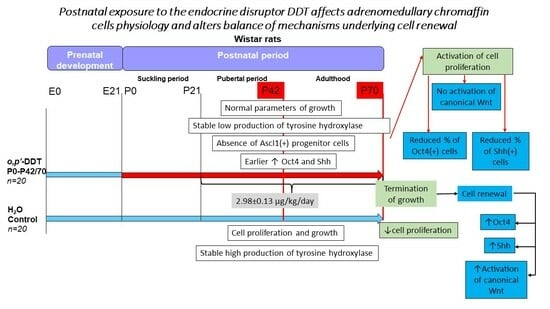
Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal
Abstract
1. Introduction
2. Results
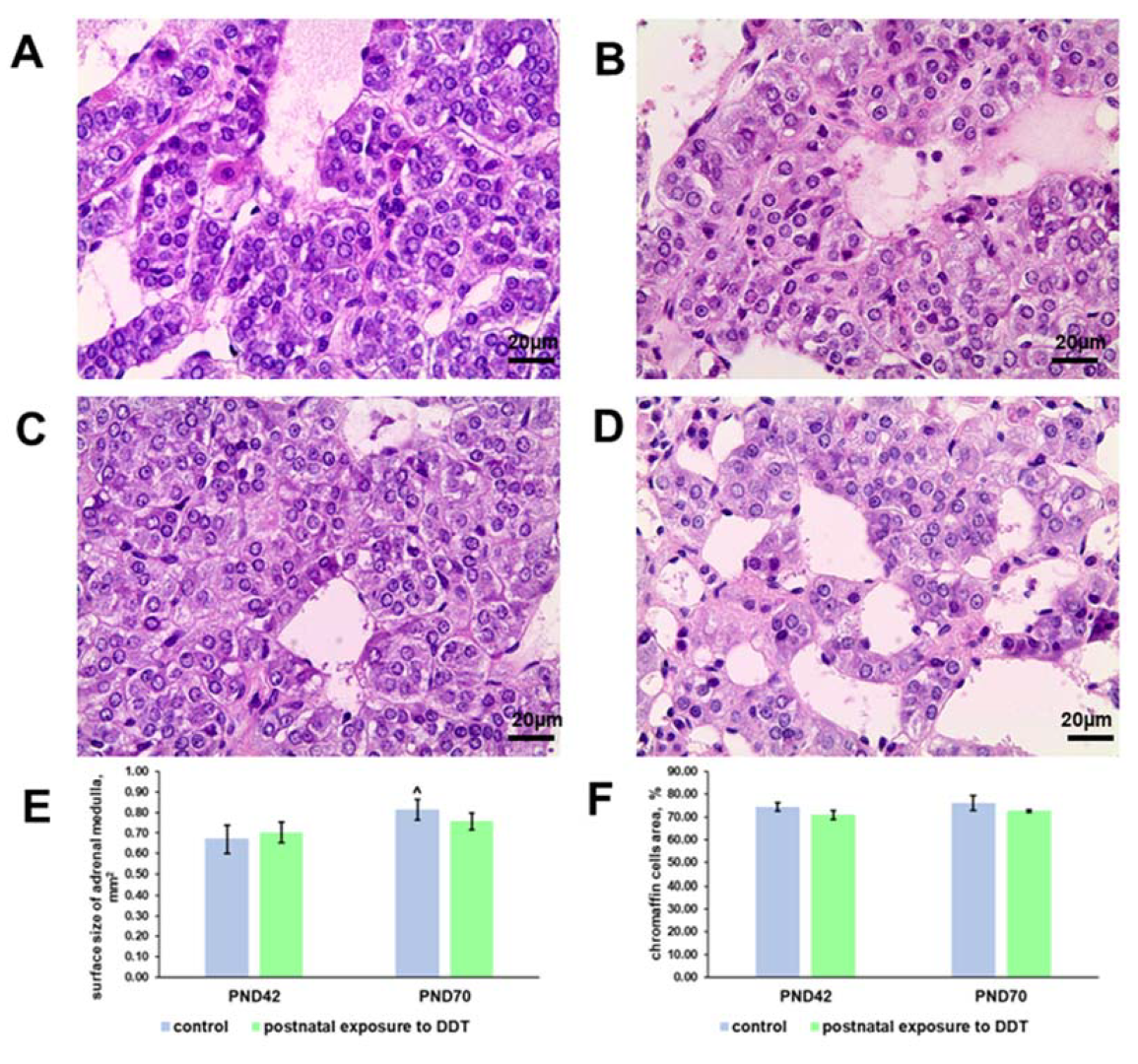
2.1. Histology and Histomorphometry of the Adrenal Medulla
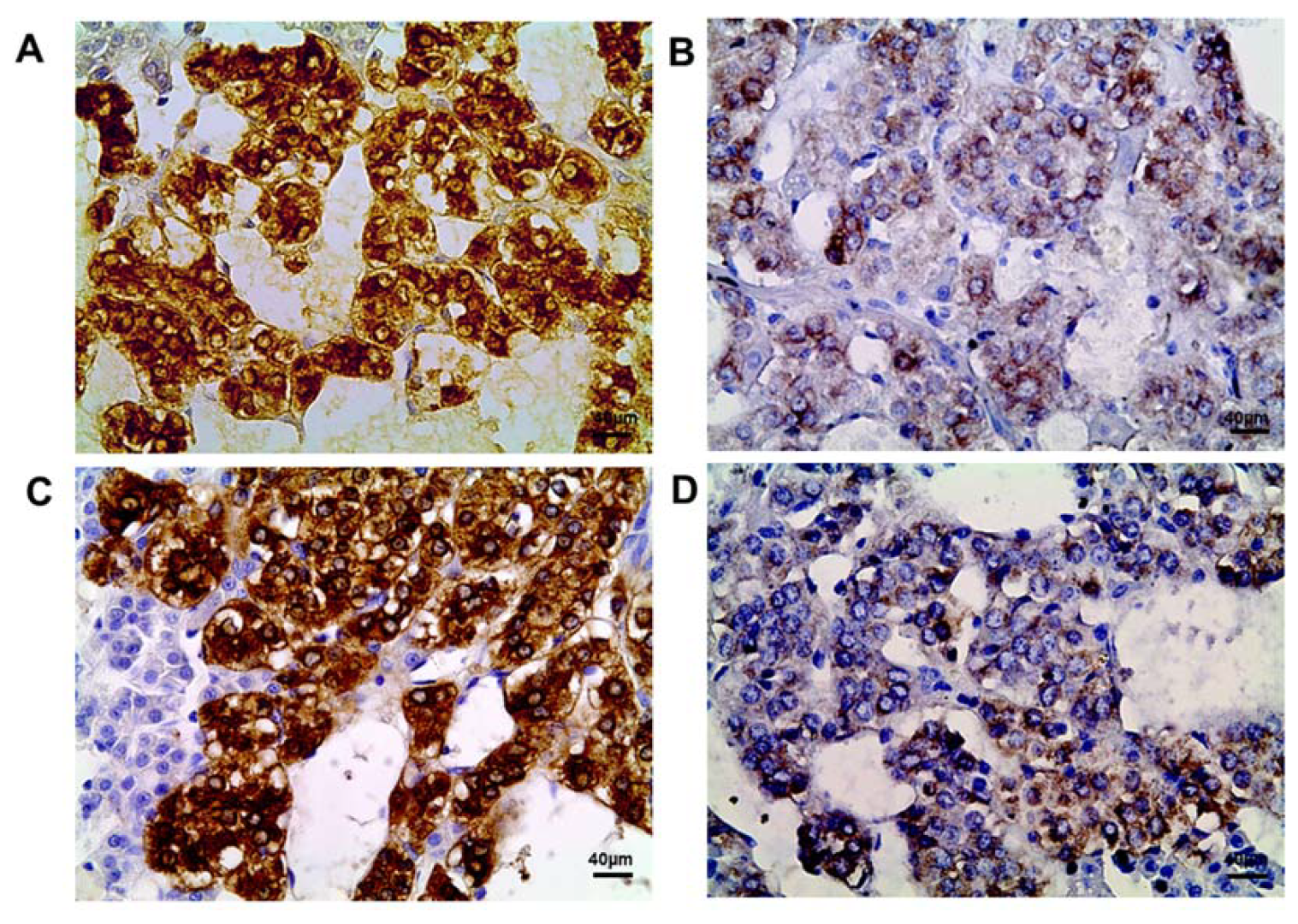
2.2. Tyrosine Hydroxylase Content in the Adrenomedullary Chromaffin Cells
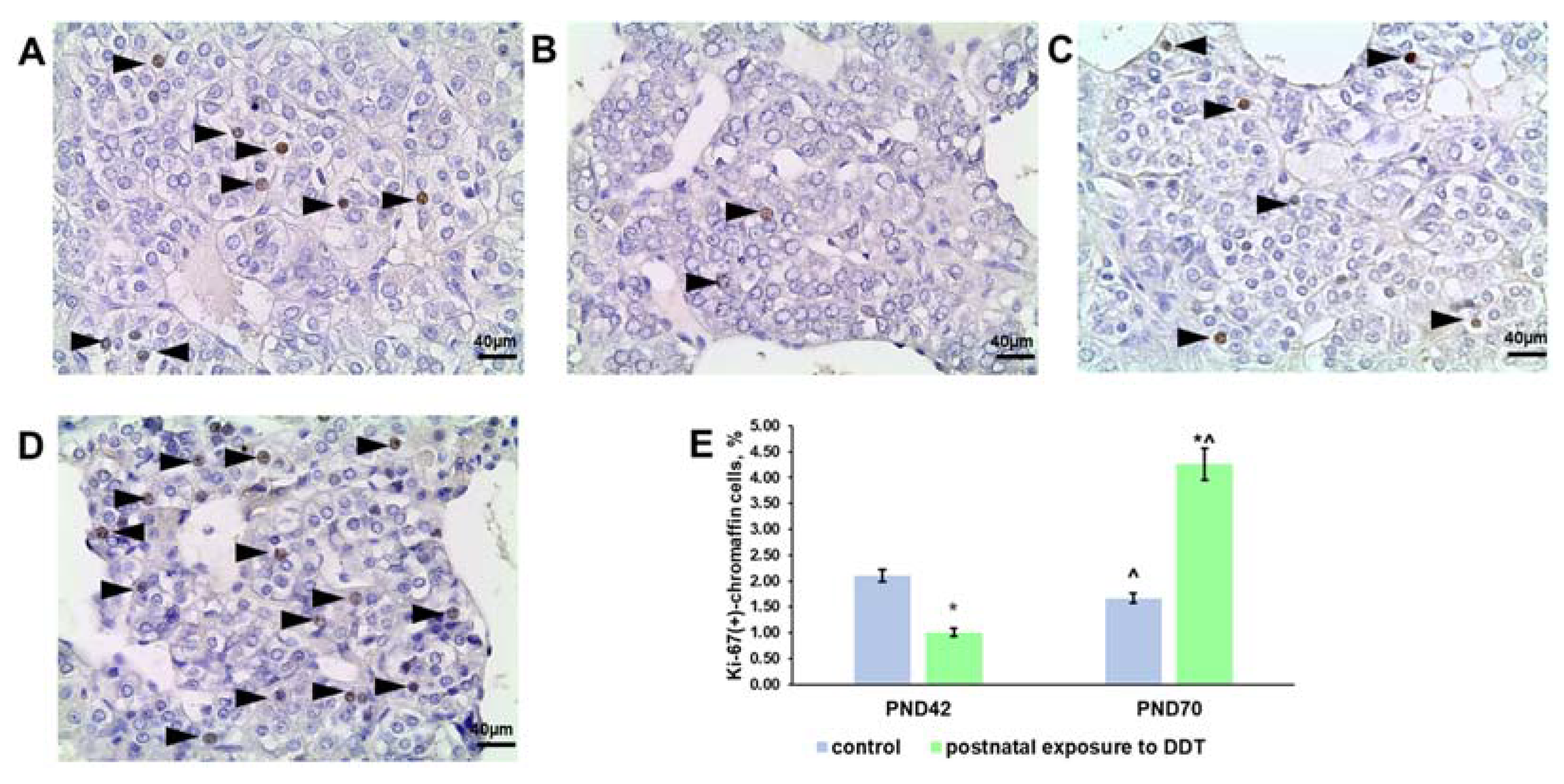
2.3. Proliferation Rate of the Adrenomedullary Chromaffin Cells
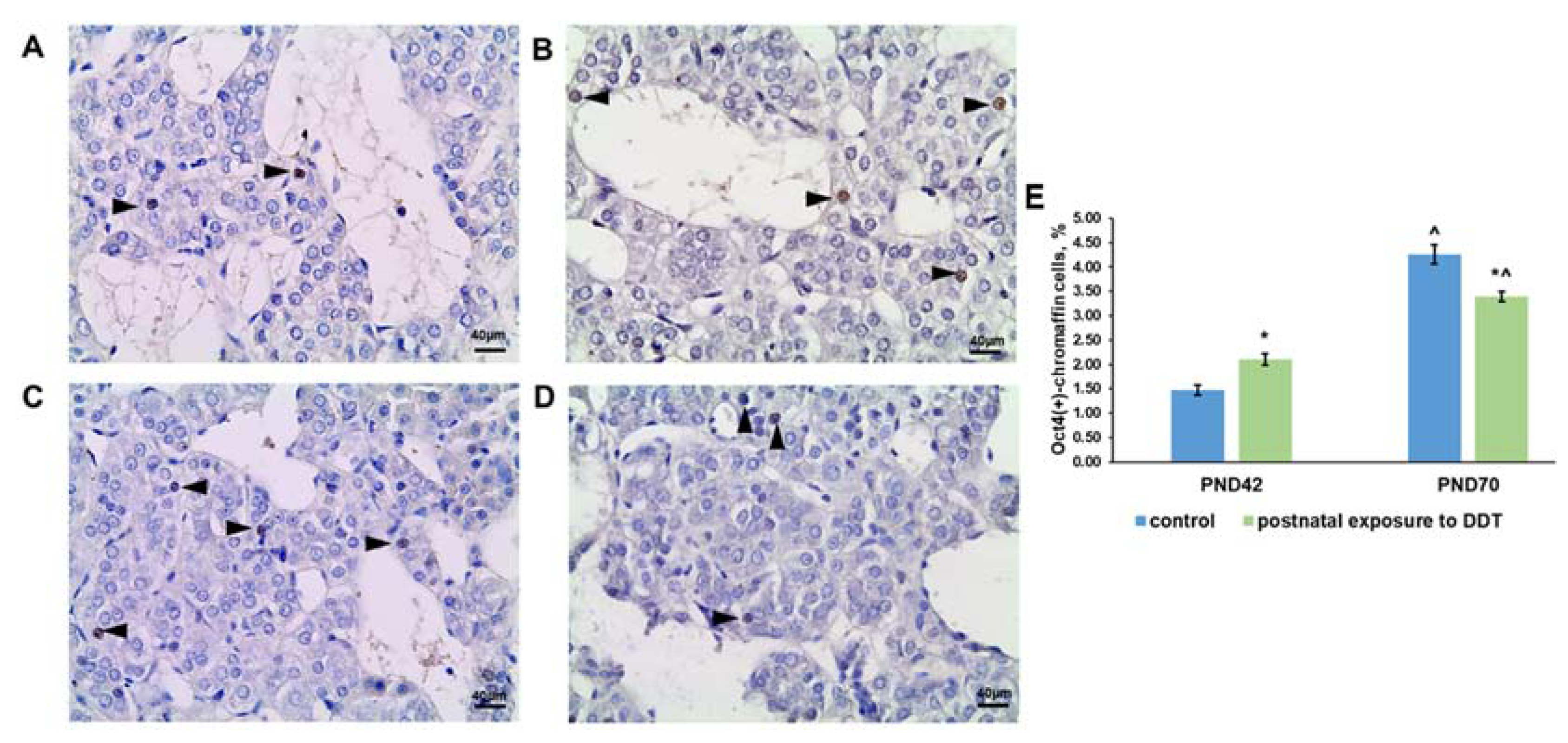
2.4. Oct4 Expression
2.5. Sox2 Expression
2.6. Ascl1 Expression
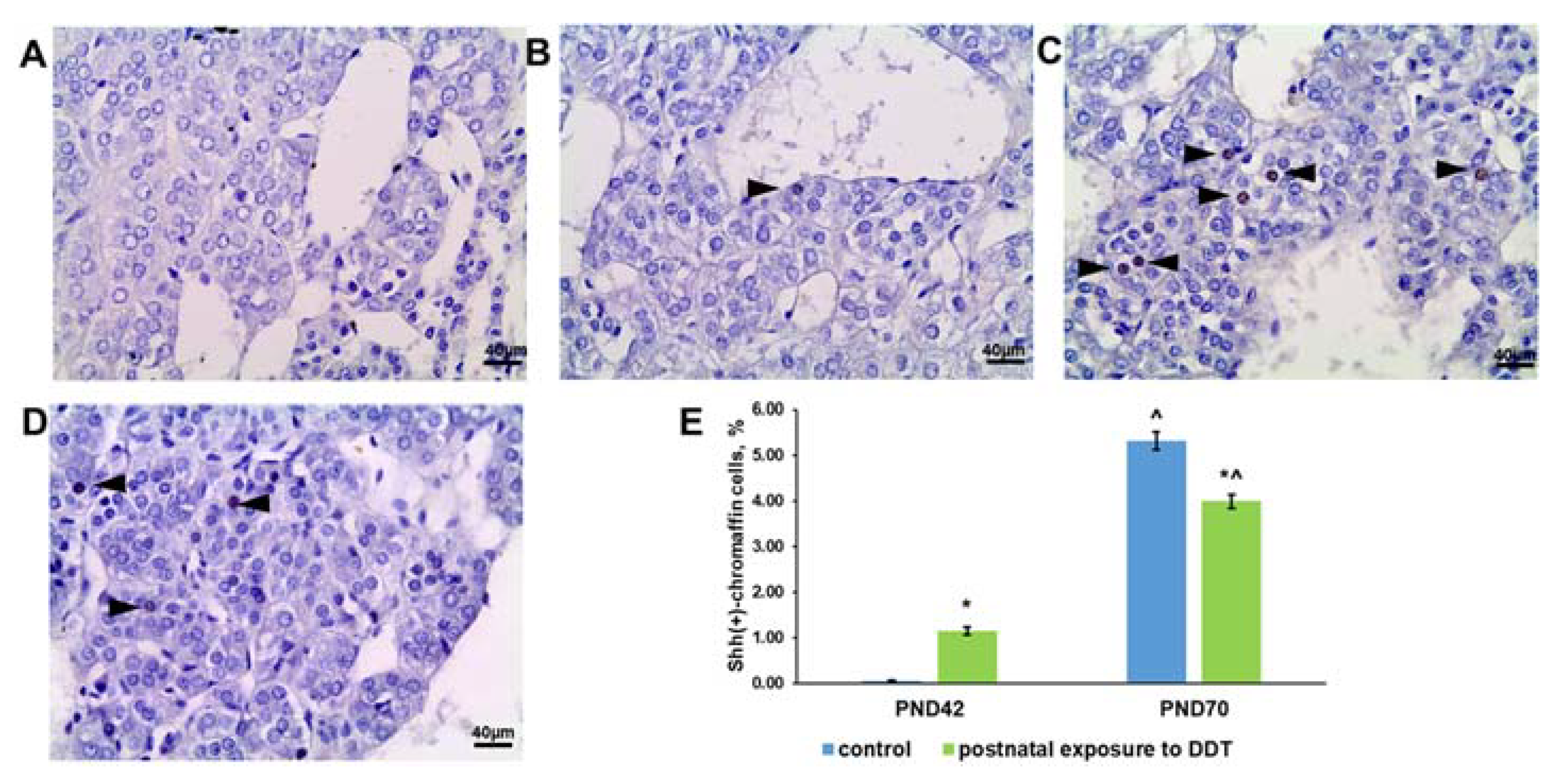
2.7. Sonic Hedgehog (Shh) Ligand Expression
2.8. Wnt Signaling Activation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Adrenal Histology
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996, 104 (Suppl. S4), 715–740. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Banke, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.K.; Sobolewski, M.; Susiarjo, M. Exposure to endocrine disrupting chemicals impacts immunological and metabolic status of women during pregnancy. Mol. Cell Endocrinol. 2023, 577, 112031. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.Y.; Arbuckle, T.E.; Janssen, P.; Lanphear, B.P.; Zhuang, L.H.; Braun, J.M.; Chen, A.; McCandless, L.C. Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: A Bayesian analysis using kernel machine regression. Environ. Res. 2021, 195, 110749. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Calafat, A.M.; Sjödin, A.; Marcus, M.; Hartman, T.J. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and early menarche in a population-based cohort of British girls. Environ. Pollut. 2021, 276, 116705. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Tannerm, E.; Gennings, C.; Lindh, C.; Kiviranta, H.; Wikström, S.; Bornehag, C.G. Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study. Sci. Rep. 2021, 11, 11036. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, E.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, D.A.; Timokhina, E.P.; Chereshneva, E.V.; Ivanova, M.Y.; Payushina, O.V. Developmental Exposure to Endocrine Disrupter DDT Interferes with Age-Related Involution of Thymus. Int. J. Mol. Sci. 2022, 23, 6678. [Google Scholar] [CrossRef]
- Bonde, J.P.; Flachs, E.M.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Ramlau-Hansen, C.H.; Hougaard, K.S.; Høyer, B.B.; Hærvig, K.K.; Petersen, S.B.; et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 23, 104–125. [Google Scholar] [CrossRef]
- Bliatka, D.; Nigdelis, M.P.; Chatzimeletiou, K.; Mastorakos, G.; Lymperi, S.; Goulis, D.G. The effects of postnatal exposure of endocrine disruptors on testicular function: A systematic review and a meta-analysis. Hormones 2020, 19, 157–169. [Google Scholar] [CrossRef]
- Uldbjerg, C.S.; Koch, T.; Lim, Y.H.; Gregersen, L.S.; Olesen, C.S.; Andersson, A.M.; Frederiksen, H.; Coull, B.A.; Hauser, R.; Juul, A.; et al. Prenatal and postnatal exposures to endocrine disrupting chemicals and timing of pubertal onset in girls and boys: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 687–716. [Google Scholar] [CrossRef]
- Ham, D.; Ha, M.; Park, H.; Hong, Y.C.; Kim, Y.; Ha, E.; Bae, S. Association of postnatal exposure to mixture of bisphenol A, Di-n-butyl phthalate and Di-(2-ethylhexyl) phthalate with Children’s IQ at 5 Years of age: Mothers and Children’s environmental health (MOCEH) study. Chemosphere 2024, 347, 140626. [Google Scholar] [CrossRef]
