Future Therapeutic Strategies for Alzheimer’s Disease: Focus on Behavioral and Psychological Symptoms
Abstract
1. Introduction
2. NPS in AD
2.1. Depression
2.2. Anxiety
2.3. Apathy
2.4. Agitation and Aggression
2.5. Psychosis
2.6. Sleep Disturbances
2.7. Social Processing
2.8. Implication from the NPS in AD
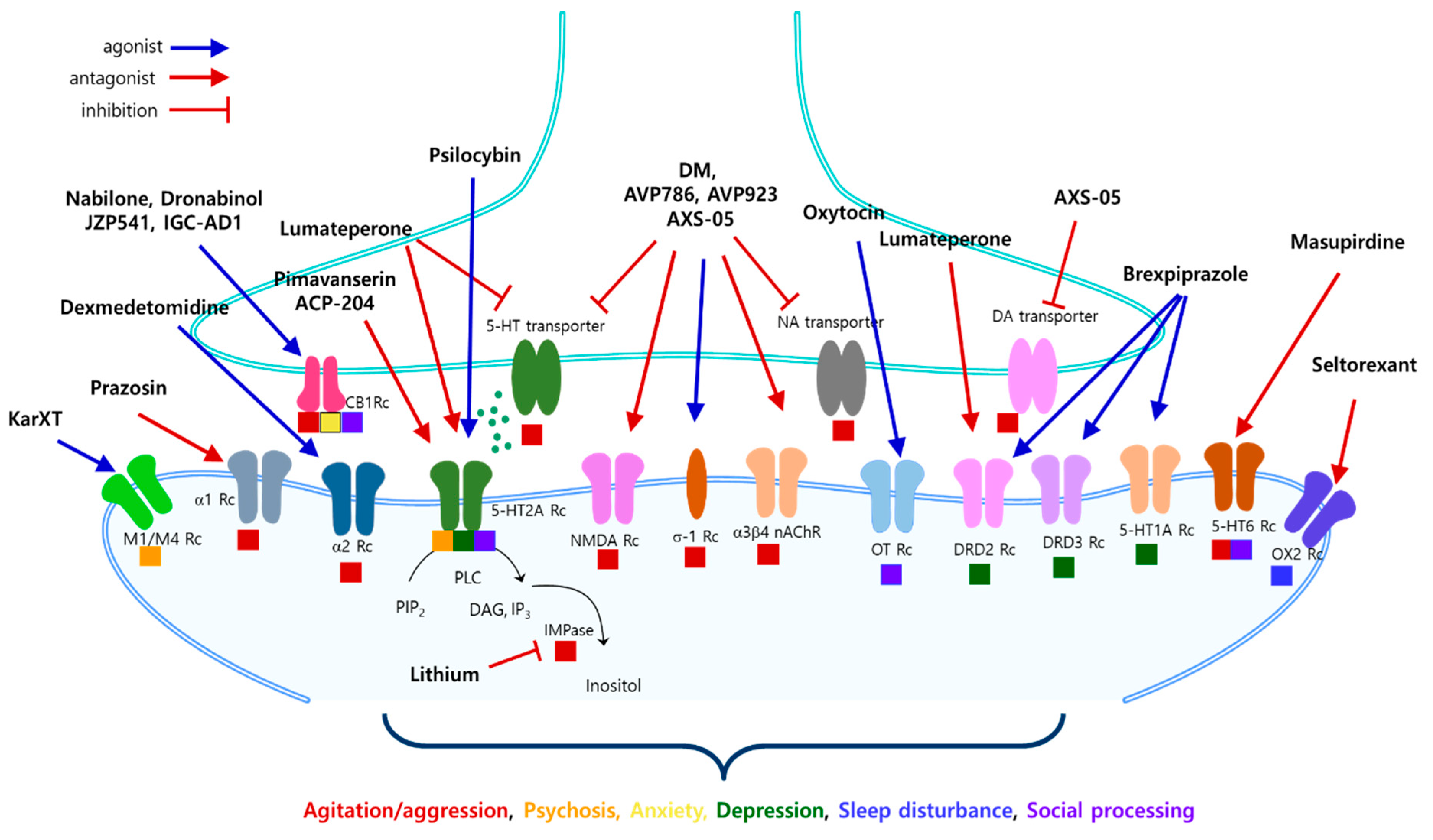
3. Treatment Pipelines, Targets, and Mechanisms of Action
3.1. An Antagonist of NMDA Receptor
3.1.1. Dextromethorphan
| Candidate Drugs | Target | Mechanism of Action | Target NPS | Disease Stage of AD | Clinical Status | References |
|---|---|---|---|---|---|---|
| Lumateperone (Caplyta) | Neurotransmitter receptors | A 5-HT2A antagonist, a SERT inhibitor, a DRD2 antagonist, a GluN2B modulator | Depression | [78,79,80,81] | ||
| Psilocybin | Neurotransmitter receptors | A modulator of 5-HT1A, 5-HT2A, and 5-HT2C receptors | Prodormal/prodromal-mild | Phase 1 | [82,83,84] | |
| Cannabidiol | Neurotransmitter receptors | Cannabinoid, an agonist against 5-HT1A, A2A, and TRP-V1 receptors, anti-inflammatory action | Anxiety | Mild–moderate dementia, prodormal/prodromal-mild | Phase 1 Phase 2 | [85,86] |
| Dextromethorphan | Neurotransmitter receptors | NMDA receptor antagonist; σ-1 receptor agonist, a SERT and NET inhibitor, a nicotinic σ3β4 receptor antagonist | Agitation | [15,75,76,77,87,88] | ||
| AVP-923 (Neudexta) | Neurotransmitter receptors | dextromethorphan/ quinidine | Mild–moderate dementia, severe dementia | [87,89,90] | ||
| AXS-05 | Neurotransmitter receptors | dextromethorphan/ bupropion NMDA receptor antagonist; σ-1 receptor agonist. A SERT and NET inhibitor | Mild–moderate dementia, severe dementia | Phase 3 | [91,92,93] | |
| Nabilone | Cannabinoid receptor | A partial agonist against CB1 and CB2 receptor | Mild–moderate dementia, severe dementia | Phase 3 | [94,95] | |
| Brexpiprazole | Neurotransmitter receptors | A partial agonist against D2, D3 receptor, and 5-HT1A. A serotonin and dopamine modulator | Mild–moderate dementia, severe dementia | [42,96,97] | ||
| AVP-786 | Neurotransmitter receptors | NMDA receptor antagonist; σ-1 receptor agonist. A SERT and NET inhibitor | Mild–moderate dementia, severe dementia | Phase 3 | [16,98] | |
| Dexmedetomidine | Neurotransmitter receptors | An α2 adrenergic agonist | Mild–moderate dementia, severe dementia | Phase 3 | [99,100,101] | |
| JZP541 | Cannabinoid receptor | An agonist against CB1 and CB2 receptor | Mild–moderate dementia Severe dementia | Phase 2 | [16] | |
| Dronabinol | Cannabinoid receptor | A weak partial agonist against CB1 and CB2 receptor | Mild–moderate dementia | Phase 2 | [102,103,104] | |
| IGC-AD1 | Cannabinoid receptor | Cannabinoid, a partial agonist against CB1 receptor | Mild–moderate dementia, severe dementia | Phase 2 | [16,86] | |
| Prazosin | Neurotransmitter receptors | An α1 adrenergic antagonist | Phase 2b | [105,106] | ||
| SCI-110 | Neurotransmitter receptors | Tetrahydrocannabinol and palmitoylethanolamide | Mild–moderate dementia | Phase 2 | [107,108] | |
| THC-Free CBD | Cannabinoid receptor | An agonist against CB1 and CB2 receptor | Mild–moderate dementia, severe dementia | Phase 2 | [109,110] | |
| Masupirdine | Neurotransmitter receptors | A 5-HT6 receptor antagonist | Mild–moderate dementia | Phase 3 | [111,112,113,114] | |
| MK-8189 | Phosphodiesterase | A PDE10a inhibitor | Mild–moderate dementia | phase 1 | [115] | |
| Pimvanserin | Neurotransmitter receptors | A selective inverse agonist of the serotonin 5-HT2A receptor | Psychosis | Phase 3 | [47,116,117] | |
| ACP-204 | Neurotransmitter receptors | A potent and selective antagonist/inverse agonist of 5-HT2A receptor | Mild–moderate dementia, severe dementia | Phase 2 Phase 3 | [15,16,83,118] | |
| KarXT | Cholinergic modulator | A dual M1/M4 muscarinic acetylcholine receptor agonist | Mild–moderate dementia | Phase 3 | [119,120,121,122,123] | |
| Seltorexant | Orexin system | A selective antagonist of the orexin-2 receptor | Sleep disturbances | Mild–moderate dementia | Phase 2 | [124,125] |
| SLV | Neurotransmitter receptors | A selective 5-HT6 receptor antagonist | Social processing | [126] | ||
| N-acetyl cysteine | Redox system | An antioxidant and glutathione inducer | [71] | |||
| BDNF | Neurotrophic factor | A member of the neurotrophin family, TrkB activation | [65,72,73] | |||
| Oxytocin | Endocrine system | a nonapeptide hormone, oxytocin receptor activation | [127,128,129] |
3.1.2. AVP-923
3.1.3. AVP-786
3.1.4. AXS-05
3.2. An Inverse Agonist and Antagonist 5-HT2A Receptor
3.2.1. Pimavanserin
3.2.2. ACP-204
3.3. A Partial Agonist of Dopamine D2 Receptor and 5-HT1A Receptor
3.4. An Antagonist of 5-HT2A and Dopamine (D1, D2, and D4) Receptor
3.5. Glutamate Receptor Modulator and an Inositol Monophosphatase Inhibitor
3.6. Norepinephrine Modulators
3.7. Cannabinoid Receptors
3.7.1. Nabilone
3.7.2. Dronabinol
3.7.3. JZP541
3.7.4. IGC-AD1
3.7.5. SCI-110
3.7.6. Cannabidiol (CBD)
3.8. Cholinergic Modulators
3.9. 5-HT6 Receptor Antagonists
3.10. Orexin-2 Receptor Antagonists
3.11. An Agonist of Alpha-2 Adrenergic Receptor
3.12. PDE10 Inhibitors
3.13. Psychedelic Compounds
3.14. Antioxidants and Anti-Inflammatory Drugs
3.15. Social Deficits in AD
4. Implication for Potential Future Research and Drug Development
4.1. Impact of NPS on Caregivers and Strategies for Support
4.2. Limitations of Current Treatments for NPS
4.3. Potential Targets for Social Function in AD
4.4. Potential Application for Biomarker Development
4.5. Non-Pharmacological Approaches for Managing NPS
4.6. Potential Benefits of Combining Therapeutic Approaches
4.7. Long-Term Outcomes of Different Treatments
4.8. Potential Therapeutic Research Directions
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol. Ther. 2022, 11, 525–551. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Steinberg, M.; Tschanz, J.T.; Norton, M.C.; Steffens, D.C.; Breitner, J.C. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am. J. Psychiatry 2000, 157, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Masterman, D.L.; Ortiz, F.; Fairbanks, L.A.; Cummings, J.L. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis. Assoc. Disord. 2004, 18, 17–21. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef]
