrome. Inflamm. Res. 2022, 71, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Shuai, W.; Meng, J.; Feng, J.; Han, Z. Macrophage Polarization and Its Role in the Pathogenesis of Acute Lung Injury/Acute Respiratory Distress Syndrome. Inflamm. Res. 2020, 69, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wei, H.; Sheng, Y.; Peng, T.; Zhao, Q.; Xie, L.; Yang, J. Circ_0006640 Transferred by Bone Marrow-Mesenchymal Stem Cell-Exosomes Suppresses Lipopolysaccharide-Induced Apoptotic, Inflammatory and Oxidative Injury in Spinal Cord Injury. J. Orthop. Surg. Res. 2024, 19, 50. [Google Scholar] [CrossRef]
- Hellström, A.; Ley, D.; Hansen-Pupp, I.; Hallberg, B.; Löfqvist, C.; van Marter, L.; van Weissenbruch, M.; Ramenghi, L.A.; Beardsall, K.; Dunger, D.; et al. Insulin-like Growth Factor 1 Has Multisystem Effects on Foetal and Preterm Infant Development. Acta Paediatr. 2016, 105, 576–586. [Google Scholar] [CrossRef]
- Catalucci, D.; Latronico, M.V.G.; Condorelli, G. MicroRNAs Control Gene Expression: Importance for Cardiac Development and Pathophysiology. Ann. N. Y. Acad. Sci. 2008, 1123, 20–29. [Google Scholar] [CrossRef]
- Neppl, R.L.; Wang, D.-Z. The Myriad Essential Roles of MicroRNAs in Cardiovascular Homeostasis and Disease. Genes Dis. 2014, 1, 18–39. [Google Scholar] [CrossRef][Green Version]
- Joris, V.; Gomez, E.L.; Menchi, L.; Lobysheva, I.; Di Mauro, V.; Esfahani, H.; Condorelli, G.; Balligand, J.-L.; Catalucci, D.; Dessy, C. MicroRNA-199a-3p and MicroRNA-199a-5p Take Part to a Redundant Network of Regulation of the NOS (NO Synthase)/NO Pathway in the Endothelium. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2345–2357. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to Increase Nitric Oxide Signalling in Cardiovascular Disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- The R Development Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org (accessed on 10 March 2021).