- Anand, N.; Chakraborty, P.; Ray, S. Human exposure to organochlorine, pyrethroid and neonicotinoid pesticides: Comparison between urban and semi-urban regions of India. Environ. Pollut. 2021, 270, 116156. [Google Scholar] [CrossRef]
- World Health Organization. Pesticide Residues in Food—2018. Toxicological Evaluations; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization: Rome, Italy, 2019; 780p. [Google Scholar]
- Almasi, H.; Takdastan, A.; Jaafarzadeh, N.; Babaei, A.A.; Birgani, Y.T.; Cheraghian, B.; Saki, A.; Jorfi, S. Spatial distribution, ecological and health risk assessment and source identification of atrazine in Shadegan international wetland, Iran. Mar. Pollut. Bull. 2020, 160, 111569. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Giwercman, A. Endocrine disruptors and testicular function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef]
- World Health Organization. The Use of DDT in Malaria Vector Control; WHO: Geneva, Switzerland, 2011; 16p. [Google Scholar]
- Dong, J.; Gao, H.; Wang, S.; Yao, H.; Ma, M. Simulation of the transfer and fate of HCHs since the 1950s in Lanzhou, China. Ecotoxicol. Environ. Saf. 2009, 72, 1950–1956. [Google Scholar] [CrossRef]
- Tang, D.; Liu, X.; He, H.; Cui, Z.; Gan, H.; Xia, Z. Distribution, sources and ecological risks of organochlorine compounds (DDTs, HCHs and PCBs) in surface sediments from the Pearl River Estuary, China. Mar. Pollut. Bull. 2020, 152, 110942. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhou, Y.; Xu, S.; Jing, J.; Zhang, H.; Huang, Y. Distribution, Transfer, and Health Risk of Organochlorine Pesticides in Soil and Water of the Huangshui River Basin. Toxics 2023, 11, 1024. [Google Scholar] [CrossRef]