- Poulin, S.P.; Bergeron, D.; Dickerson, B.C.; Alzheimer’s Disease Neuroimaging, I. Risk Factors, Neuroanatomical Correlates, and Outcome of Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 483–493. [Google Scholar] [CrossRef]
- Jost, B.C.; Grossberg, G.T. The evolution of psychiatric symptoms in Alzheimer’s disease: A natural history study. J. Am. Geriatr. Soc. 1996, 44, 1078–1081. [Google Scholar] [CrossRef]
- Avitan, I.; Halperin, Y.; Saha, T.; Bloch, N.; Atrahimovich, D.; Polis, B.; Samson, A.O.; Braitbard, O. Towards a Consensus on Alzheimer’s Disease Comorbidity? J. Clin. Med. 2021, 10, 4360. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimer’s Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Butler, L.M.; Houghton, R.; Abraham, A.; Vassilaki, M.; Duran-Pacheco, G. Comorbidity Trajectories Associated With Alzheimer’s Disease: A Matched Case-Control Study in a United States Claims Database. Front. Neurosci. 2021, 15, 749305. [Google Scholar] [CrossRef] [PubMed]
- Olazaran, J.; Carnero-Pardo, C.; Fortea, J.; Sanchez-Juan, P.; Garcia-Ribas, G.; Vinuela, F.; Martinez-Lage, P.; Boada, M. Prevalence of treated patients with Alzheimer’s disease: Current trends and COVID-19 impact. Alzheimer’s Res. Ther. 2023, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.J.; Suemoto, C.K.; Franca Resende, E.P.; Petersen, C.; Leite, R.E.P.; Rodriguez, R.D.; Ferretti-Rebustini, R.E.L.; You, M.; Oh, J.; Nitrini, R.; et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 66, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lanctot, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef]
- Ajenikoko, M.K.; Ajagbe, A.O.; Onigbinde, O.A.; Okesina, A.A.; Tijani, A.A. Review of Alzheimer’s disease drugs and their relationship with neuron-glia interaction. IBRO Neurosci. Rep. 2023, 14, 64–76. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimer’s Dement. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Li, X.L.; Hu, N.; Tan, M.S.; Yu, J.T.; Tan, L. Behavioral and psychological symptoms in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 927804. [Google Scholar] [CrossRef]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: Challenges and advances in diagnosis and treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef]
- Asmer, M.S.; Kirkham, J.; Newton, H.; Ismail, Z.; Elbayoumi, H.; Leung, R.H.; Seitz, D.P. Meta-Analysis of the Prevalence of Major Depressive Disorder Among Older Adults With Dementia. J. Clin. Psychiatry 2018, 79, 15460. [Google Scholar] [CrossRef]
- Chi, S.; Wang, C.; Jiang, T.; Zhu, X.C.; Yu, J.T.; Tan, L. The prevalence of depression in Alzheimer’s disease: A systematic review and meta-analysis. Curr. Alzheimer Res. 2015, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Sutin, A.R.; Robinson, E. Depression reported by US adults in 2017-2018 and March and April 2020. J. Affect. Disord. 2021, 278, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.T.; Wang, H.F.; Meng, X.F.; Wang, C.; Tan, C.C.; Tan, L. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 101–109. [Google Scholar] [CrossRef]
- Simic, G.; Babic Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of Antidepressants for Depression in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2017, 58, 725–733. [Google Scholar] [CrossRef]
- Dudas, R.; Malouf, R.; McCleery, J.; Dening, T. Antidepressants for treating depression in dementia. Cochrane Database Syst. Rev. 2018, 8, CD003944. [Google Scholar] [CrossRef]
- Ismail, Z.; Gatchel, J.; Bateman, D.R.; Barcelos-Ferreira, R.; Cantillon, M.; Jaeger, J.; Donovan, N.J.; Mortby, M.E. Affective and emotional dysregulation as pre-dementia risk markers: Exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int. Psychogeriatrics 2018, 30, 185–196. [Google Scholar] [CrossRef]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 171–177. [Google Scholar] [CrossRef]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Kohler, S.; Oude Voshaar, R.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef]
- Santabarbara, J.; Lipnicki, D.M.; Olaya, B.; Villagrasa, B.; Bueno-Notivol, J.; Nuez, L.; Lopez-Anton, R.; Gracia-Garcia, P. Does Anxiety Increase the Risk of All-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies. J. Clin. Med. 2020, 9, 1791. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Li, J.; Liu, S.; Ding, H.; Wang, G.; Li, X. Assessing Cannabidiol as a Therapeutic Agent for Preventing and Alleviating Alzheimer’s Disease Neurodegeneration. Cells 2023, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Drijgers, R.L.; Aalten, P.; Winogrodzka, A.; Verhey, F.R.; Leentjens, A.F. Pharmacological treatment of apathy in neurodegenerative diseases: A systematic review. Dement. Geriatr. Cogn. Disord. 2009, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, D.; Drake, K.; Bostwick, J. Diagnosis and Management of Neuropsychiatric Symptoms in Alzheimer’s Disease. Curr. Psychiatry Rep. 2018, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.; Husain, M.; Roiser, J.P. Apathy and Motivation: Biological Basis and Drug Treatment. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 313–338. [Google Scholar] [CrossRef]
- Fahed, M.; Steffens, D.C. Apathy: Neurobiology, Assessment and Treatment. Clin. Psychopharmacol. Neurosci. 2021, 19, 181–189. [Google Scholar] [CrossRef]
- Ruthirakuhan, M.T.; Herrmann, N.; Abraham, E.H.; Chan, S.; Lanctot, K.L. Pharmacological interventions for apathy in Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 5, CD012197. [Google Scholar] [CrossRef]
- Kverno, K.S.; Rabins, P.V.; Blass, D.M.; Hicks, K.L.; Black, B.S. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J. Gerontol. Nurs. 2008, 34, 8–15, quiz 16–17. [Google Scholar] [CrossRef]
- Ravyts, S.G.; Perez, E.; Donovan, E.K.; Soto, P.; Dzierzewski, J.M. Measurement of aggression in older adults. Aggress. Violent Behav. 2021, 57, 101484. [Google Scholar] [CrossRef]
- Cummings, J.; Mintzer, J.; Brodaty, H.; Sano, M.; Banerjee, S.; Devanand, D.P.; Gauthier, S.; Howard, R.; Lanctot, K.; Lyketsos, C.G.; et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int. Psychogeriatr. 2015, 27, 7–17. [Google Scholar] [CrossRef]
