GmbZIP4a/b Positively Regulate Nodule Number by Affecting Cytokinin Biosynthesis in Glycine max
Abstract
1. Introduction
2. Results
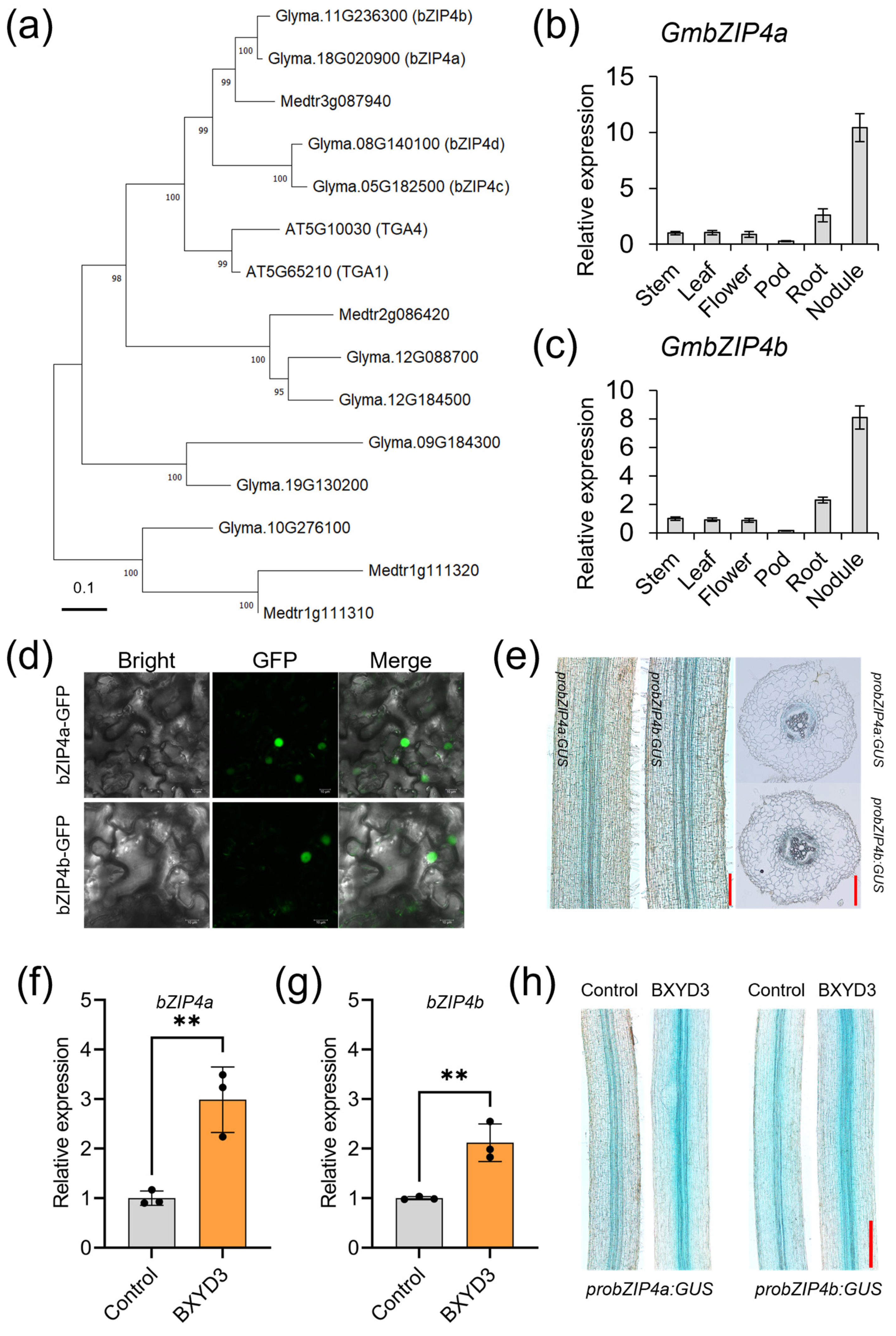
2.1. Transcriptional Levels of GmbZIP4a/b Were Rhizobia-Inducible
2.2. Generation of Gmbzip4a/b Double Mutants Using CRISPR/Cas9
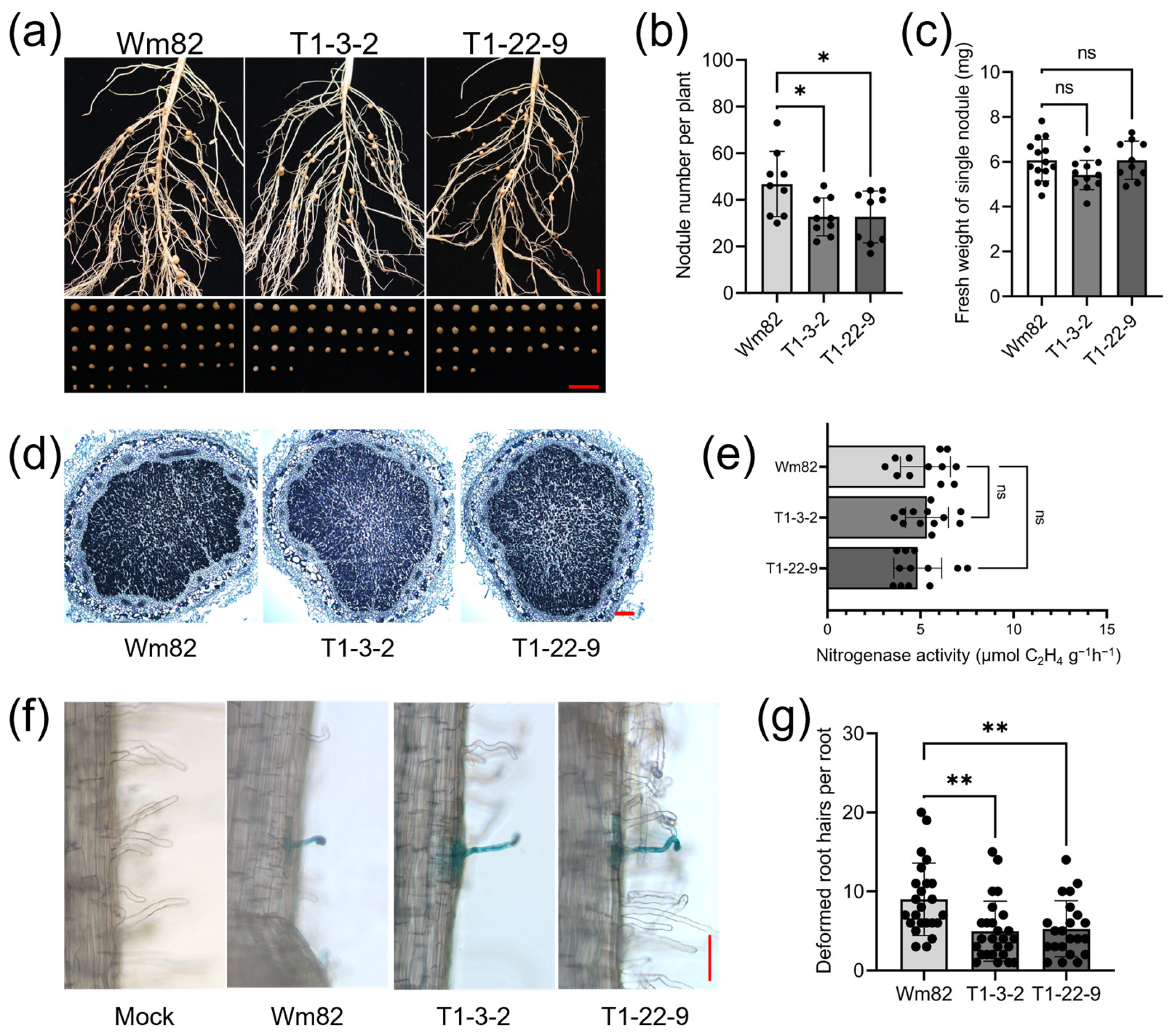
2.3. Gmbzip4a/b Double Mutants Showed Decreased Nodule Numbers
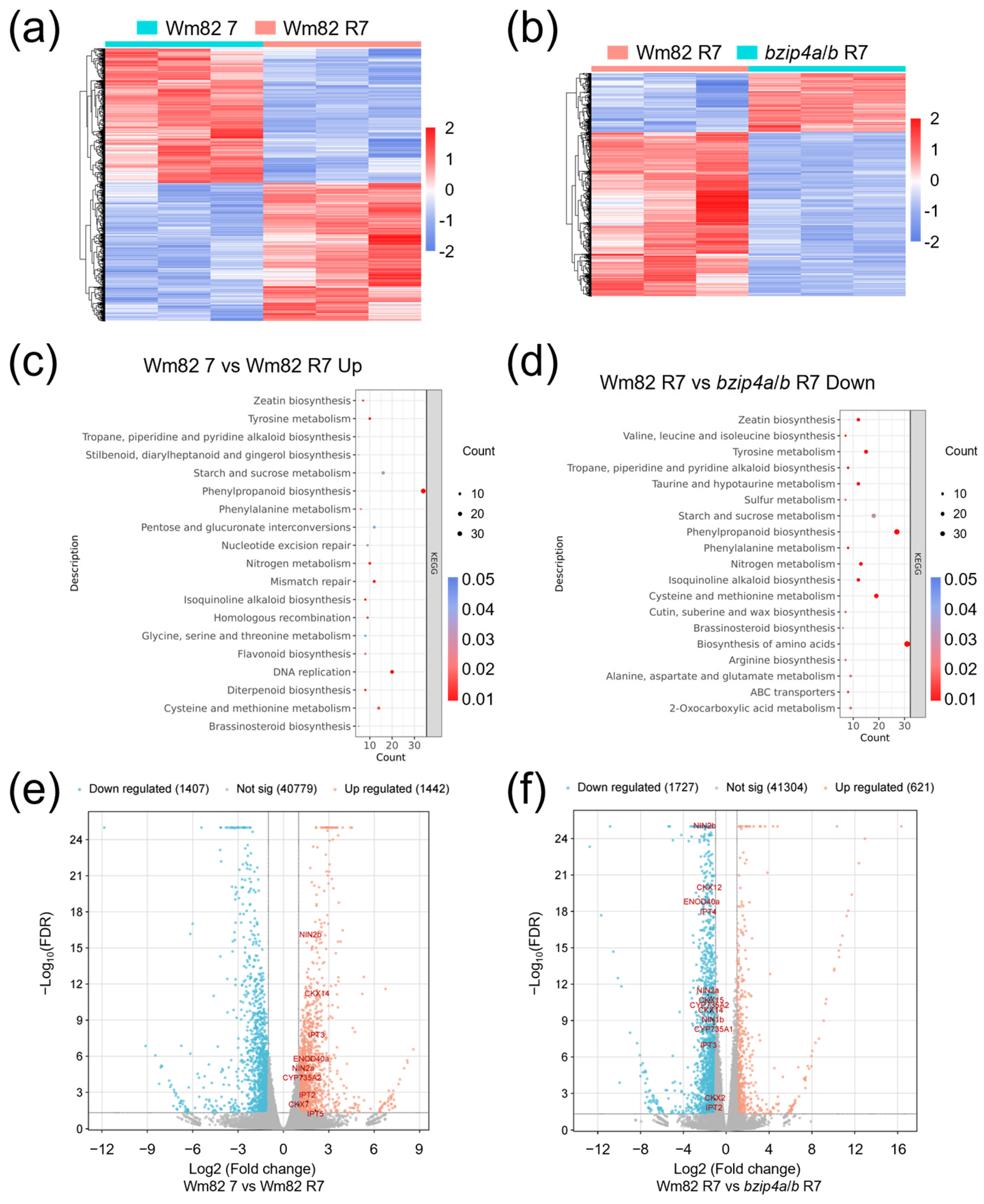
2.4. The Impaired Nodule Establishment in Gmbzip4a/b Is Associated with Zeatin Biosynthesis
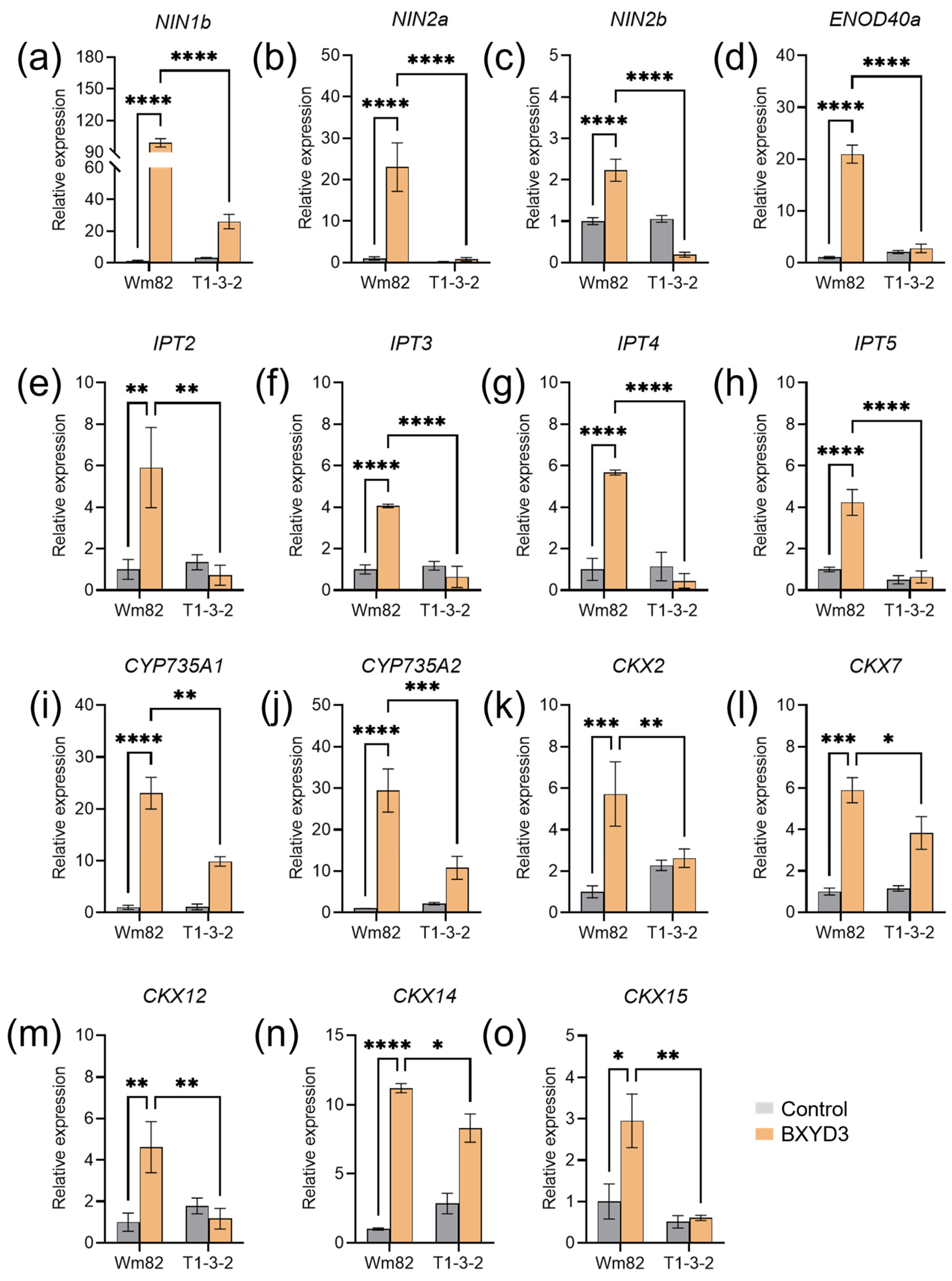
2.5. Zeatin Biosynthesis Genes Were Not Rhizobia-Responsive in Gmbzip4a/b Mutants
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. RNA Extraction and qRT-PCR Analysis
4.3. Transient Expression of bZIP4a/b CDS Sequence in Nicotiana benthamiana
4.4. Histochemical GUS Staining of Tissue Sections
4.5. Generation of Gmbzip4a/b Double Mutants via CRISPR-Cas9
4.6. Nodule and Infection Thread Traits Analysis
4.7. Transcriptome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gautrat, P.; Mortier, V.; Laffont, C.; De Keyser, A.; Fromentin, J.; Frugier, F.; Goormachtig, S. Unraveling new molecular players involved in the autoregulation of nodulation in Medicago truncatula. J. Exp. Bot. 2019, 70, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.-C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.-Q.; et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wang, X. Energy sensors: Emerging regulators of symbiotic nitrogen fixation. Trends Plant Sci. 2024, 29, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; McNeil, D.L.; Gresshoff, P.M. A Supernodulation and Nitrate-Tolerant Symbiotic (nts) Soybean Mutant. Plant Physiol. 1985, 78, 34–40. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Shen, D.; Bisseling, T. Soybean breeders can count on nodules. Trends Plant Sci. 2024. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, J.; Shi, X.; Bai, M.; Yuan, C.; Cai, C.; Wang, N.; Zhu, X.; Kuang, H.; Wang, X.; et al. Genetically optimizing soybean nodulation improves yield and protein content. Nat. Plants 2024, 10, 736–742. [Google Scholar] [CrossRef]