- RStudio Team. RStudio: Integrated Development for R. RStudio. Available online: http://www.rstudio.com/ (accessed on 23 March 2021).

| miRNA | BaseMean | log2FoldChange | lfcSE* | p-Value | |
|---|---|---|---|---|---|
| 1 | hsa-miR-215-5p | 98.7 | 5.8 | 1.2 | 4.2 × 10−6 |
| 2 | hsa-miR-516b-5p | 215.1 | 5.2 | 1.1 | 6.8 × 10−6 |
| 3 | hsa-miR-182-5p | 55.2 | 4.7 | 1.1 | 2.0 × 10−5 |
| 4 | hsa-miR-183-5p | 143.4 | 4.1 | 1.0 | 6.6 × 10−5 |
| 5 | hsa-miR-192-5p | 503.9 | 1.6 | 0.4 | <0.001 |
| 6 | hsa-miR-1323 | 30.6 | 3.8 | 1.1 | 0.001 |
| 7 | hsa-miR-760 | 15.0 | −3.2 | 1.0 | 0.001 |
| 8 | hsa-let-7f-5p | 992.7 | 2.2 | 0.7 | 0.002 |
| 9 | hsa-miR-26a-5p | 1195.9 | 1.7 | 0.6 | 0.003 |
| 10 | hsa-miR-199a-3p | 320.7 | −1.8 | 0.6 | 0.004 |
| 11 | hsa-miR-200c-3p | 121.4 | −4.1 | 1.4 | 0.004 |
| 12 | hsa-miR-199b-3p | 160.3 | −1.7 | 0.6 | 0.004 |
| 13 | hsa-let-7g-5p | 1207.6 | 1.8 | 0.6 | 0.005 |
| 14 | hsa-miR-10a-5p | 1121.6 | 2.7 | 1.0 | 0.006 |
| 15 | hsa-miR-146b-5p | 130.2 | 1.4 | 0.5 | 0.007 |
| 16 | hsa-miR-99b-3p | 8.9 | −3.4 | 1.2 | 0.008 |
| 17 | hsa-miR-218-5p | 9.3 | −4.0 | 1.6 | 0.011 |
| 18 | hsa-miR-150-5p | 24.7 | 1.4 | 0.6 | 0.019 |
| 19 | hsa-miR-29a-3p | 35.6 | 1.9 | 0.8 | 0.021 |
| 20 | hsa-miR-181b-5p | 124.9 | −2.3 | 1.0 | 0.028 |
| 21 | hsa-miR-378c | 8.8 | 1.8 | 0.8 | 0.029 |
| 22 | hsa-miR-26b-5p | 102.9 | 1.2 | 0.5 | 0.029 |
| 23 | hsa-miR-30e-3p | 45.6 | 1.5 | 0.7 | 0.031 |
| 24 | hsa-miR-483-3p | 37.4 | 2.1 | 0.9 | 0.032 |
| 25 | hsa-miR-194-5p | 209.4 | 1.6 | 0.7 | 0.033 |
| 26 | hsa-miR-99a-5p | 1362.0 | −1.4 | 0.7 | 0.037 |
| 27 | hsa-miR-2110 | 38.6 | −1.9 | 0.9 | 0.038 |
| 28 | hsa-let-7d-3p | 244.3 | 1.2 | 0.6 | 0.041 |
| 29 | hsa-miR-382-5p | 125.1 | −2.2 | 1.2 | 0.045 |