- Ballard, C.; Orrell, M.; YongZhong, S.; Moniz-Cook, E.; Stafford, J.; Whittaker, R.; Woods, B.; Corbett, A.; Garrod, L.; Khan, Z.; et al. Impact of Antipsychotic Review and Nonpharmacological Intervention on Antipsychotic Use, Neuropsychiatric Symptoms, and Mortality in People With Dementia Living in Nursing Homes: A Factorial Cluster-Randomized Controlled Trial by the Well-Being and Health for People With Dementia (WHELD) Program. Am. J. Psychiatry 2016, 173, 252–262. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F. A comprehensive review on pharmacological applications and drug-induced toxicity of valproic acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Slomkowski, M.; Hefting, N.; Chen, D.; Larsen, K.G.; Kohegyi, E.; Hobart, M.; Cummings, J.L.; Grossberg, G.T. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023, 80, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.S.; Kumar, S.; Demichele-Sweet, M.A.; Sweet, R.A. Psychosis in Alzheimer’s disease. Biol. Psychiatry 2014, 75, 542–552. [Google Scholar] [CrossRef]
- Woodward, M. Aspects of communication in Alzheimer’s disease: Clinical features and treatment options. Int. Psychogeriatr. 2013, 25, 877–885. [Google Scholar] [CrossRef]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef]
- Khoury, R.; Marx, C.; Mirgati, S.; Velury, D.; Chakkamparambil, B.; Grossberg, G.T. AVP-786 as a promising treatment option for Alzheimer’s Disease including agitation. Expert Opin. Pharmacother. 2021, 22, 783–795. [Google Scholar] [CrossRef]
- Kurhan, F.; Akin, M. A New Hope in Alzheimer’s Disease Psychosis: Pimavanserin. Curr. Alzheimer Res. 2023, 20, 403–408. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Okhravi, H.R.; Vitiello, M.V.; Sanford, L.D.; Tang, X. Sleep in Alzheimer’s disease: A systematic review and meta-analysis of polysomnographic findings. Transl. Psychiatry 2022, 12, 136. [Google Scholar] [CrossRef]
- Wu, T.T.; Zou, Y.L.; Xu, K.D.; Jiang, X.R.; Zhou, M.M.; Zhang, S.B.; Song, C.H. Insomnia and multiple health outcomes: Umbrella review of meta-analyses of prospective cohort studies. Public Health 2023, 215, 66–74. [Google Scholar] [CrossRef]
- Ju, Y.E.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Z.; Hu, N.; Yang, Y.; Xiong, R.; Fu, Z. Cognition effectiveness of continuous positive airway pressure treatment in obstructive sleep apnea syndrome patients with cognitive impairment: A meta-analysis. Exp. Brain Res. 2021, 239, 3537–3552. [Google Scholar] [CrossRef] [PubMed]
- Di Meco, A.; Joshi, Y.B.; Pratico, D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol. Aging 2014, 35, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J.A. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef]
- McCurry, S.M.; Reynolds, C.F.; Ancoli-Israel, S.; Teri, L.; Vitiello, M.V. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med. Rev. 2000, 4, 603–628. [Google Scholar] [CrossRef]
- Burke, S.L.; Hu, T.; Spadola, C.E.; Burgess, A.; Li, T.; Cadet, T. Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease. J. Aging Health 2019, 31, 322–342. [Google Scholar] [CrossRef]
- McCleery, J.; Sharpley, A.L. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst. Rev. 2020, 11, CD009178. [Google Scholar] [CrossRef]
- Bediou, B.; Ryff, I.; Mercier, B.; Milliery, M.; Henaff, M.A.; D’Amato, T.; Bonnefoy, M.; Vighetto, A.; Krolak-Salmon, P. Impaired social cognition in mild Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2009, 22, 130–140. [Google Scholar] [CrossRef]
- Moreau, N.; Rauzy, S.; Viallet, F.; Champagne-Lavau, M. Theory of mind in Alzheimer disease: Evidence of authentic impairment during social interaction. Neuropsychology 2016, 30, 312–321. [Google Scholar] [CrossRef]
- Ellis Weismer, S.; Tomblin, J.B.; Durkin, M.S.; Bolt, D.; Palta, M. A preliminary epidemiologic study of social (pragmatic) communication disorder in the context of developmental language disorder. Int. J. Lang. Commun. Disord. 2021, 56, 1235–1248. [Google Scholar] [CrossRef]
- Sommerlad, A.; Kivimaki, M.; Larson, E.B.; Rohr, S.; Shirai, K.; Singh-Manoux, A.; Livingston, G. Social participation and risk of developing dementia. Nat. Aging 2023, 3, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Chang, C.H.; Gean, P.W. Impact of social relationships on Alzheimer’s memory impairment: Mechanistic studies. J. Biomed. Sci. 2018, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Leser, N.; Wagner, S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 2015, 124, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, L.; Cao, M.; Marshall, C.; Gao, J.; Xiao, N.; Hu, G.; Xiao, M. Isolation Housing Exacerbates Alzheimer’s Disease-Like Pathophysiology in Aged APP/PS1 Mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu116. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hung, H.C.; Yu, Y.J.; Su, C.L.; Chen, S.H.; Gean, P.W. Co-housing reverses memory decline by epigenetic regulation of brain-derived neurotrophic factor expression in an animal model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2017, 141, 1–8. [Google Scholar] [CrossRef]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16574–16579. [Google Scholar] [CrossRef]
- Azzinnari, D.; Sigrist, H.; Staehli, S.; Palme, R.; Hildebrandt, T.; Leparc, G.; Hengerer, B.; Seifritz, E.; Pryce, C.R. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology 2014, 85, 328–341. [Google Scholar] [CrossRef]
- Djordjevic, A.; Adzic, M.; Djordjevic, J.; Radojcic, M.B. Chronic social isolation is related to both upregulation of plasticity genes and initiation of proapoptotic signaling in Wistar rat hippocampus. J. Neural Transm. 2009, 116, 1579–1589. [Google Scholar] [CrossRef]