- Tsikou, D.; Yan, Z.; Holt, D.B.; Abel, N.B.; Reid, D.E.; Madsen, L.H.; Bhasin, H.; Sexauer, M.; Stougaard, J.; Markmann, K. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 2018, 362, 233–236. [Google Scholar] [CrossRef]
- Nishida, H.; Suzaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant. Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Z.; Zhu, W.; Wang, N.; Bai, M.; Kuang, H.; Cai, C.; Zhong, X.; Kong, F.; Lü, P.; et al. The NAC transcription factors SNAP1/2/3/4 are central regulators mediating high nitrogen responses in mature nodules of soybean. Nat. Commun. 2023, 14, 4711. [Google Scholar] [CrossRef]
- Lin, J.S.; Li, X.; Luo, Z.; Mysore, K.S.; Wen, J.; Xie, F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants 2018, 4, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tanaka, S.; Handa, Y.; Ito, M.; Sakamoto, Y.; Matsunaga, S.; Betsuyaku, S.; Miura, K.; Soyano, T.; Kawaguchi, M.; et al. A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat. Plants 2020, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Misawa, F.; Ito, M.; Nosaki, S.; Nishida, H.; Watanabe, M.; Suzuki, T.; Miura, K.; Kawaguchi, M.; Suzaki, T. Nitrate transport via NRT2.1 mediates NIN-LIKE PROTEIN-dependent suppression of root nodulation in Lotus japonicus. Plant Cell 2022, 34, 1844–1862. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, A.; Wu, J.; Li, H.; Duan, Y.; Chen, Q.; Zhu, H.; Cao, Y. GmNAC039 and GmNAC018 activate the expression of cysteine protease genes to promote soybean nodule senescence. Plant Cell 2023, 35, 2929–2951. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Wang, N.; Li, S.; Yao, X.; Kuang, H.; Qiu, Z.; Ke, D.; Yang, W.; Guan, Y. Nitrogen inhibition of nitrogenase activity involves the modulation of cytosolic invertase in soybean nodule. J. Genet. Genom. 2024, 51, 1404–1412. [Google Scholar] [CrossRef]