- Lopez-Espinosa, M.J.; Vizcaino, E.; Murcia, M.; Llop, S.; Espada, M.; Seco, V.; Marco, A.; Rebagliato, M.; Grimalt, J.O.; Ballester, F. Association between thyroid hormone levels and 4,40-DDE concentrations in pregnant women (Valencia, Spain). Environ. Res. 2009, 109, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid disrupting chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Yaglov, V.V. Differences in production of adrenal steroid hormones in pubertal rats exposed to low doses of the endocrine disrupter DDT during prenatal and postnatal development. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2018, 12, 80–86. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Tsomartova, D.A.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Differential Disrupting Effects of Prolonged Low-Dose Exposure to Dichlorodiphenyltrichloroethane on Androgen and Estrogen Production in Males. Int. J. Mol. Sci. 2021, 22, 3155. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Nazimova, S.V.; Yaglov, V.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Impaired Morphogenesis and Function of Rat Adrenal Zona Glomerulosa by Developmental Low-Dose Exposure to DDT Is Associated with Altered Oct4 Expression. Int. J. Mol. Sci. 2021, 22, 6324. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Yaglov, V.V.; Nazimova, S.V.; Timokhina, E.P.; Tsomartova, D.A. Low-Dose Exposure to Endocrine Disrupter Dichlorodiphenyltrichloroethane (DDT) Affects Transcriptional Regulation of Adrenal Zona Reticularis in Male Rats. Bull. Exp. Biol. Med. 2021, 170, 682–685. [Google Scholar] [CrossRef]
- Yaglova, N.; Tsomartova, D.; Yaglov, V. Effect of prenatal and postnatal exposure to low doses of DDT on catecholamine secretion in rats in different period of ontogeny. Bull. Exp. Biol. Med. 2017, 163, 422–424. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Obernikhin, S.S.; Tsomartova, D.A.; Yaglov, V.V.; Nazimova, S.V.; Tsomartova, E.S.; Timokhina, E.P.