- Murinova, J.; Hlavacova, N.; Chmelova, M.; Riecansky, I. The Evidence for Altered BDNF Expression in the Brain of Rats Reared or Housed in Social Isolation: A Systematic Review. Front. Behav. Neurosci. 2017, 11, 101. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Kuo, J.R.; Chen, S.H.; Gean, P.W. Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiol. Dis. 2012, 45, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, W.; Shao, F.; Wang, W. Cognitive dysfunction and epigenetic alterations of the BDNF gene are induced by social isolation during early adolescence. Behav. Brain Res. 2016, 313, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; D’Andrea, I.; Fiore, M.; Di Fausto, V.; Aloe, L.; Alleva, E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry 2006, 60, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Zahodne, L.B.; Brandt, J.; Blacker, D.; Albert, M.; Dubois, B.; Stern, Y. Social cognition in Alzheimer’s disease: A separate construct contributing to dependence. Alzheimer’s Dement. 2014, 10, 818–826. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chung, C.H.; Chien, W.C.; Chen, H.C. The Association Between Dextromethorphan Use and the Risk of Dementia. Am. J. Alzheimer’s Dis. Other Dementiasr 2022, 37, 15333175221124952. [Google Scholar] [CrossRef]
- Malar, D.S.; Thitilertdecha, P.; Ruckvongacheep, K.S.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Targeting Sigma Receptors for the Treatment of Neurodegenerative and Neurodevelopmental Disorders. CNS Drugs 2023, 37, 399–440. [Google Scholar] [CrossRef]
- Ohi, Y.; Tsunekawa, S.; Haji, A. Dextromethorphan inhibits the glutamatergic synaptic transmission in the nucleus tractus solitarius of guinea pigs. J. Pharmacol. Sci. 2011, 116, 54–62. [Google Scholar] [CrossRef]
- Blair, H.A. Lumateperone: First Approval. Drugs 2020, 80, 417–423. [Google Scholar] [CrossRef]
- Mazza, M.; Marano, G.; Traversi, G.; Sani, G.; Janiri, L. Evidence on the New Drug Lumateperone (ITI-007) for Psychiatric and Neurological Disorders. CNS Neurol. Disord. Drug Targets 2020, 19, 243–247. [Google Scholar] [CrossRef]
- Vanover, K.E.; Davis, R.E.; Zhou, Y.; Ye, W.; Brasic, J.R.; Gapasin, L.; Saillard, J.; Weingart, M.; Litman, R.E.; Mates, S.; et al. Dopamine D(2) receptor occupancy of lumateperone (ITI-007): A Positron Emission Tomography Study in patients with schizophrenia. Neuropsychopharmacology 2019, 44, 598–605. [Google Scholar] [CrossRef]
- Vyas, P.; Hwang, B.J.; Brasic, J.R. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin. Pharmacother. 2020, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.J.; Wilson, C.; Hannan, A.J.; Renoir, T. Psilocybin as a lead candidate molecule in preclinical therapeutic studies of psychiatric disorders: A systematic review. J. Neurochem. 2023, 168, 1687–1720. [Google Scholar] [CrossRef] [PubMed]
- Kozak, Z.; Johnson, M.W.; Aaronson, S.T. Assessing potential of psilocybin for depressive disorders. Expert Opin. Investig. Drugs 2023, 32, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Nguyen, Q.A.; Matthews, S.J.; Carpenter, E.; Mathews, D.B.; Patten, C.A.; Hammond, C.J. Psilocybin history, action and reaction: A narrative clinical review. J. Psychopharmacol. 2023, 37, 849–865. [Google Scholar] [CrossRef]
- Chen, K.; Li, H.; Yang, L.; Jiang, Y.; Wang, Q.; Zhang, J.; He, J. Comparative efficacy and safety of antidepressant therapy for the agitation of dementia: A systematic review and network meta-analysis. Front. Aging Neurosci. 2023, 15, 1103039. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. 2023, 9, e12385. [Google Scholar] [CrossRef]
- Pioro, E.P.; Brooks, B.R.; Cummings, J.; Schiffer, R.; Thisted, R.A.; Wynn, D.; Hepner, A.; Kaye, R.; Safety, T.; Efficacy Results Trial of, A.V.P.i.P.B.A.I. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann. Neurol. 2010, 68, 693–702. [Google Scholar] [CrossRef]
- Taylor, C.P.; Traynelis, S.F.; Siffert, J.; Pope, L.E.; Matsumoto, R.R. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta(R)) clinical use. Pharmacol. Ther. 2016, 164, 170–182. [Google Scholar] [CrossRef]
- Chez, M.; Kile, S.; Lepage, C.; Parise, C.; Benabides, B.; Hankins, A. A Randomized, Placebo-Controlled, Blinded, Crossover, Pilot Study of the Effects of Dextromethorphan/Quinidine for the Treatment of Neurobehavioral Symptoms in Adults with Autism. J. Autism Dev. Disord. 2020, 50, 1532–1538. [Google Scholar] [CrossRef]
- Cummings, J.L.; Lyketsos, C.G.; Peskind, E.R.; Porsteinsson, A.P.; Mintzer, J.E.; Scharre, D.W.; De La Gandara, J.E.; Agronin, M.; Davis, C.S.; Nguyen, U.; et al. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA 2015, 314, 1242–1254. [Google Scholar] [CrossRef]
- Devanand, D.P. Management of neuropsychiatric symptoms in dementia. Curr. Opin. Neurol. 2023, 36, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, E.C. Treatment Resistant Depression with Loss of Antidepressant Response: Rapid-Acting Antidepressant Action of Dextromethorphan, A Possible Treatment Bridging Molecule. Psychopharmacol Bull 2016, 46, 53–58. [Google Scholar] [PubMed]
- Willett, K.C.; Bond, L.R.; Morrill, A.M.; Lorena, D.; Petru, I. Dextromethorphan/Bupropion: A Novel Treatment for Patients With Major Depressive Disorder. Am. J. Ther. 2024, 31, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.; Kiss, A.; Black, S.E.; Lanctot, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- Ruthirakuhan, M.; Herrmann, N.; Andreazza, A.C.; Verhoeff, N.; Gallagher, D.; Black, S.E.; Kiss, A.; Lanctot, K.L. Agitation, Oxidative Stress, and Cytokines in Alzheimer Disease: Biomarker Analyses From a Clinical Trial With Nabilone for Agitation. J. Geriatr. Psychiatry Neurol. 2020, 33, 175–184. [Google Scholar] [CrossRef]
- Diefenderfer, L.A.; Iuppa, C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment. Health Clin. 2017, 7, 207–212. [Google Scholar] [CrossRef]
- Eaves, S.; Rey, J.A. Brexpiprazole (Rexulti): A New Monotherapy for Schizophrenia and Adjunctive Therapy for Major Depressive Disorder. Pharm. Ther. 2016, 41, 418–422. [Google Scholar]
- Khoury, R. Deuterated dextromethorphan/quinidine for agitation in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1013–1014. [Google Scholar] [CrossRef]
- Luo, S.M.; Li, L.Y.; Guo, L.Z.; Wang, L.; Wang, Y.F.; Chen, N.; Wang, E. Dexmedetomidine exerts an anti-inflammatory effect via alpha2 adrenoceptors to alleviate cognitive dysfunction in 5xFAD mice. Front. Aging Neurosci. 2022, 14, 978768. [Google Scholar] [CrossRef]
- Deiner, S.; Luo, X.; Lin, H.M.; Sessler, D.I.; Saager, L.; Sieber, F.E.; Lee, H.B.; Sano, M.; and the Dexlirium Writing, G.; Jankowski, C.; et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg. 2017, 152, e171505. [Google Scholar] [CrossRef]