- Lin, J.; Bjørk, P.K.; Kolte, M.V.; Poulsen, E.; Dedic, E.; Drace, T.; Andersen, S.U.; Nadzieja, M.; Liu, H.; Castillo-Michel, H.; et al. Zinc mediates control of nitrogen fixation via transcription factor filamentation. Nature 2024, 631, 164–169. [Google Scholar] [CrossRef]
- Xiao, A.; Wu, J.; Wang, W.; Guan, Y.; Zhuang, M.; Guo, X.; Zhu, H.; Yu, H.; Cao, Y. Soybean ethylene response factors GmENS1 and GmENS2 promote nodule senescence. Plant Physiol. 2024, 196, 1029–1041. [Google Scholar] [CrossRef]
- Katagiri, F.; Lam, E.; Chua, N.-H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature 1989, 340, 727–730. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Kesarwani, M.; Yoo, J.; Dong, X. Genetic Interactions of TGA Transcription Factors in the Regulation of Pathogenesis-Related Genes and Disease Resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Busta, L.; Zhang, Q.; Ding, P.; Jetter, R.; Zhang, Y. TGACG-BINDING FACTOR 1 (TGA1) and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULIN-BINDING PROTEIN 60g (CBP60g). New Phytol. 2018, 217, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Yang, B.; Ruan, R.; Li, K.; Menzo, V.; Fu, D.; Chern, M.; Ronald, P.C.; Dubcovsky, J. Comparative analysis of protein-protein interactions in the defense response of rice and wheat. BMC Genom. 2013, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Li, K.; Kang, Z.; Cantu, D.; Dubcovsky, J. A Conserved Puccinia striiformis Protein Interacts with Wheat NPR1 and Reduces Induction of Pathogenesis-Related Genes in Response to Pathogens. Mol. Plant Microbe Interact. 2016, 29, 977–989. [Google Scholar] [CrossRef]
