Glucocorticoids Selectively Inhibit Hippocampal CA1 Pyramidal Neurons Activity Through HCN Channels
Abstract
:1. Introduction
2. Results
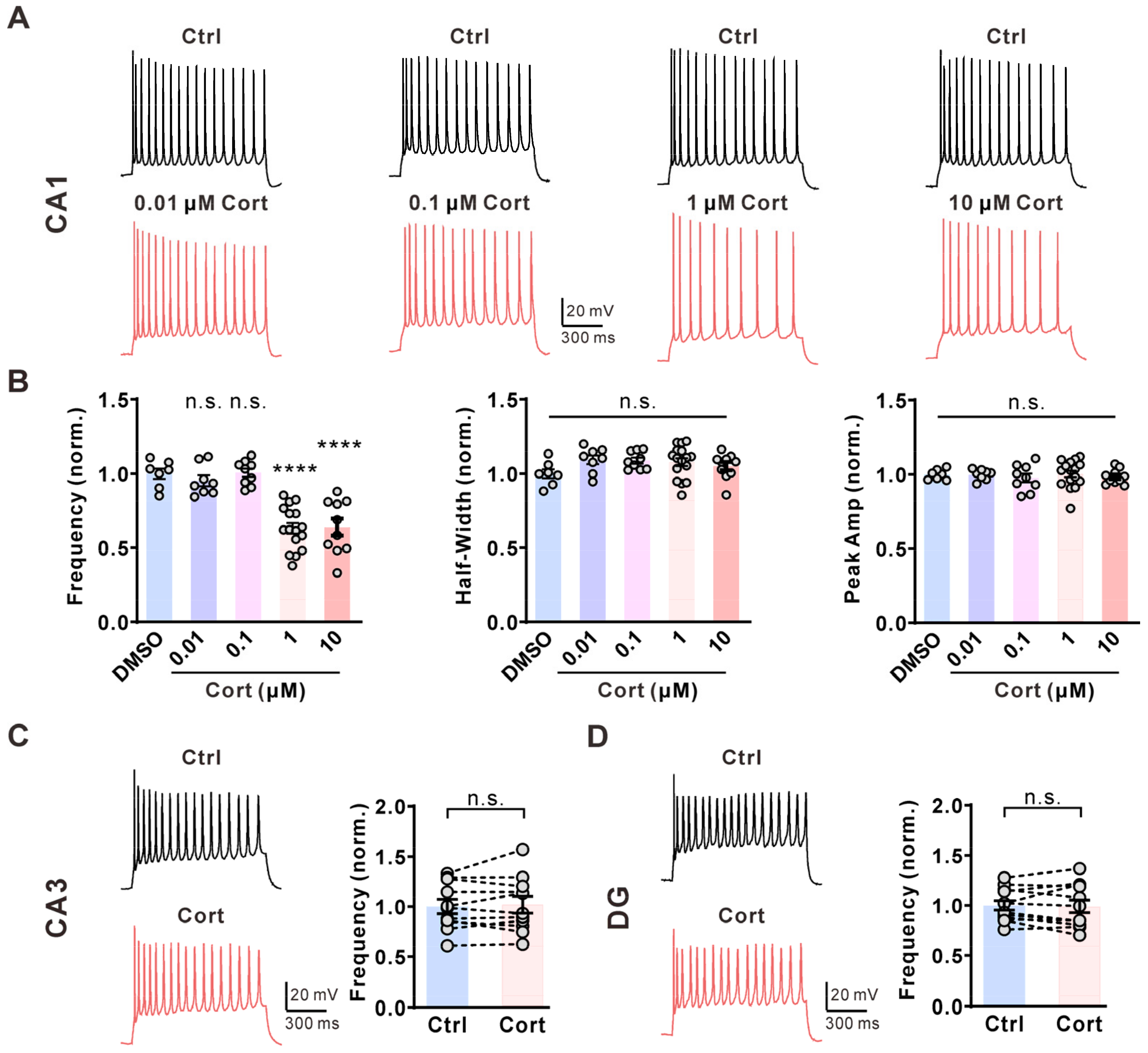
2.1. Corticosterone Selectively Inhibits the Activity of CA1 Pyramidal Neurons in the Hippocampus
2.2. Corticosterone Inhibits AP Firing Frequency via the Membrane-Associated Glucocorticoid Receptor
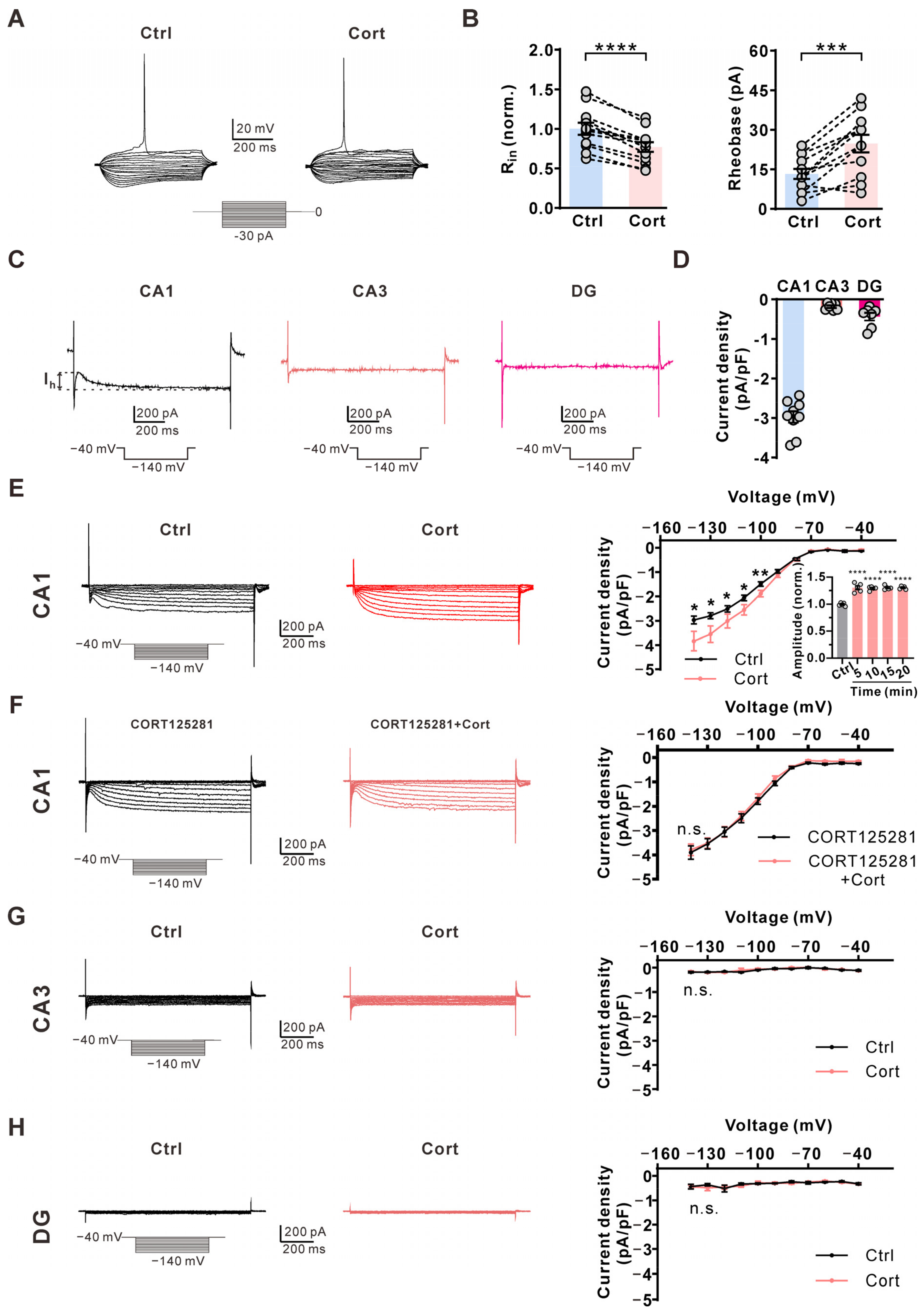
2.3. Corticosterone Increases Ih Amplitude Specifically in CA1 Pyramidal Neurons via Glucocorticoid Receptor
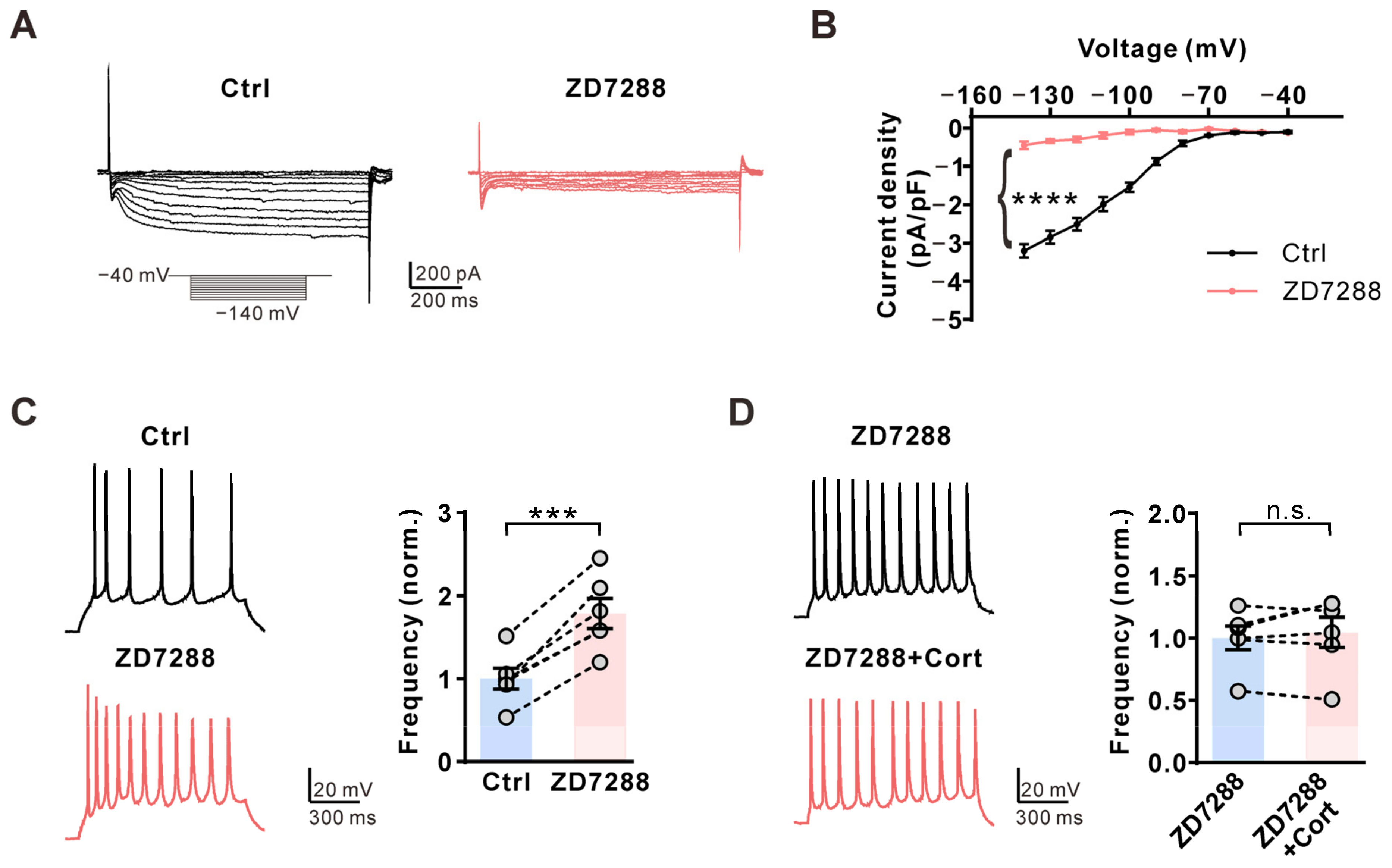
2.4. Corticosterone Inhibits AP Firing Frequency in CA1 Pyramidal Neurons via the HCN Channel
3. Discussion
4. Materials and Methods
4.1. Animal and Slice Preparation
4.2. Electrophysiology
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Frank, M. Glucocorticoids in neurology: Mechanism of action, applications and side effects. Fortschr. Neurol. Psychiatr. 2014, 82, 311–322. [Google Scholar] [CrossRef]
- Spiga, F.; Walker, J.J.; Terry, J.R.; Lightman, S.L. HPA axis-rhythms. Compr. Physiol. 2014, 4, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Reul, J.M.; de Kloet, E.R. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res. 2016, 1645, 50–54. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Pego, J.M.; Taipa, R.; Bessa, J.M.; Almeida, O.F.; Sousa, N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005, 25, 7792–7800. [Google Scholar] [CrossRef]
- Bennur, S.; Shankaranarayana Rao, B.S.; Pawlak, R.; Strickland, S.; McEwen, B.S.; Chattarji, S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 2007, 144, 8–16. [Google Scholar] [CrossRef]