| Clinical Parameters | Control, Without CT (n = 11), I Group | PAS, Without CT (n = 10), II Group | PAS, CT More Than 14 Days Before Delivery (n = 13), III Group | PAS, CT During 7–14 Days Before Delivery (n = 25), IV Group | PAS, CT During 2–7 Days Before Delivery (n = 21), V Group | Wilcoxon–Mann–Whitney U Test, p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| I Group vs. II Group | I Group vs. III Group | I Group vs. IV Group | I Group vs. V Group | ||||||
| Weight of newborn, g | 2250.0 (1965.0; 2437.5) | 2795.5 (2542.0; 3042.2) | 2520.0 (2390.0; 2652.0) | 2863.0 (2780.0; 3030.0) | 2850.0 (2730.0; 2960.0) | 0.001 | 0.089 | <0.001 | 0.001 |
| Apgar score, 1 min | 8.0 (7.0; 8.0) | 7.0 (7.0; 8.0) | 8.0 (7.0; 8.0) | 8.0 (7.0; 8.0) | 8.0 (7.0; 8.0) | 0.205 | 0.702 | 0.606 | 0.973 |
| Apgar score, 5 min | 8.0 (8.0; 9.0) | 8.0 (8.0; 8.0) | 8.0 (8.0; 8.0) | 8.0 (8.0; 9.0) | 8.0 (8.0; 9.0) | 0.084 | 0.067 | 0.425 | 0.447 |
| WBC | 11.4 (9.7; 12.6) | 12.2 (9.9; 18.0) | 10.4 (9.3; 13.3) | 14.1 (9.5; 16.9) | 13.2 (10.6; 16.5) | 0.417 | 0.757 | 0.207 | 0.189 |
| ACHN | 4225.0 (3806.5; 4561.0) | 4776.5 (3236.2; 8941.0) | 3872.0 (3448.0; 5440.0) | 5664.0 (4323.0; 7874.0) | 6190.0 (4131.0; 7722.0) | 0.475 | 0.937 | 0.148 | 0.155 |
| Ni | 0.07 (0.04; 0.08) | 0.05 (0.02; 0.11) | 0.06 (0.03; 0.09) | 0.07 (0.03; 0.11) | 0.06 (0.05; 0.09) | 0.659 | 0.781 | 0.714 | 0.979 |
| RBC | 4.5 (4.3; 4.8) | 4.7 (4.1; 4.9) | 4.7 (4.4; 4.8) | 4.4 (4.0; 4.8) | 4.6 (4.4; 4.8) | 1.000 | 0.938 | 0.48 | 0.75 |
| RDW-CV | 16.0 (15.3; 17.2) | 15.7 (15.2; 16.2) | 15.8 (15.4; 16.6) | 15.8 (15.4; 16.1) | 15.8 (15.3; 16.5) | 0.769 | 0.721 | 0.437 | 0.652 |
| RDW-SD | 63.1 (61.9; 67.9) | 57.4 (51.8; 59.3) | 58.8 (55.9; 60.4) | 58.9 (56.7; 59.7) | 60.1 (57.7; 62.9) | 0.007 | 0.047 | 0.009 | 0.08 |
| MCV | 105.8 (105.0; 108.3) | 98.0 (95.3; 102.1) | 101.4 (99.4; 103.2) | 102.2 (98.5; 103.3) | 101.9 (100.4; 105.6) | 0.001 | 0.008 | 0.002 | 0.027 |