- Preskorn, S.H.; Zeller, S.; Citrome, L.; Finman, J.; Goldberg, J.F.; Fava, M.; Kakar, R.; De Vivo, M.; Yocca, F.D.; Risinger, R. Effect of Sublingual Dexmedetomidine vs Placebo on Acute Agitation Associated With Bipolar Disorder: A Randomized Clinical Trial. JAMA 2022, 327, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Woodward, M.R.; Harper, D.G.; Stolyar, A.; Forester, B.P.; Ellison, J.M. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am. J. Geriatr. Psychiatry 2014, 22, 415–419. [Google Scholar] [CrossRef]
- Cohen, L.M.; Ash, E.; Outen, J.D.; Vandrey, R.; Amjad, H.; Agronin, M.; Burhanullah, M.H.; Walsh, P.; Wilkins, J.M.; Leoutsakos, J.M.; et al. Study rationale and baseline data for pilot trial of dronabinol adjunctive treatment of agitation in Alzheimer’s dementia (THC-AD). Int. Psychogeriatrics 2021, 1–6. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Norepinephrine May Oppose Other Neuromodulators to Impact Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7364. [Google Scholar] [CrossRef]
- Gutierrez, I.L.; Dello Russo, C.; Novellino, F.; Caso, J.R.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Noradrenaline in Alzheimer’s Disease: A New Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 6143. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Jonsson, K.O.; Vandevoorde, S.; Lambert, D.M.; Tiger, G.; Fowler, C.J. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br. J. Pharmacol. 2001, 133, 1263–1275. [Google Scholar] [CrossRef]
- Outen, J.D.; Burhanullah, M.H.; Vandrey, R.; Amjad, H.; Harper, D.G.; Patrick, R.E.; May, R.L.; Agronin, M.E.; Forester, B.P.; Rosenberg, P.B. Cannabinoids for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2021, 29, 1253–1263. [Google Scholar] [CrossRef]
- Goveas, J.S. Commentary on “Cannabinoids for Agitation in Alzheimer’s Disease”. Am. J. Geriatr. Psychiatry 2021, 29, 1264–1266. [Google Scholar] [CrossRef]
- Nirogi, R.; Ieni, J.; Goyal, V.K.; Ravula, J.; Jetta, S.; Shinde, A.; Jayarajan, P.; Benade, V.; Palacharla, V.R.C.; Dogiparti, D.K.; et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: A randomized, double-blind, phase 2, proof-of-concept study. Alzheimer’s Dementia 2022, 8, e12307. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Jayarajan, P.; Benade, V.; Shinde, A.; Goyal, V.K.; Jetta, S.; Ravula, J.; Abraham, R.; Grandhi, V.R.; Subramanian, R.; et al. Potential beneficial effects of masupirdine (SUVN-502) on agitation/aggression and psychosis in patients with moderate Alzheimer’s disease: Exploratory post hoc analyses. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- de Jong, I.E.M.; Mork, A. Antagonism of the 5-HT(6) receptor—Preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology 2017, 125, 50–63. [Google Scholar] [CrossRef]
- Ferrero, H.; Solas, M.; Francis, P.T.; Ramirez, M.J. Serotonin 5-HT(6) Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status. CNS Drugs 2017, 31, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Meng, X.; Haley, H.D.; Harrell, C.M.; McDonald, T.P.; Miller, C.O.; Smith, S.M. Magnetic resonance imaging detects white adipose tissue beiging in mice following PDE10A inhibitor treatment. J. Lipid Res. 2023, 64, 100408. [Google Scholar] [CrossRef]
- Baker, M.; Song, W.; Fusick, A. Pimavanserin Use in Lewy Body Dementia: A Case Report Demonstrating the Medication’s Efficacy. Cureus 2023, 15, e46356. [Google Scholar] [CrossRef]
- Tariot, P.N.; Ballard, C.; Devanand, D.P.; Cummings, J.L.; Sultzer, D.L. Pimavanserin and dementia-related psychosis. Lancet Neurol. 2022, 21, 114–115. [Google Scholar] [CrossRef]
- Ballard, C.; O’Brien, J.; Coope, B.; Fairbairn, A.; Abid, F.; Wilcock, G. A prospective study of psychotic symptoms in dementia sufferers: Psychosis in dementia. Int. Psychogeriatr. 1997, 9, 57–64. [Google Scholar] [CrossRef]
- Thorn, C.A.; Moon, J.; Bourbonais, C.A.; Harms, J.; Edgerton, J.R.; Stark, E.; Steyn, S.J.; Butter, C.R.; Lazzaro, J.T.; O’Connor, R.E.; et al. Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline. ACS Chem. Neurosci. 2019, 10, 1753–1764. [Google Scholar] [CrossRef]
- Kaul, I.; Sawchak, S.; Correll, C.U.; Kakar, R.; Breier, A.; Zhu, H.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: Results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 2024, 403, 160–170. [Google Scholar] [CrossRef]
- Sauder, C.; Allen, L.A.; Baker, E.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: Post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study. Transl. Psychiatry 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, N.; Elsayed, O.H.; El-Mallakh, R.S. Xanomeline-Trospium and Muscarinic Involvement in Schizophrenia. Neuropsychiatr. Dis. Treat. 2023, 19, 1145–1151. [Google Scholar] [CrossRef]
- Leber, A.; Ramachandra, R.; Ceban, F.; Kwan, A.T.H.; Rhee, T.G.; Wu, J.; Cao, B.; Jawad, M.Y.; Teopiz, K.M.; Ho, R.; et al. Efficacy, safety, and tolerability of xanomeline for schizophrenia spectrum disorders: A systematic review. Expert Opin. Pharmacother. 2024, 25, 467–476. [Google Scholar] [CrossRef]
- Bergamini, G.; Coloma, P.; Massinet, H.; Steiner, M.A. What evidence is there for implicating the brain orexin system in neuropsychiatric symptoms in dementia? Front. Psychiatry 2022, 13, 1052233. [Google Scholar] [CrossRef]
- Kron, J.O.J.; Keenan, R.J.; Hoyer, D.; Jacobson, L.H. Orexin Receptor Antagonism: Normalizing Sleep Architecture in Old Age and Disease. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 359–386. [Google Scholar] [CrossRef]
- de Bruin, N.; van Loevezijn, A.; Wicke, K.M.; de Haan, M.; Venhorst, J.; Lange, J.H.M.; de Groote, L.; van der Neut, M.A.W.; Prickaerts, J.; Andriambeloson, E.; et al. The selective 5-HT6 receptor antagonist SLV has putative cognitive- and social interaction enhancing properties in rodent models of cognitive impairment. Neurobiol. Learn. Mem. 2016, 133, 100–117. [Google Scholar] [CrossRef]