- Vandael, D.; Jonas, P. Structure, biophysics, and circuit function of a “giant” cortical presynaptic terminal. Science 2024, 383, eadg6757. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef]
- Combe, C.L.; Gasparini, S. I(h) from synapses to networks: HCN channel functions and modulation in neurons. Prog. Biophys. Mol. Biol. 2021, 166, 119–132. [Google Scholar] [CrossRef]
- Brewster, A.L.; Chen, Y.; Bender, R.A.; Yeh, A.; Shigemoto, R.; Baram, T.Z. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb. Cortex 2007, 17, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Santoro, B.; Chen, S.; Luthi, A.; Pavlidis, P.; Shumyatsky, G.P.; Tibbs, G.R.; Siegelbaum, S.A. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 2000, 20, 5264–5275. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, D.V.; Barish, M.E. Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons. J. Neurosci. 2002, 22, 8992–9004. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Abbott, L.F.; Siegelbaum, S.A. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat. Neurosci. 2009, 12, 577–584. [Google Scholar] [CrossRef]
- Kim, J.; Lei, Y.; Lu, X.Y.; Kim, C.S. Glucocorticoid-glucocorticoid receptor-HCN1 channels reduce neuronal excitability in dorsal hippocampal CA1 neurons. Mol. Psychiatry 2022, 27, 4035–4049. [Google Scholar] [CrossRef]
- Han, J.Z.; Lin, W.; Chen, Y.Z. Inhibition of ATP-induced calcium influx in HT4 cells by glucocorticoids: Involvement of protein kinase A. Acta Pharmacol. Sin. 2005, 26, 199–204. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, C.; Chen, Y. Glucocorticoid rapidly enhances NMDA-evoked neurotoxicity by attenuating the NR2A-containing NMDA receptor-mediated ERK1/2 activation. Mol. Endocrinol. 2010, 24, 497–510. [Google Scholar] [CrossRef]
