Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy
Abstract
1. Introduction
2. Results
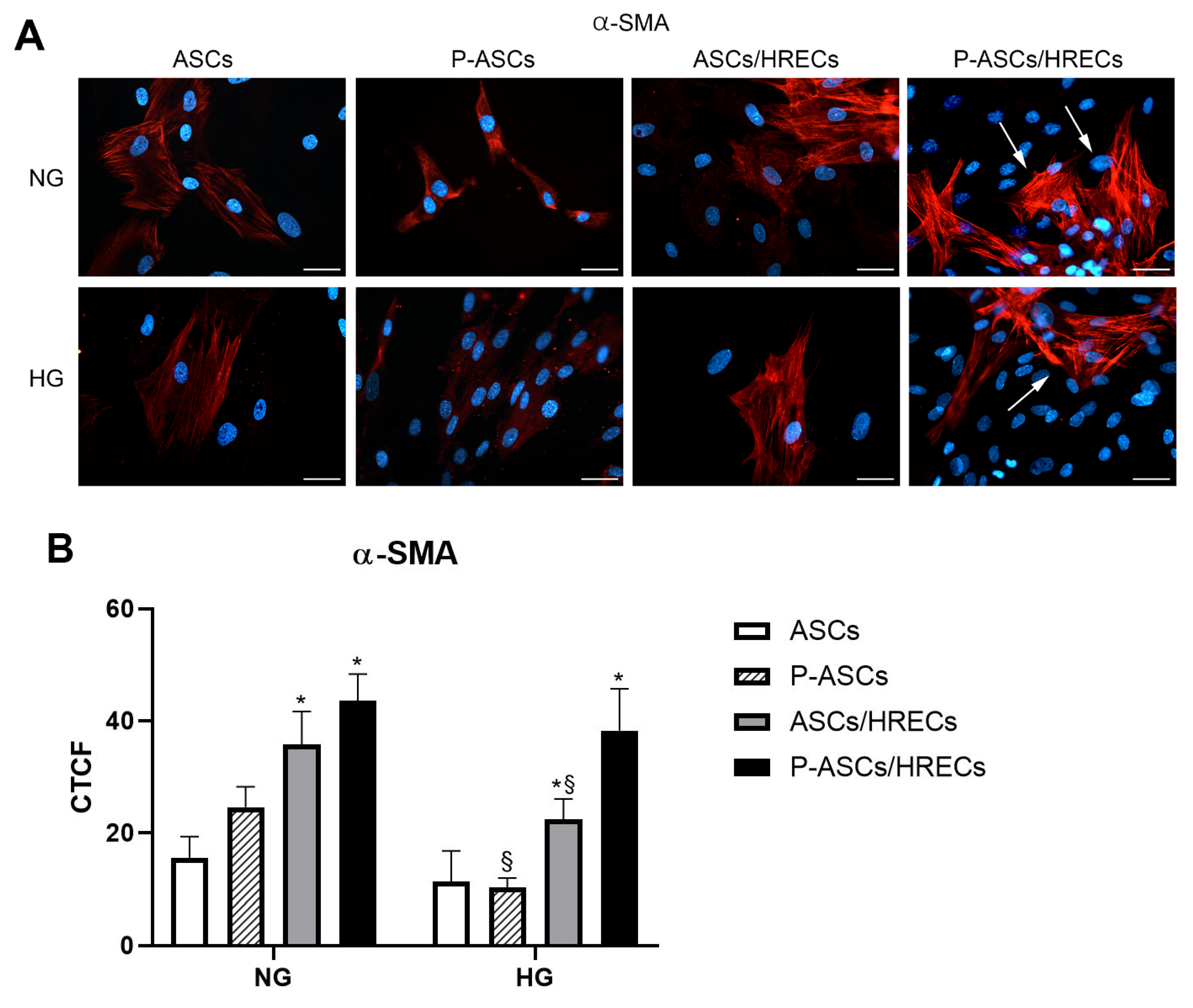
2.1. ASC Pericyte-like Differentiation
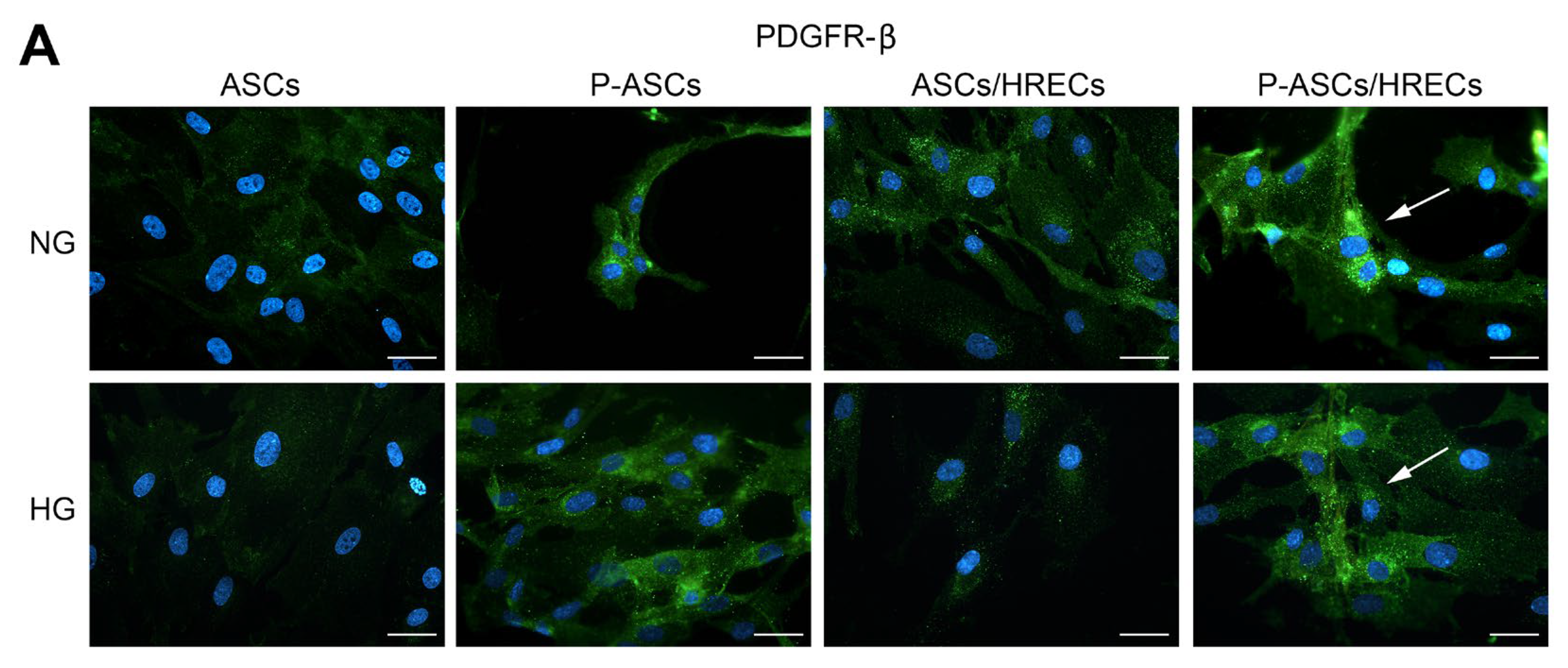