- Lardenoije, R.; Roubroeks, J.A.Y.; Pishva, E.; Leber, M.; Wagner, H.; Iatrou, A.; Smith, A.R.; Smith, R.G.; Eijssen, L.M.T.; Kleineidam, L.; et al. Alzheimer’s disease-associated (hydroxy)methylomic changes in the brain and blood. Clin. Epigenetics 2019, 11, 164. [Google Scholar] [CrossRef]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Koulousakis, P.; Willems, E.; Schepers, M.; Rombaut, B.; Prickaerts, J.; Vanmierlo, T.; van den Hove, D. Exogenous Oxytocin Administration Restores Memory in Female APP/PS1 Mice. J. Alzheimer’s Dis. 2023, 96, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Journey, J.D.; Agrawal, S.; Stern, E. Dextromethorphan Toxicity. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Suneil Agrawal declares no relevant financial relationships with ineligible companies. Disclosure: Evan Stern declares no relevant financial relationships with ineligible companies; Publisher; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Raiborde, M.D.; Kumar, G.; Singh, P.; Sharma, S. Dextromethorphan: An emerging drug of abuse. J. Pharm. Negat. Results 2022, 13, 13. [Google Scholar]
- Akbar, D.; Rhee, T.G.; Ceban, F.; Ho, R.; Teopiz, K.M.; Cao, B.; Subramaniapillai, M.; Kwan, A.T.H.; Rosenblat, J.D.; McIntyre, R.S. Dextromethorphan-Bupropion for the Treatment of Depression: A Systematic Review of Efficacy and Safety in Clinical Trials. CNS Drugs 2023, 37, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; Grossberg, G.T. AVP-786 for the treatment of agitation in dementia of the Alzheimer’s type. Expert Opin. Investig. Drugs 2017, 26, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Tampi, R.R.; Joshi, P.; Marpuri, P.; Tampi, D.J. Evidence for using dextromethorphan-quinidine for the treatment of agitation in dementia. World J. Psychiatry 2020, 10, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Citrome, L. AXS-05: An investigational treatment for Alzheimer’s disease-associated agitation. Expert Opin. Investig. Drugs 2022, 31, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Alva, G.; Cubala, W.J.; Berrio, A.; Coate, B.; Abler, V.; Pathak, S. Safety Profile of Pimavanserin Therapy in Elderly Patients with Neurodegenerative Disease-Related Neuropsychiatric Symptoms: A Phase 3B Study. J. Alzheimer’s Dis. 2024, 98, 265–274. [Google Scholar] [CrossRef]
- Quiroz, J.A.; Machado-Vieira, R.; Zarate, C.A., Jr.; Manji, H.K. Novel insights into lithium’s mechanism of action: Neurotrophic and neuroprotective effects. Neuropsychobiology 2010, 62, 50–60. [Google Scholar] [CrossRef]
- Devanand, D.P.; Crocco, E.; Forester, B.P.; Husain, M.M.; Lee, S.; Vahia, I.V.; Andrews, H.; Simon-Pearson, L.; Imran, N.; Luca, L.; et al. Low Dose Lithium Treatment of Behavioral Complications in Alzheimer’s Disease: Lit-AD Randomized Clinical Trial. Am. J. Geriatr. Psychiatry 2022, 30, 32–42. [Google Scholar] [CrossRef]
- Hidding, U.; Mainka, T.; Buhmann, C. Therapeutic use of medical Cannabis in neurological diseases: A clinical update. J. Neural Transm. 2024, 131, 117–126. [Google Scholar] [CrossRef]
- Xiong, Y.; Lim, C.S. Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease. Brain Sci. 2021, 11, 1211. [Google Scholar] [CrossRef]
- Dawson, L.A. The central role of 5-HT6 receptors in modulating brain neurochemistry. Int. Rev. Neurobiol. 2011, 96, 1–26. [Google Scholar] [CrossRef]
- Zagorska, A.; Partyka, A.; Bucki, A.; Gawalskax, A.; Czopek, A.; Pawlowski, M. Phosphodiesterase 10 Inhibitors—Novel Perspectives for Psychiatric and Neurodegenerative Drug Discovery. Curr. Med. Chem. 2018, 25, 3455–3481. [Google Scholar] [CrossRef] [PubMed]
- Layton, M.E.; Kern, J.C.; Hartingh, T.J.; Shipe, W.D.; Raheem, I.; Kandebo, M.; Hayes, R.P.; Huszar, S.; Eddins, D.; Ma, B.; et al. Discovery of MK-8189, a Highly Potent and Selective PDE10A Inhibitor for the Treatment of Schizophrenia. J. Med. Chem. 2023, 66, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, D.; Abt, M.; Tamburri, P.; Chatham, C.; Holiga, S.; Frank, M.J.; Collins, A.G.E.; Walling, D.P.; Mofsen, R.; Gruener, D.; et al. Proof-of-Mechanism Study of the Phosphodiesterase 10 Inhibitor RG7203 in Patients With Schizophrenia and Negative Symptoms. Biol. Psychiatry Glob. Open Sci. 2021, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Rogers, J.C.; Katshu, M.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Klinedinst, N.J.; Regenold, W.T. A mitochondrial bioenergetic basis of depression. J. Bioenerg. Biomembr. 2015, 47, 155–171. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J. Neurosci. 2019, 39, 5594–5605. [Google Scholar] [CrossRef]
- Elamin, M.; Pender, N.; Hardiman, O.; Abrahams, S. Social cognition in neurodegenerative disorders: A systematic review. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; de Carvalho, R.L.S.; Nogueira, M.; Baptista, M.A.T.; Kimura, N.; Lacerda, I.B.; Dourado, M.C.N. The Relationship between Social Cognition and Executive Functions in Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2020, 17, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Setien-Suero, E.; Murillo-Garcia, N.; Sevilla-Ramos, M.; Abreu-Fernandez, G.; Pozueta, A.; Ayesa-Arriola, R. Exploring the Relationship Between Deficits in Social Cognition and Neurodegenerative Dementia: A Systematic Review. Front. Aging Neurosci. 2022, 14, 778093. [Google Scholar] [CrossRef]
- Saris, I.M.J.; Aghajani, M.; Jongs, N.; Reus, L.M.; van der Wee, N.J.A.; Bilderbeck, A.C.; Winter van Rossum, I.; Arango, C.; de la Torre-Luque, A.; Malik, A.; et al. Cross-disorder and disorder-specific deficits in social functioning among schizophrenia and alzheimer’s disease patients. PLoS ONE 2022, 17, e0263769. [Google Scholar] [CrossRef]
- Legaz, A.; Prado, P.; Moguilner, S.; Baez, S.; Santamaria-Garcia, H.; Birba, A.; Barttfeld, P.; Garcia, A.M.; Fittipaldi, S.; Ibanez, A. Social and non-social working memory in neurodegeneration. Neurobiol. Dis. 2023, 183, 106171. [Google Scholar] [CrossRef]
- Stam, D.; Rosseel, S.; De Winter, F.L.; Van den Bossche, M.J.A.; Vandenbulcke, M.; Van den Stock, J. Facial expression recognition deficits in frontotemporal dementia and Alzheimer’s disease: A meta-analytic investigation of effects of phenotypic variant, task modality, geographical region and symptomatic specificity. J. Neurol. 2023, 270, 5731–5755. [Google Scholar] [CrossRef]
- Singleton, E.H.; Fieldhouse, J.L.P.; van ‘t Hooft, J.J.; Scarioni, M.; van Engelen, M.E.; Sikkes, S.A.M.; de Boer, C.; Bocancea, D.I.; van den Berg, E.; Scheltens, P.; et al. Social cognition deficits and biometric signatures in the behavioural variant of Alzheimer’s disease. Brain 2023, 146, 2163–2174. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Choi, J.; Lee, S.; Gurland, B.; Devanand, D.P. Effects of restriction of activities and social isolation on risk of dementia in the community. Int. Psychogeriatr. 2021, 33, 1207–1215. [Google Scholar] [CrossRef]