- Harris, C.; Weiss, G.L.; Di, S.; Tasker, J.G. Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiol. Stress 2019, 10, 100158. [Google Scholar] [CrossRef]
- Ffrench-Mullen, J.M. Cortisol inhibition of calcium currents in guinea pig hippocampal CA1 neurons via G-protein-coupled activation of protein kinase C. J. Neurosci. 1995, 15, 903–911. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hu, J.; Pan, C.; Gao, Y.; Liu, Q.; Xu, W.; Xue, L.; Hu, C. Glucocorticoids modulate neural activity via a rapid non-genomic effect on Kv2.2 channels in the central nervous system. Neurobiol. Stress 2024, 28, 100593. [Google Scholar] [CrossRef]
- Zaki, A.; Barrett-Jolley, R. Rapid neuromodulation by cortisol in the rat paraventricular nucleus: An in vitro study. Br. J. Pharmacol. 2002, 137, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zolles, G.; Klocker, N.; Wenzel, D.; Weisser-Thomas, J.; Fleischmann, B.K.; Roeper, J.; Fakler, B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 2006, 52, 1027–1036. [Google Scholar] [CrossRef]
- Herrmann, S.; Rajab, H.; Christ, I.; Schirdewahn, C.; Hofler, D.; Fischer, M.J.M.; Bruno, A.; Fenske, S.; Gruner, C.; Kramer, F.; et al. Protein kinase A regulates inflammatory pain sensitization by modulating HCN2 channel activity in nociceptive sensory neurons. Pain 2017, 158, 2012–2024. [Google Scholar] [CrossRef]
- Zong, X.; Eckert, C.; Yuan, H.; Wahl-Schott, C.; Abicht, H.; Fang, L.; Li, R.; Mistrik, P.; Gerstner, A.; Much, B.; et al. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J. Biol. Chem. 2005, 280, 34224–34232. [Google Scholar] [CrossRef]