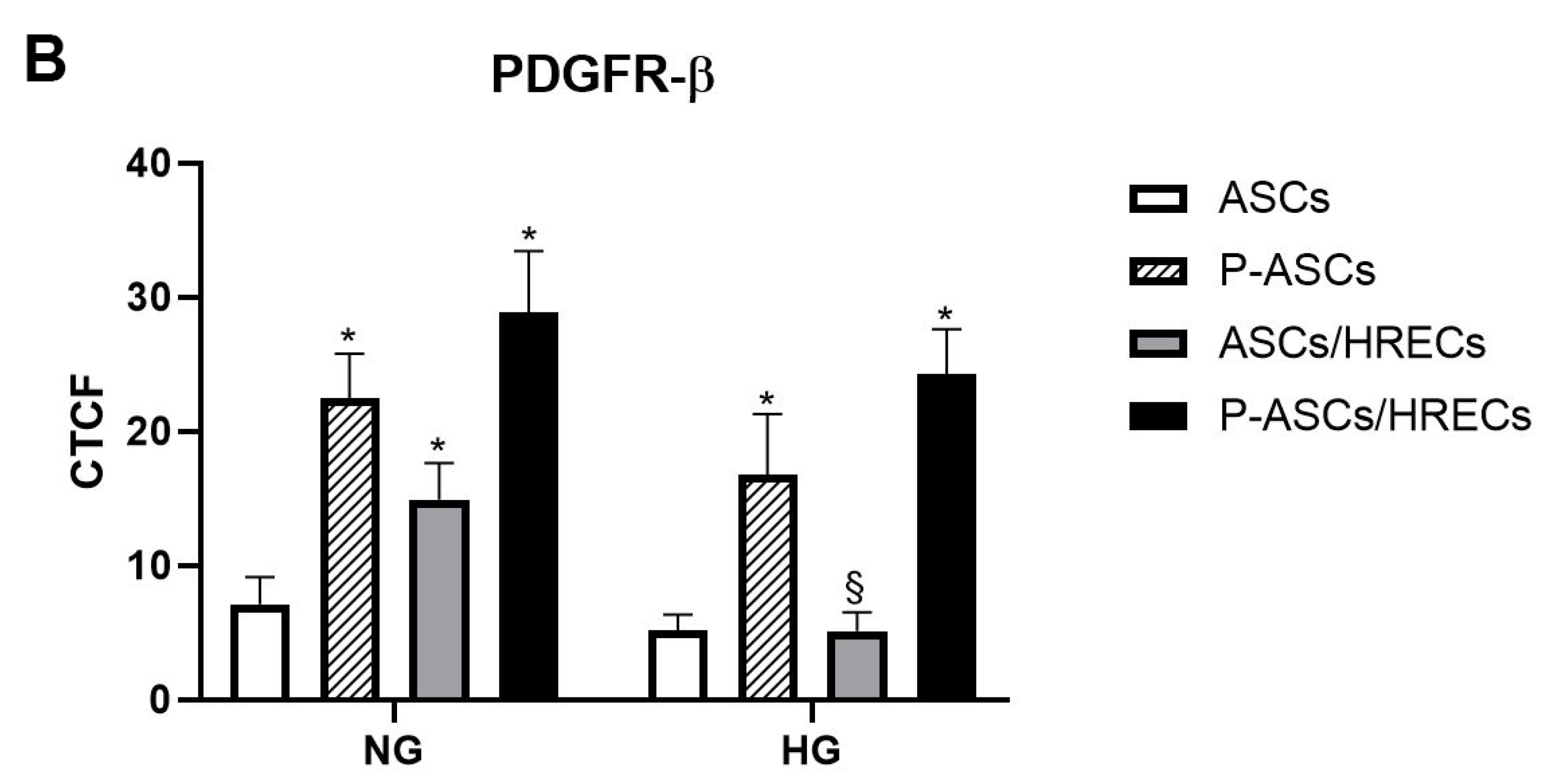
2.2. PDGFR-β and Phospho-PDGFR-β Immunofluorescence in ASCs and P-ASCs