- Drinkwater, E.; Davies, C.; Spires-Jones, T.L. Potential neurobiological links between social isolation and Alzheimer’s disease risk. Eur. J. Neurosci. 2022, 56, 5397–5412. [Google Scholar] [CrossRef]
- Ren, Y.; Savadlou, A.; Park, S.; Siska, P.; Epp, J.R.; Sargin, D. The impact of loneliness and social isolation on the development of cognitive decline and Alzheimer’s Disease. Front. Neuroendocr. 2023, 69, 101061. [Google Scholar] [CrossRef]
- Joshi, P.; Hendrie, K.; Jester, D.J.; Dasarathy, D.; Lavretsky, H.; Ku, B.S.; Leutwyler, H.; Torous, J.; Jeste, D.V.; Tampi, R.R. Social connections as determinants of cognitive health and as targets for social interventions in persons with or at risk of Alzheimer’s disease and related disorders: A scoping review. Int. Psychogeriatrics 2024, 36, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.C.; Robert, V.; Bouisset, G.; Loisy, M.; Lopez, S.; Cattaud, V.; Lejards, C.; Piskorowski, R.A.; Rampon, C.; Chevaleyre, V.; et al. Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer’s mouse model. iScience 2022, 25, 103895. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L.; Huang, M.; Mahjour, S.; Ryan, C.; Elzokaky, A.; Svensson, K.A.; Meltzer, H.Y. The dopamine D1 receptor positive allosteric modulator, DETQ, improves cognition and social interaction in aged mice and enhances cortical and hippocampal acetylcholine efflux. Behav. Brain Res. 2024, 459, 114766. [Google Scholar] [CrossRef]
- Michaelian, J.C.; McCade, D.; Hoyos, C.M.; Brodaty, H.; Harrison, F.; Henry, J.D.; Guastella, A.J.; Naismith, S.L. Pilot Randomized, Double-Blind, Placebo-Controlled Crossover Trial Evaluating the Feasibility of an Intranasal Oxytocin in Improving Social Cognition in Individuals Living with Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2023, 7, 715–729. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Hosawi, S.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S.; Murtaza, B.N.; Kazmi, I. Symptomatic, Genetic, and Mechanistic Overlaps between Autism and Alzheimer’s Disease. Biomolecules 2021, 11, 1635. [Google Scholar] [CrossRef]
- Steubler, V.; Erdinger, S.; Back, M.K.; Ludewig, S.; Fassler, D.; Richter, M.; Han, K.; Slomianka, L.; Amrein, I.; von Engelhardt, J.; et al. Loss of all three APP family members during development impairs synaptic function and plasticity, disrupts learning, and causes an autism-like phenotype. EMBO J. 2021, 40, e107471. [Google Scholar] [CrossRef]
- Sokol, D.K.; Lahiri, D.K. APPlications of amyloid-beta precursor protein metabolites in macrocephaly and autism spectrum disorder. Front. Mol. Neurosci. 2023, 16, 1201744. [Google Scholar] [CrossRef]
- Sokol, D.K.; Lahiri, D.K. Neurodevelopmental disorders and microcephaly: How apoptosis, the cell cycle, tau and amyloid-beta precursor protein APPly. Front. Mol. Neurosci. 2023, 16, 1201723. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, F.; Lu, R.; Xing, X.; Xu, L.; Wu, K.; Gong, Z.; Zhang, Q.; Zhang, Y.; Xing, M.; et al. CNTNAP2 intracellular domain (CICD) generated by gamma-secretase cleavage improves autism-related behaviors. Signal Transduct. Target. Ther. 2023, 8, 219. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Bai, Y.; Chen, P.; Xing, M.; Cai, F.; Wu, Y.; Song, W. Intermittent hypoxia-induced enhancement of sociability and working memory associates with CNTNAP2 upregulation. Front. Mol. Neurosci. 2023, 16, 1155047. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Sokol, D.K.; Erickson, C.; Ray, B.; Ho, C.Y.; Maloney, B. Autism as early neurodevelopmental disorder: Evidence for an sAPPalpha-mediated anabolic pathway. Front. Cell. Neurosci. 2013, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Sokol, D.K.; Maloney, B.; Lahiri, D.K. Finding novel distinctions between the sAPPalpha-mediated anabolic biochemical pathways in Autism Spectrum Disorder and Fragile X Syndrome plasma and brain tissue. Sci. Rep. 2016, 6, 26052. [Google Scholar] [CrossRef] [PubMed]
- Allegri, R.F.; Sarasola, D.; Serrano, C.M.; Taragano, F.E.; Arizaga, R.L.; Butman, J.; Lon, L. Neuropsychiatric symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2006, 2, 105–110. [Google Scholar] [PubMed]
- Gitlin, L.N.; Winter, L.; Dennis, M.P.; Hodgson, N.; Hauck, W.W. Targeting and managing behavioral symptoms in individuals with dementia: A randomized trial of a nonpharmacological intervention. J. Am. Geriatr. Soc. 2010, 58, 1465–1474. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Szekely, C.A.; Mielke, M.M.; Rosenberg, P.B.; Zandi, P.P. Developing new treatments for Alzheimer’s disease: The who, what, when, and how of biomarker-guided therapies. Int. Psychogeriatr. 2008, 20, 871–889. [Google Scholar] [CrossRef]
- Chintalapudi, N.; Dhulipalla, V.R.; Battineni, G.; Rucco, C.; Amenta, F. Voice Biomarkers for Parkinson’s Disease Prediction Using Machine Learning Models with Improved Feature Reduction Techniques. J. Data Sci. Intell. Syst. 2023, 1, 92–98. [Google Scholar] [CrossRef]
- Milner, H.; Baron, M. Establishing an Optimal Online Phishing Detection Method: Evaluating Topological NLP Transformers on Text Message Data. J. Data Sci. Intell. Syst. 2023, 2, 173–181. [Google Scholar] [CrossRef]
- Livingston, G.; Johnston, K.; Katona, C.; Paton, J.; Lyketsos, C.G.; Old Age Task Force of the World Federation of Biological Psychiatry. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am. J. Psychiatry 2005, 162, 1996–2021. [Google Scholar] [CrossRef]
| NPS | Category | General Patients | Patients with AD |
|---|---|---|---|
| Depression | Prevalence | 5–8.3% | 14.8–40% |
| Symptoms | Feeling sad, irritable, empty, disrupted sleep, hopelessness about the future, poor concentration, weight change, low energy, excessive guilt, or low self-worth | Persistent feelings of worthlessness, excessive/inappropriate guilt, a pervasive loss of interest or pleasure in all activities | |
| Treatment | SSRIs (citalopram, fluoxetine, paroxetine, sertraline), SNRIs (duloxetine, vanlafaxine, levomilnacipran), Atypical antidepressants (bupropion, mirtazapine, trazodone, vortioxetine), TCAs (imipramine, desipramine, amitriptyline), MAOIs (selegiline) | SSRIs (fluoxetine, paroxetine, sertraline, citalopram, escitalopram) | |