- Arinsburg, S.S.; Cohen, I.S.; Yu, H.G. Constitutively active Src tyrosine kinase changes gating of HCN4 channels through direct binding to the channel proteins. J. Cardiovasc. Pharmacol. 2006, 47, 578–586. [Google Scholar] [CrossRef]
- Sartiani, L.; Mannaioni, G.; Masi, A.; Novella Romanelli, M.; Cerbai, E. The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: From Biophysics to Pharmacology of a Unique Family of Ion Channels. Pharmacol. Rev. 2017, 69, 354–395. [Google Scholar] [CrossRef]
- Garratt, J.C.; Alreja, M.; Aghajanian, G.K. LSD has high efficacy relative to serotonin in enhancing the cationic current Ih: Intracellular studies in rat facial motoneurons. Synapse 1993, 13, 123–134. [Google Scholar] [CrossRef]
- Gasparini, S.; DiFrancesco, D. Action of serotonin on the hyperpolarization-activated cation current (Ih) in rat CA1 hippocampal neurons. Eur. J. Neurosci. 1999, 11, 3093–3100. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Li, D.; Chang, C.; Weng, X. Upregulation of HCN2 in ventral tegmental area is involved in morphine-induced conditioned place preference in rats. FEBS Open Bio 2024. ahead of print. [Google Scholar] [CrossRef]
- Mu, L.; Liu, X.; Yu, H.; Vickstrom, C.R.; Friedman, V.; Kelly, T.J.; Hu, Y.; Su, W.; Liu, S.; Mantsch, J.R.; et al. cAMP-mediated upregulation of HCN channels in VTA dopamine neurons promotes cocaine reinforcement. Mol. Psychiatry 2023, 28, 3930–3942. [Google Scholar] [CrossRef]