- Shimizu, K.; Suzuki, H.; Uemura, T.; Nozawa, A.; Desaki, Y.; Hoshino, R.; Yoshida, A.; Abe, H.; Nishiyama, M.; Nishiyama, C.; et al. Immune gene activation by NPR and TGA transcriptional regulators in the model monocot Brachypodium distachyon. Plant J. 2022, 110, 470–481. [Google Scholar] [CrossRef]
- Kumar, N.; Galli, M.; Dempsey, D.; Imani, J.; Moebus, A.; Kogel, K.H. NPR1 is required for root colonization and the establishment of a mutualistic symbiosis between the beneficial bacterium Rhizobium radiobacter and barley. Environ. Microbiol. 2021, 23, 2102–2115. [Google Scholar] [CrossRef]
- Tóth, K.; Stacey, G. Does plant immunity play a critical role during initiation of the legume-rhizobium symbiosis? Front. Plant Sci. 2015, 6, 401. [Google Scholar]
- Ullah, I.; Magdy, M.; Wang, L.; Liu, M.; Li, X. Genome-wide identification and evolutionary analysis of TGA transcription factors in soybean. Sci. Rep. 2019, 9, 11186. [Google Scholar] [CrossRef]
- De Cuyper, C.; Fromentin, J.; Yocgo, R.E.; De Keyser, A.; Guillotin, B.; Kunert, K.; Boyer, F.-D.; Goormachtig, S. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2014, 66, 137–146. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Downie, J.A. Calcium, kinases and nodulation signalling in legumes. Nat. Rev. Mol. Cell Biol. 2004, 5, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant. Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.E. FER meets the Nod factor pathway. Nat. Plants 2023, 9, 1581–1582. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-López, O.; Tamayo, K.P.; Aceituno, F.; Gómez, I.; Ruffel, S.; Lejay, L.; et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014, 80, 1–13. [Google Scholar] [CrossRef]
- Canales, J.; Contreras-López, O.; Álvarez, J.M.; Gutiérrez, R.A. Nitrate induction of root hair density is mediated by TGA1/TGA4 and CPC transcription factors in Arabidopsis thaliana. Plant J. 2017, 92, 305–316. [Google Scholar] [CrossRef]
- Frugier, F.; Kosuta, S.; Murray, J.D.; Crespi, M.; Szczyglowski, K. Cytokinin: Secret agent of symbiosis. Trends Plant Sci. 2008, 13, 115–120. [Google Scholar] [CrossRef]