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Impact of Prenatal and Postnatal Exposure to Endocrine Disrupter DDT on Adrenal Medulla Function. Int. J. Mol. Sci. 2022, 23, 4912. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Nazimova, S.V.; Obernikhin, S.S.; Tsomartova, D.A.; Yaglov, V.V.; Timokhina, E.P.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Developmental Exposure to DDT Disrupts Transcriptional Regulation of Postnatal Growth and Cell Renewal of Adrenal Medulla. Int. J. Mol. Sci. 2023, 24, 2774. [Google Scholar] [CrossRef] [PubMed]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.; Joyner, A. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Obernikhin, S.S.; Yaglova, N.V.; Timokhina, E.P.; Nazimova, S.V.; Yaglov, V.V. Regulation of Morphogenetic Processes during Postnatal Development and Physiological Regeneration of the Adrenal Medulla. Bull. Exp. Biol. Med. 2023, 175, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.S.; Ruzicka, L.A.; Donahue, S.R.; DeLellis, R.A. Chromaffin cell proliferation in the adult rat adrenal medulla. Int. J. Dev. Neurosci. 1989, 7, 439–448. [Google Scholar] [CrossRef]
- Verhofstad, A.A. Kinetics of adrenal medullary cells. J. Anat. 1993, 183 Pt 2, 315–326. [Google Scholar]
- Chung, K.F.; Sicard, F.; Vukicevic, V.; Hermann, A.; Storch, A.; Huttner, W.B.; Bornstein, S.R.; Ehrhart-Bornstein, M. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem. Cells 2009, 27, 2602–2613. [Google Scholar] [CrossRef]
- Rubin De Celis, M.F.; Garcia-Martin, R.; Wittig, D.; Valencia, G.D.; Enikolopov, G.; Funk, R.H.; Chavakis, T.; Bornstein, S.R.; Androutsellis-Theotokis, A.; Ehrhart-Bornstein, M. Multipotent glia-like stem cells mediate stress adaptation. Stem. Cells 2015, 33, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.M.; Chung, K.