| HGB, g/L | 163.0 (155.5; 180.5) | 161.0 (145.5; 167.7) | 168.0 (158.0; 179.0) | 158.0 (146.0; 173.0) | 168.0 (161.0; 171.0) | 0.806 | 0.936 | 0.583 | 0.121 |
| MCH | 36.6 (35.8; 38.2) | 35.0 (34.0; 35.4) | 36.2 (35.2; 36.7) | 35.5 (35.1; 36.5) | 35.9 (35.1; 36.6) | 0.010 | 0.427 | 0.068 | 0.185 |
| MCHC | 34.6 (34.5; 34.9) | 35.4 (35.0; 36.2) | 35.7 (35.2; 36.1) | 35.4 (35.0; 35.7) | 35.1 (34.6; 35.6) | 0.050 | 0.039 | 0.079 | 0.287 |
| HTC | 47.3 (45.1; 52.1) | 42.7 (40.0; 49.5) | 47.2 (45.1; 49.8) | 44.8 (41.2; 50.6) | 47.7 (46.4; 48.9) | 0.130 | 0.606 | 0.171 | 0.958 |
| Platelets | 324.0 (288.0; 356.0) | 323.0 (280.2; 399.0) | 281.0 (224.0; 335.0) | 354.0 (317.0; 402.0) | 339.0 (296.0; 413.0) | 0.696 | 0.428 | 0.092 | 0.533 |
| MPV | 9.7 (9.0; 9.9) | 9.4 (9.2; 9.6) | 9.8 (9.4; 10.0) | 9.5 (8.9; 10.0) | 9.6 (9; 10.1) | 0.302 | 0.720 | 1.000 | 0.811 |
| PTC | 0.3 (0.2; 0.3) | 0.3 (0.2; 0.3) | 0.2 (0.2; 0.3) | 0.3 (0.3; 0.4) | 0.3 (0.2; 0.4) | 0.883 | 0.341 | 0.283 | 0.594 |
| PDW | 10.4 (9.5; 10.5) | 9.7 (8.9; 10.8) | 10.2 (9.5; 10.7) | 9.1 (8.6; 10.0) | 9.8 (9; 10.1) | 0.807 | 0.873 | 0.273 | 0.381 |
| PLCR | 22.3 (17.6; 24.0) | 19.9 (18.5; 23.1) | 22.8 (19.2; 24.5) | 19.7 (15.9; 24.2) | 21.0 (17.8; 25.1) | 0.660 | 0.751 | 0.789 | 1.000 |
| DHR | 2.0 (1.0; 4.0) | 4.5 (3.0; 6.0) | 5.0 (2.0; 6.0) | 2.0 (2.0; 4.0) | 2.0 (2.0; 3.0) | 0.115 | 0.118 | 0.591 | 0.978 |
| HD | 13.0 (9.0; 14.5) | 10.0 (8.0; 14.0) | 11.0 (11.0; 13.0) | 10.0 (7.0; 15.0) | 9.0 (7.0; 11.0) | 0.305 | 0.937 | 0.315 | 0.770 |
| miR-382-5p | |||
|---|---|---|---|
| ID Group | Group Name | RT-PCR Data | Control Group (1) vs. Groups (2–7) |
| Me (Q1; Q3) | Wilcoxon-Mann-Whitney U Test, p-Value * | ||
| 1 | Control, wo CT | −13.2 (−13.3; −12.9) | 1.000 |
| 2 | Accreta, wo CT | −11.1 (−11.4; −10.1) | 0.006 |
| 3 | Accreta, 2 < CT < 14 days | −11.9 (−12.8; −11.4) | 0.148 |