- Wang, D.; Dong, W.; Murray, J.; Wang, E. Innovation and appropriation in mycorrhizal and rhizobial Symbioses. Plant Cell 2022, 34, 1573–1599. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, Y.; Chang, H.; Wang, C.; Yang, J.; Shi, J.; Gao, J.; Yang, W.; Lan, L.; Wang, Y.; et al. An SHR-SCR module specifies legume cortical cell fate to enable nodulation. Nature 2021, 589, 586–590. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of Plant Cytokinin Biosynthetic Enzymes as Dimethylallyl Diphosphate:ATP/ADP Isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Li, X.; Jiang, H.; Wu, P.; Xia, K.; Yang, Y.; Wu, G. Knockdown of LjIPT3 influences nodule development in Lotus japonicus. Plant Cell Physiol. 2014, 55, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-derived cytokinins systemically regulate root nodulation. Nat. Commun. 2014, 5, 4983. [Google Scholar] [CrossRef]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin Biosynthesis Promotes Cortical Cell Responses during Nodule Development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Azarakhsh, M.; Lebedeva, M.A.; Lutova, L.A. Identification and Expression Analysis of Medicago truncatula Isopentenyl Transferase Genes (IPTs) Involved in Local and Systemic Control of Nodulation. Front. Plant Sci. 2018, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Montiel, J.; Reid, D.; Grønbæk, T.H.; Benfeldt, C.M.; James, E.K.; Ott, T.; Ditengou, F.A.; Nadzieja, M.; Kelly, S.; Stougaard, J. Distinct signaling routes mediate intercellular and intracellular rhizobial infection in Lotus japonicus. Plant Physiol. 2020, 185, 1131–1147. [Google Scholar] [CrossRef]
- Mortier, V.; Wasson, A.; Jaworek, P.; De Keyser, A.; Decroos, M.; Holsters, M.; Tarkowski, P.; Mathesius, U.; Goormachtig, S. Role of LONELY GUY genes in indeterminate nodulation on Medicago truncatula. New Phytol. 2014, 202, 582–593. [Google Scholar] [CrossRef]