-F.; Vukicevic, V.; Rosmaninho-Salgado, J.; Kanczkowski, W.; Cortez, V.; Hackmann, K.; Bastos, C.A.; Mota, A.; Schrock, E. Isolation, Characterization, and Differentiation of Progenitor Cells from Human Adult Adrenal Medulla. Stem. Cells Transl. Med. 2012, 1, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Castro, D.S.; Guillemot, F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002, 3, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Greenberg, M.E.; Stiles, C.D. Basic helix–loop–helix factors in cortical development. Neuron 2003, 39, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.S.; Martynoga, B.; Parras, C.; Ramesh, V.; Pacary, E.; Johnston, C.; Drechsel, D.; Lebel-Potter, M.; Garcia, L.G.; Hunt, C.; et al. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 2011, 25, 930–945. [Google Scholar] [CrossRef]
- Szemes, M.; Greenhough, A.; Melegh, Z.; Malik, S.; Yuksel, A.; Catchpoole, D.; Gallacher, K.; Kollareddy, M.; Park, J.H.; Malik, K. Wnt Signalling Drives Context-Dependent Differentiation or Proliferation in Neuroblastoma. Neoplasia 2018, 20, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Izpisua Belmonte, J.C. Deconstructing the pluripotency gene regulatory network. Nat. Cell Biol. 2018, 20, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; Edwards, M.; Langerman, J.; Sridharan, R.; Plath, K.; Smale, S.T. Oct4:Sox2 binding is essential for establishing but not maintaining active and silent states of dynamically regulated genes in pluripotent cells. Genes Dev. 2022, 36, 1079–1095. [Google Scholar] [CrossRef]