| 4 | Increta, wo CT | −10.5 (−10.9; −9.9) | 0.005 |
| 5 | Increta, 2 < CT < 14 days | −11.6 (−12.1; −11.1) | 0.036 |
| 6 | Percreta, wo CT | −11.3 (−12.3; −10.8) | 0.075 |
| 7 | Percreta, 2 < CT < 14 days | −11.9 (−12.5; −11.6) | 0.061 |
| miR-199a-3p | |||
| 1 | Control, wo CT | −11.4 (−11.8; −10.9) | 1.000 |
| 2 | Accreta, wo CT | −9.8 (−9.9; −9.7) | 0.006 |
| 3 | Accreta, 2 < CT < 14 days | −10.0 (−10.4; −9.9) | 0.106 |
| 4 | Increta, wo CT | −9.5 (−10.0; −9.0) | 0.005 |
| 5 | Increta, 2 < CT < 14 days | −10.1 (−10.4; −9.7) | 0.062 |
| 6 | Percreta, wo CT | −11.0 (−11.4; −10.3) | 0.330 |
| 7 | Percreta, 2 < CT < 14 days | −10.5 (−11.7; −10.1) | 0.470 |
| miR-382-5p | |||
|---|---|---|---|
| ID Group | Group Name | RT-PCR Data | Control Group (1) vs. Groups (2–7) |
| Me (Q1; Q3) | Wilcoxon-Mann-Whitney U test, p-Value * | ||
| 1 | Control, wo CT | −19.2 (−19.3; −19.0) | 1.000 |
| 2 | Accreta, wo CT | −18.0 (−19.1; −17.0) | 0.180 |
| 3 | Accreta, 2 < CT < 14 days | −18.9 (−19.1; −18.2) | 0.070 |
| 4 | Increta, wo CT | −19.0 (−19.2; −18.8) | 0.110 |
| 5 | Increta, 2 < CT < 14 days | −18.7 (−19.0; −16.2) | 0.020 |
| 6 | Percreta, wo CT | −19.1 (−19.2; −18.9) | 0.470 |
| 7 | Percreta, 2 < CT < 14 days | −18.9 (−19.0; −16.5) | 0.024 |
| miR-199a-3p | |||
| 1 | Control, wo CT | −15.5 (−15.8; −15.3) | 1.000 |
| 2 | Accreta, wo CT | −13.3 (−13.8; −12.9) | <0.001 |
| 3 | Accreta, 2 < CT < 14 days | −13.3 (−13.8; −13.1) | <0.001 |
| 4 | Increta, wo CT | −13.0 (−14.0; −12.3) | <0.001 |
| 5 | Increta, 2 < CT < 14 days | −14.0 (−14.9; −13.3) | 0.002 |
| 6 | Percreta, wo CT | −14.3 (−14.8; −13.6) | <0.001 |
| 7 | Percreta, 2 < CT < 14 days | −14.2 (−15.1; −13.8) | 0.015 |
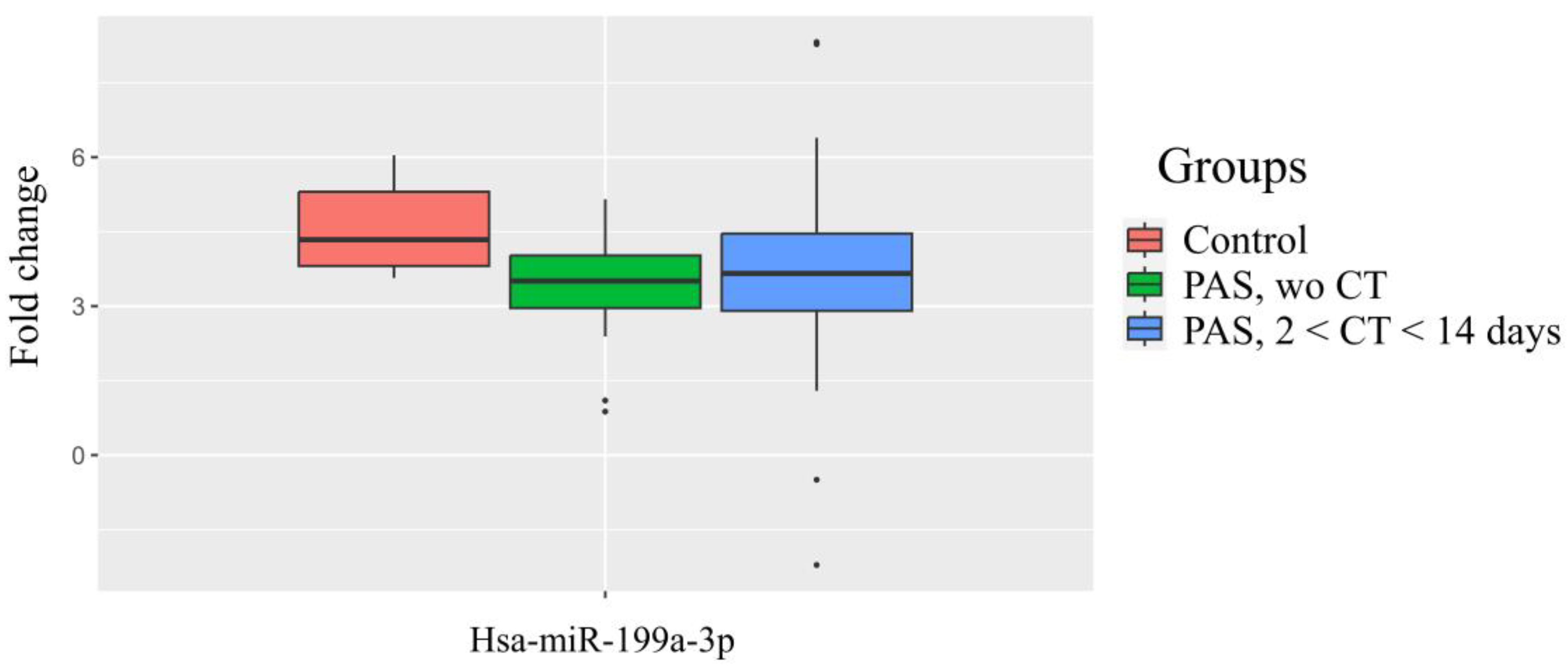
| miR-199a-3p | Control Group (1) vs. Groups (2,3) | ||
|---|---|---|---|
| ID Group | Group Name | Me (Q1; Q3) | p-Value * |
| 1 | Control, wo CT | 4.3 (3.8; 5.3) | 1.000 |