- Reid, D.E.; Heckmann, A.B.; Novák, O.; Kelly, S.; Stougaard, J. CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus japonicus. Plant Physiol. 2016, 170, 1060–1074. [Google Scholar] [CrossRef]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.-H.; Hwang, I. The Cytokinin-Activated Transcription Factor ARR2 Promotes Plant Immunity via TGA3/NPR1-Dependent Salicylic Acid Signaling in Arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef]
- Bai, M.; Yuan, C.; Kuang, H.; Sun, Q.; Hu, X.; Cui, L.; Lin, W.; Peng, C.; Yue, P.; Song, S.; et al. Combination of two multiplex genome-edited soybean varieties enables customization of protein functional properties. Mol. Plant 2022, 15, 1081–1083. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.-J.; Zhang, K. Spatially distributed cytokinins: Metabolism, signaling, and transport. Plant Commun. 2024, 5, 100936. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr. Biol. 2023, 33, R543–R559. [Google Scholar] [CrossRef] [PubMed]
- Kawade, K.; Sugiura, D.; Oikawa, A.; Kawaguchi, M. Control of root nodule formation ensures sufficient shoot water availability in Lotus japonicus. Plant Physiol. 2024, 195, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yao, X.; Li, X.; Liu, J.; Bai, M.; Fang, Z.; Gong, J.; Guan, Y.; Xie, F. GmNLP1 and GmNLP4 activate nitrate-induced CLE peptides NIC1a/b to mediate nitrate-regulated root nodulation. Plant J. 2024, 119, 783–795. [Google Scholar] [CrossRef]
- Yu, L.; Di, Q.; Zhang, D.; Liu, Y.; Li, X.; Mysore, K.S.; Wen, J.; Yan, J.; Luo, L. A legume-specific novel type of phytosulfokine, PSK-δ, promotes nodulation by enhancing nodule organogenesis. J. Exp. Bot. 2022, 73, 2698–2713. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Yan, J.; Li, C.; Lin, X.; Lin, L.; Cao, Y.; Xu, T.; Li, J.; Yuan, Y.; et al. VPT-like genes modulate Rhizobium–legume symbiosis and phosphorus adaptation. Plant J. 2023, 116, 112–127. [Google Scholar] [CrossRef]