- Gray, J.D.; Kogan, J.F.; Marrocco, J.; McEwen, B.S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 2017, 13, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.; Villegas, M.; Martinez, C.; McEwen, B.S. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994, 639, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, K.; Russell, G.M.; Harmer, C.J.; Munafo, M.R.; Marchant, N.; Wilson, A.; Brooks, J.C.; Durant, C.; Thakrar, J.; Murphy, P.; et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc. Natl. Acad. Sci. USA 2018, 115, E4091–E4100. [Google Scholar] [CrossRef] [PubMed]
- Tasker, J.G.; Herman, J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011, 14, 398–406. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lu, T.; Pan, C.; Hu, C. Glucocorticoids Selectively Inhibit Hippocampal CA1 Pyramidal Neurons Activity Through HCN Channels. Int. J. Mol. Sci. 2024, 25, 11971. https://doi.org/10.3390/ijms252211971
Li C, Lu T, Pan C, Hu C. Glucocorticoids Selectively Inhibit Hippocampal CA1 Pyramidal Neurons Activity Through HCN Channels. International Journal of Molecular Sciences. 2024; 25(22):11971. https://doi.org/10.3390/ijms252211971
Chicago/Turabian StyleLi, Chenyang, Tongchuang Lu, Chengfang Pan, and Changlong Hu. 2024. "Glucocorticoids Selectively Inhibit Hippocampal CA1 Pyramidal Neurons Activity Through HCN Channels" International Journal of Molecular Sciences 25, no. 22: 11971. https://doi.org/10.3390/ijms252211971
APA StyleLi, C., Lu, T., Pan, C., & Hu, C. (2024). Glucocorticoids Selectively Inhibit Hippocampal CA1 Pyramidal Neurons Activity Through HCN Channels. International Journal of Molecular Sciences, 25(22), 11971. https://doi.org/10.3390/ijms252211971