| 2 | PAS, wo CT | 3.5 (2.9; 4.0) | 0.007 |
| 3 | PAS, 2 < CT < 14 days | 3.6 (2.9; 4.4) | 0.122 |
| miR-382-5p | miR-199a-3p | |||||||
|---|---|---|---|---|---|---|---|---|
| RT-PCR Data, −ΔCt | p-Value *, Mann-Whitney U Test | RT-PCR Data, −ΔCt | p-Value *, Mann-Whitney U Test | |||||
| Groups According to the Neomod Scale | Me | Q1 | Q3 | Neomod, 0 | Me | Q1 | Q3 | Neomod, 0 |
| Neomod, 0 | −12.1 | −12.8 | −11.8 | 1.000 | −10.3 | −11.0 | −10.1 | 1.000 |
| Neomod, 1 | −11.7 | −12.8 | −11.0 | 0.251 | −10.3 | −11.1 | −9.6 | 0.672 |
| Neomod, 2 | −11.2 | −11.5 | −10.1 | 0.073 | −9.7 | −10.1 | −9.3 | 0.180 |
| Neomod, 4 | −11.2 | −11.6 | −10.8 | 0.013 | −10.2 | −10.5 | −9.8 | 0.886 |
| Neomod, 5 | −11.4 | −11.6 | −10.8 | 0.050 | −10.1 | −10.9 | −9.4 | 0.927 |
| Neomod, >4 | −11.2 | −11.6 | −10.8 | 0.009 | −10.2 | −10.5 | −9.8 | 0.855 |
| Group | miR-181a-5p | miR-199a-3p | miR-382-5p | ||||
|---|---|---|---|---|---|---|---|
| ID Group | Group Name | Me (Q1; Q3) | Control Group (1) vs. Groups (2,3), p-Value * | Me (Q1; Q3) | Control Group (1) vs. Groups (2,3), p-Value * | Me (Q1; Q3) | Control Group (1) vs. Groups (2,3), p-Value * |
| 1 | Control, wo CT | −16.1 (−17.0; −15.9) | 1.000 | −15.5 (−15.8; −15.3) | 1.000 | −19.2 (−19.3; −19.0) | 1.000 |
| 2 | PAS, wo CT | −15.6 (−19.0; −14.3) | 0.340 | −13.7 (−14.7; −12.9) | <0.001 | −18.3 (−19.0; −17.1) | <0.001 |
| 3 | PAS, 2 < CT < 14 days | −17.9 (−19.0; −15.0) | 0.690 | −14.1 (−14.9; −13.3) | <0.001 | −18.9 (−19.0; −17.9) | 0.011 |
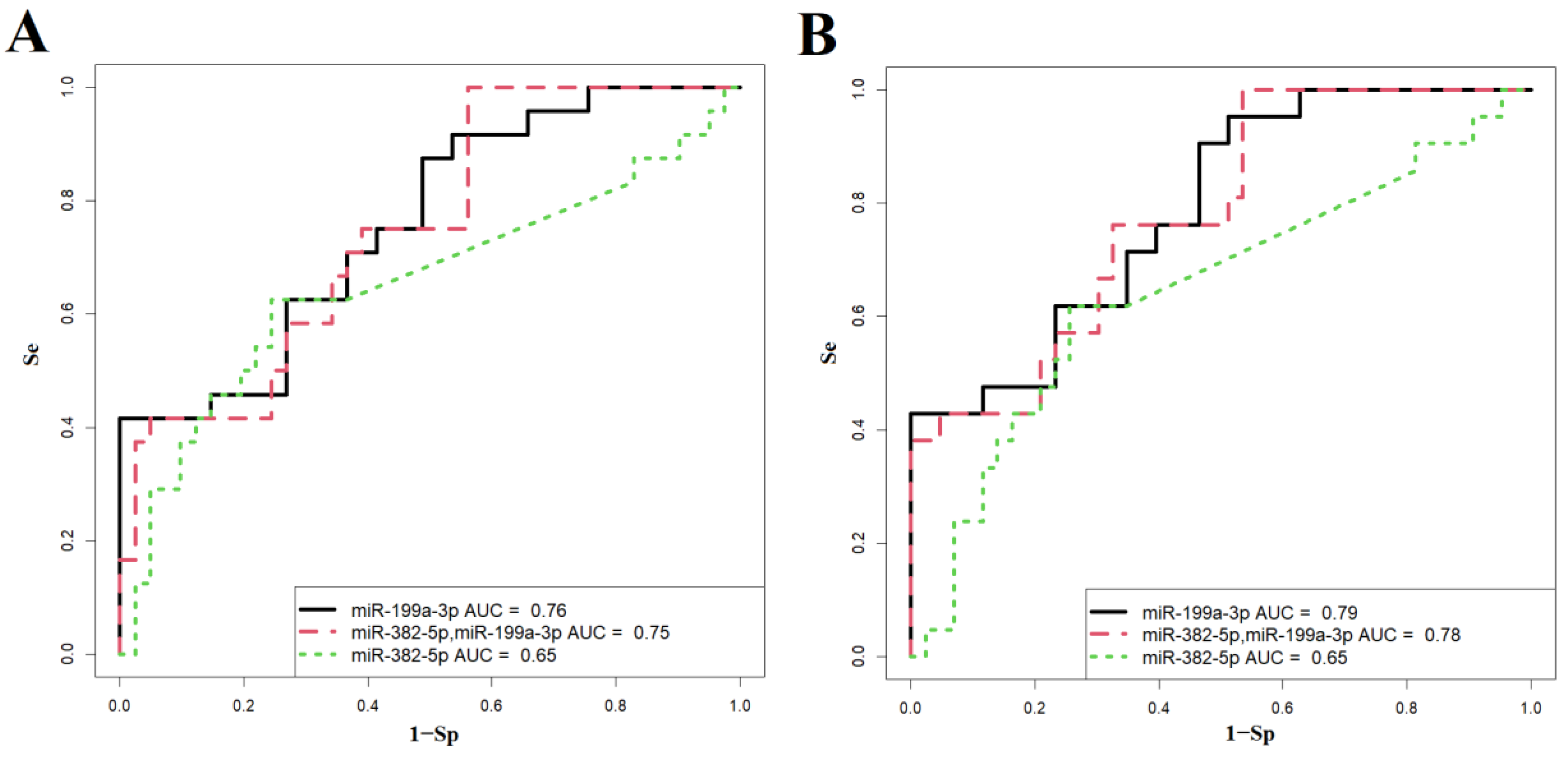
| Figure 6A | Wald | p-Value | Coefficients | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 1 Model | 0.642 | 0.42 | 1.00 | |||
| (Intercept) | 1.879 | 0.060 | 0.974 | |||
| miR-199a-3p | −3.281 | 0.001 | −0.548 | |||
| 2 Model | 0.202 | 1.00 | 0.44 | |||
| (Intercept) | 1.706 | 0.088 | 1.540 | |||