- Ivanovici, A.; Laffont, C.; Larrainzar, E.; Patel, N.; Winning, C.S.; Lee, H.-C.; Imin, N.; Frugier, F.; Djordjevic, M.A. The Medicago SymCEP7 hormone increases nodule number via shoots without compromising lateral root number. Plant Physiol. 2023, 191, 2012–2026. [Google Scholar] [CrossRef]
- Liu, B.; Wu, J.; Yang, S.; Schiefelbein, J.; Gan, Y. Nitrate regulation of lateral root and root hair development in plants. J. Exp. Bot. 2019, 71, 4405–4414. [Google Scholar] [CrossRef]
- Triozzi, P.M.; Irving, T.B.; Schmidt, H.W.; Keyser, Z.P.; Chakraborty, S.; Balmant, K.; Pereira, W.J.; Dervinis, C.; Mysore, K.S.; Wen, J.; et al. Spatiotemporal cytokinin response imaging and ISOPENTENYLTRANSFERASE 3 function in Medicago nodule development. Plant Physiol. 2021, 188, 560–575. [Google Scholar] [CrossRef]
- Gamas, P.; Brault, M.; Jardinaud, M.-F.; Frugier, F. Cytokinins in Symbiotic Nodulation: When, Where, What For? Trends Plant Sci. 2017, 22, 792–802. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone modulation of legume-rhizobial symbiosis. J. Inter. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant. Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- van Zeijl, A.; Op den Camp, R.H.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; Op den Camp, H.J.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium Lipo-chitooligosaccharide Signaling Triggers Accumulation of Cytokinins in Medicago truncatula Roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Khandal, H.; Gupta, S.K.; Dwivedi, V.; Mandal, D.; Sharma, N.K.; Vishwakarma, N.K.; Pal, L.; Choudhary, M.; Francis, A.; Malakar, P.; et al. Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotechnol. J. 2020, 18, 2225–2240. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, G.; Wang, X.; Zhao, J.; Yan, X.; Liao, H. Isolation and application of effective nitrogen fixation rhizobial strains on low-phosphorus acid soils in South China. Chin. Sci. Bull. 2009, 54, 412–420. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qiu, Z.; Bai, M.; Kuang, H.; Wang, X.; Yu, X.; Zhong, X.; Guan, Y. Cytosolic Fructose-1,6-bisphosphate Aldolases Modulate Primary Metabolism and Phytohormone Homeostasis in Soybean. Agronomy 2023, 13, 1383. [Google Scholar] [CrossRef]
- Bai, M.; Yuan, J.; Kuang, H.; Gong, P.; Li, S.; Zhang, Z.; Liu, B.; Sun, J.; Yang, M.; Yang, L.; et al. Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol. J. 2020, 18, 721–731. [Google Scholar] [CrossRef]
- Fu, M.; Sun, J.; Li, X.; Guan, Y.; Xie, F. Asymmetric redundancy of soybean Nodule Inception (NIN) genes in root nodule symbiosis. Plant Physiol. 2022, 188, 477–489. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genom. Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wang, N.; Wang, X.; Qiu, Z.; Kuang, H.; Guan, Y. GmbZIP4a/b Positively Regulate Nodule Number by Affecting Cytokinin Biosynthesis in Glycine max. Int. J. Mol. Sci. 2024, 25, 13311. https://doi.org/10.3390/ijms252413311
Meng Y, Wang N, Wang X, Qiu Z, Kuang H, Guan Y. GmbZIP4a/b Positively Regulate Nodule Number by Affecting Cytokinin Biosynthesis in Glycine max. International Journal of Molecular Sciences. 2024; 25(24):13311. https://doi.org/10.3390/ijms252413311
Chicago/Turabian StyleMeng, Yongjie, Nan Wang, Xin Wang, Zhimin Qiu, Huaqin Kuang, and Yuefeng Guan. 2024. "GmbZIP4a/b Positively Regulate Nodule Number by Affecting Cytokinin Biosynthesis in Glycine max" International Journal of Molecular Sciences 25, no. 24: 13311. https://doi.org/10.3390/ijms252413311
APA StyleMeng, Y., Wang, N., Wang, X., Qiu, Z., Kuang, H., & Guan, Y. (2024). GmbZIP4a/b Positively Regulate Nodule Number by Affecting Cytokinin Biosynthesis in Glycine max. International Journal of Molecular Sciences, 25(24), 13311. https://doi.org/10.3390/ijms252413311