- Santambrogio, A.; Kemkem, Y.; Willis, T.; Berger, I.; Kastriti, M.; Faure, L.; Russell, J.; Lodge, E.; Yianni, V.; Oakey, R.; et al. SOX2+ 1 sustentacular cells are stem cells of the postnatal adrenal medulla. bioRxiv 2023. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2013, 14, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R.; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Fuerer, C.; Ching, W.; Harnish, K.; Logan, C.; Zeng, A.; ten Berge, D.; Kalani, Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 59–66. [Google Scholar] [CrossRef]
- Lluis, F.; Pedone, E.; Pepe, S.; Cosma, M.P. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell 2008, 3, 493–507. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P.E. (Eds.) Handbook of Pollution Prevention and Cleaner Production: Best Practices in the Agrochemical Industry; Elsevier: Amsterdam, The Netherlands, 2010; 313p. [Google Scholar] [CrossRef]
- Pignatelli, D.; Xiao, F.; Gouvtia, A.; Ferreira, J.; Vinson, G. Adrenarche in the rat. J. Endocrinol. 2006, 191, 301–308. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takano, R.; Shimizu, M.; Murayama, N.; Kitajima, M.; Shono, M. Human blood concentrations of dichlorodiphenyltrichloroethane (DDT) extrapolated from metabolism in rats and humans and physiologically based pharmacokinetic modeling. J. Health Sci. 2010, 56, 566–575. [Google Scholar] [CrossRef][Green Version]
- Technical Regulation of the Customs Union TR CU 021/2011 Concerning Safety of Food Products. St. Petersburg. 2015. Available online: https://www.rustandard.com/images/CU_TR/TR_CU_021.2011_Safety_of_Food_Products.pdf (accessed on 27 December 2023).
- Kim, W.; Kim, M.; Jho, E.H. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef] [PubMed]

| 42nd Day | 70th Day | |||
|---|---|---|---|---|
| Control Group | Postnatal Exposure to DDT | Control Group | Postnatal Exposure to DDT | |
| Chromaffin cells with diffuse distribution of TH in cytoplasm | 93.20 ± 2.16 | 12.75 ± 4.89 * | 85.30 ± 4.01 | 11.69 ± 2.79 * |