| miR-382-5p | 0.796 | 0.426 | 0.119 | |||
| miR-199a-3p | −2.662 | 0.008 | −0.699 | |||
| 3 Model | 0.422 | 0.63 | 0.76 | |||
| (Intercept) | −2.616 | 0.009 | −0.804 | |||
| miR-382-5p | −2.049 | 0.040 | −0.206 | |||
| Figure 6B | Wald | p-Value | Coefficients | Threshold | Sensitivity | Specificity |
| 1 Model | 0.160 | 0.95 | 0.49 | |||
| (Intercept) | 1.887 | 0.050 | 1.046 | |||
| miR-199a-3p | −3.473 | 0.001 | −0.635 | |||
| 2 Model | 0.150 | 1.00 | 0.47 | |||
| (Intercept) | 2.005 | 0.045 | 2.127 | |||
| miR-382-5p | 1.282 | 0.200 | 0.217 | |||
| miR-199a-3p | −2.940 | 0.003 | −0.924 | |||
| 3 Model | 0.380 | 0.62 | 0.74 | |||
| (Intercept) | −3.092 | 0.002 | −1.002 | |||
| miR-382-5p | −2.031 | 0.042 | −0.217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.V.; Fedorov, I.S.; Nikonets, A.D.; Tarasova, A.M.; Balashova, E.N.; Degtyarev, D.N.; Sukhikh, G.T. Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum. Int. J. Mol. Sci. 2024, 25, 13309. https://doi.org/10.3390/ijms252413309
Timofeeva AV, Fedorov IS, Nikonets AD, Tarasova AM, Balashova EN, Degtyarev DN, Sukhikh GT. Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum. International Journal of Molecular Sciences. 2024; 25(24):13309. https://doi.org/10.3390/ijms252413309
Chicago/Turabian StyleTimofeeva, Angelika V., Ivan S. Fedorov, Anastasia D. Nikonets, Alla M. Tarasova, Ekaterina N. Balashova, Dmitry N. Degtyarev, and Gennady T. Sukhikh. 2024. "Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum" International Journal of Molecular Sciences 25, no. 24: 13309. https://doi.org/10.3390/ijms252413309
APA StyleTimofeeva, A. V., Fedorov, I. S., Nikonets, A. D., Tarasova, A. M., Balashova, E. N., Degtyarev, D. N., & Sukhikh, G. T. (2024). Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum. International Journal of Molecular Sciences, 25(24), 13309. https://doi.org/10.3390/ijms252413309