| Side effects | Nausea and vomiting, agitation, anxiety, indigestion, diarrhea or constipation, loss of appetite and weight loss, dizziness, blurred vision, dry mouth, excessive sweating, insomnia or drowsiness, headaches, and sexual side effects | No significant effects | |
| Anxiety | Prevalence | 4% | 39% |
| Symptoms | Trouble concentrating or making decisions, feeling irritable, tense or restless, nausea or abdominal distress, heart palpitations, sweating, trembling or shaking, and sleep problem | Excessive worry, fear, restlessness, and irritability | |
| Treatment | Benzodiazepines (alprazolam, diazepam, lorazepam), SSRIs, buspirone, TCAs (imipramine, clomipramine), MAOIs | SSRIs, SNRIs (venlafaxine), serotonergic atypical anxiolytics (buspirone), citalopram | |
| Side effects | Problems with balance and memory, drowsiness, confusion, vision problems, headaches, feelings of depression, dizziness, nausea, constipation, urinary retention, constipation | Abnormal bleeding, seizures, headaches, nausea, sleep trouble | |
| Apathy | Prevalence | 26–82% | 49% |
| Symptoms | Feeling flat, blunt, or numb emotionally, lack of emotional reaction, low energy and motivation, lack of goal setting, less interest in pleasure activities, hobbies, and relationships, anhedonia, lethargy | A decline in motivation and interest across emotional, goal-directed behavior, and cognitive activity | |
| Treatment | Antidepressants (trazodone, deprenyl, fluvoxamine), psychostimulants (methylphenidate, amphetamine), antipsychotics (risperidone), acetylcholinesterase inhibitors (donepezil, rivastigmine), NMDA receptor antagonists (memantine) | Cholinesterase inhibitors (donepezil, galantamine, rivastigmine), methylphenidate, Ginkgo biloba, modafinil, and SSRIs | |
| Side effects | Modest weight loss, no change in depression score | Weight loss and increased anxiety, no improvement, high blood pressure, cough, and osteoarticular pain | |
| Agitation and Aggression | Prevalence | 1–30% | 30–60% |
| Symptoms | Road rage, child abuse, sexual abuse, and domestic violence, verbal (swearing, shouting, or threatening), physical (hitting, punching, scratching, or biting) | Emotional distress, excessive psychomotor activity, aggressive behaviors, disruptive irritability, and disinhibition | |
| Treatment | Haloperidol, aripiprazole, droperidol, olanzapine, | Valproic acid, brexpiprazole, carbamazepine, SSRIs | |
| Side effects | Dizziness and nausea, paradoxical excitation, constipation, dry mouth, problems sleeping | Hepatotoxicity, GI upset, thrombocytopenia, coagulopathies, metabolic disorders, worsening cognitive dysfunction, agranulocytosis, cardiac arrhythmias | |
| Psychosis | Prevalence | 1.5–3.5% | 50% |
| Symptoms | Delusions, hallucinations, disorganized thought and behavior, poverty of speech, lack of energy, anhedonia, psychomotor retardation, catatonia | Delusions, hallucinations, disorganized thought and speech | |
| Treatment | Antipsychotics (clozapine and olanzapine), Benzodiazepines | Aripiprazole, risperidone, quetiapine, brexpiprazole | |
| Side effects | Drowsiness, dizziness, dry mouth, blurred vision, tiredness, nausea, constipation, weight gain, trouble sleeping, or muscle or nervous system problems (anxiety, agitation, jitteriness, drooling, trouble swallowing, restlessness, shaking, or stiffness) | Stroke, myocardial infarction | |
| Sleep disturbances | Prevalence | 20–41.7% | 45% |
| Symptoms | Excessive daytime sleepiness, irregular breathing or increased movement during sleep, depression, weight gain, lack of concentration, daytime fatigue, irritability, anxiety | Insomnia, excessive daytime sleepiness, nighttime awakenings, and alterations in sleep–wake patterns | |
| Treatment | Melatonin, zolpidem, zaleplon, eszopiclone, ramelteon, suvorexant, lamborexant or doxepin | Melatonin, trazodone, suvorexant, lemborexant | |
| Side effects | Changes in appetite, constipation or diarrhea, dizziness, headache, daytime drowsiness, heartburn, stomach pain, burning or tingling in the hands, arms, feet or legs, mental impairment | Various results, unclear effects | |
| Social processing | Prevalence | 7–11% | 30–50%, |
| Symptoms | Difficulty using appropriate greetings, changing language and communication style, telling and understanding stories, engaging in conversation, repairing communication breakdowns, using appropriated verbal and nonverbal signals, interpreting the verbal and nonverbal signals of others, making inferences, and forming and maintaining close relationships | Language problems, personality changes, and irritability | |
| Treatment | Behavior interventions, social communication treatments (comic strip conversations), social communication intervention, online speech therapy, social skills strengthening activities | N-acetyl cysteine, BDNF, NGF |
| Alzheimer’s Disease | Autism Spectrum Disorder | |
|---|---|---|
| Symptoms |
|
|
| Mechanisms |
|
|
| Therapeutic candidates |
|
|
| Common symptoms for social deficits |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, K.J.; Kim, H.Y.; Han, S.-H.; Shin, C.Y. Future Therapeutic Strategies for Alzheimer’s Disease: Focus on Behavioral and Psychological Symptoms. Int. J. Mol. Sci. 2024, 25, 11338. https://doi.org/10.3390/ijms252111338
Kwon KJ, Kim HY, Han S-H, Shin CY. Future Therapeutic Strategies for Alzheimer’s Disease: Focus on Behavioral and Psychological Symptoms. International Journal of Molecular Sciences. 2024; 25(21):11338. https://doi.org/10.3390/ijms252111338
Chicago/Turabian StyleKwon, Kyoung Ja, Hahn Young Kim, Seol-Heui Han, and Chan Young Shin. 2024. "Future Therapeutic Strategies for Alzheimer’s Disease: Focus on Behavioral and Psychological Symptoms" International Journal of Molecular Sciences 25, no. 21: 11338. https://doi.org/10.3390/ijms252111338
APA StyleKwon, K. J., Kim, H. Y., Han, S.-H., & Shin, C. Y. (2024). Future Therapeutic Strategies for Alzheimer’s Disease: Focus on Behavioral and Psychological Symptoms. International Journal of Molecular Sciences, 25(21), 11338. https://doi.org/10.3390/ijms252111338