| Chromaffin cells with local distribution of TH in cytoplasm | 6.80 ± 2.14 | 59.18 ± 9.13 * | 14.70 ± 0.92 | 64.64 ± 3.24 * |
| TH-negative cells | 0 | 28.1 ± 6.04 * | 0 | 23.67 ± 2.92 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaglova, N.V.; Obernikhin, S.S.; Nazimova, S.V.; Tsomartova, D.A.; Timokhina, E.P.; Yaglov, V.V.; Tsomartova, E.S.; Chereshneva, E.V.; Ivanova, M.Y.; Lomanovskaya, T.A. Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal. Int. J. Mol. Sci. 2024, 25, 1494. https://doi.org/10.3390/ijms25031494
Yaglova NV, Obernikhin SS, Nazimova SV, Tsomartova DA, Timokhina EP, Yaglov VV, Tsomartova ES, Chereshneva EV, Ivanova MY, Lomanovskaya TA. Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal. International Journal of Molecular Sciences. 2024; 25(3):1494. https://doi.org/10.3390/ijms25031494
Chicago/Turabian StyleYaglova, Nataliya V., Sergey S. Obernikhin, Svetlana V. Nazimova, Dibakhan A. Tsomartova, Ekaterina P. Timokhina, Valentin V. Yaglov, Elina S. Tsomartova, Elizaveta V. Chereshneva, Marina Y. Ivanova, and Tatiana A. Lomanovskaya. 2024. "Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal" International Journal of Molecular Sciences 25, no. 3: 1494. https://doi.org/10.3390/ijms25031494
APA StyleYaglova, N. V., Obernikhin, S. S., Nazimova, S. V., Tsomartova, D. A., Timokhina, E. P., Yaglov, V. V., Tsomartova, E. S., Chereshneva, E. V., Ivanova, M. Y., & Lomanovskaya, T. A. (2024). Postnatal Exposure to the Endocrine Disruptor Dichlorodiphenyltrichloroethane Affects Adrenomedullary Chromaffin Cell Physiology and Alters the Balance of Mechanisms Underlying Cell Renewal. International Journal of Molecular Sciences, 25(3), 1494. https://doi.org/10.3390/ijms25031494