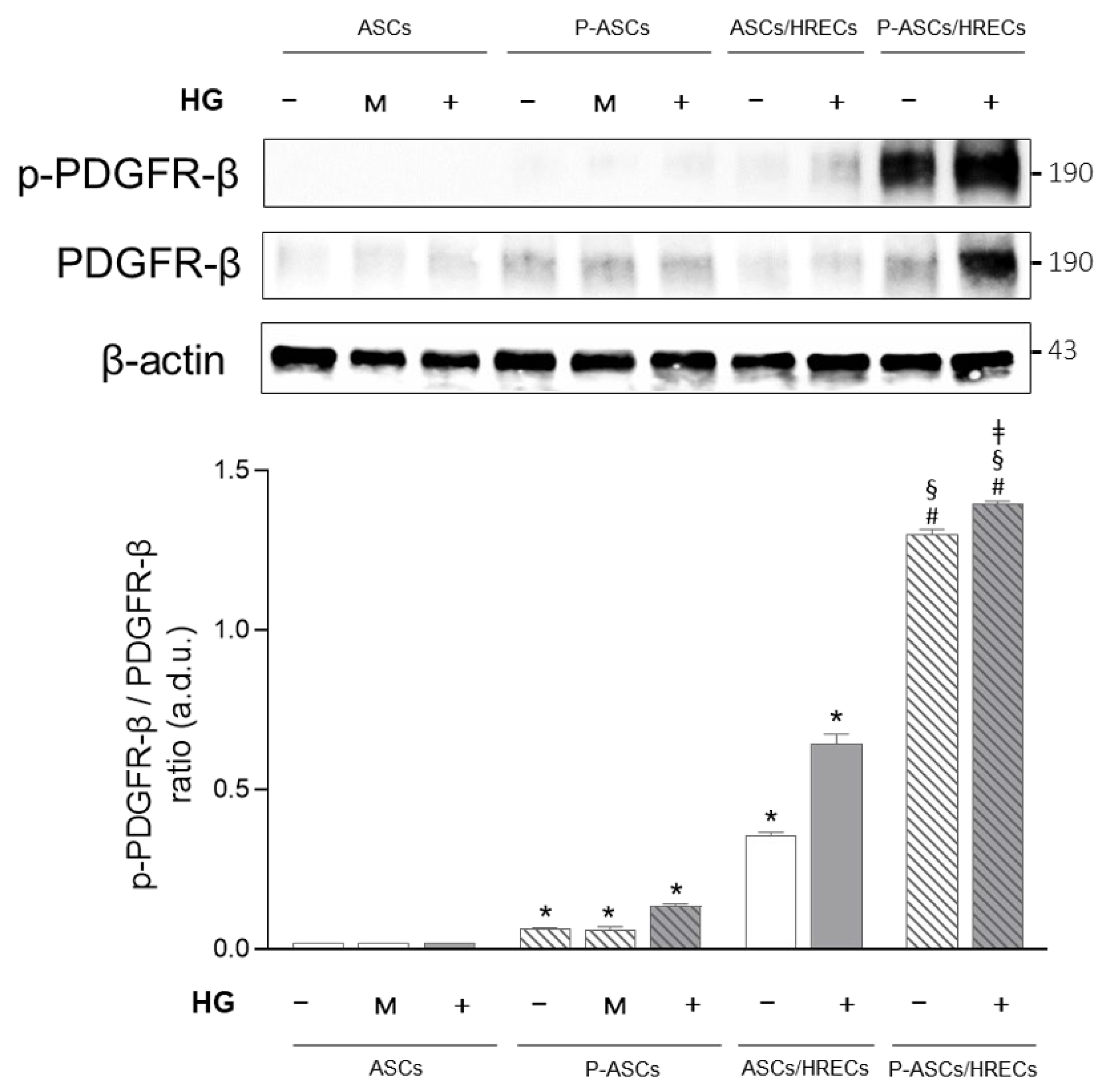
2.3. PDGFR-β and Phospho-PDGFR-β Western Blot Analyses
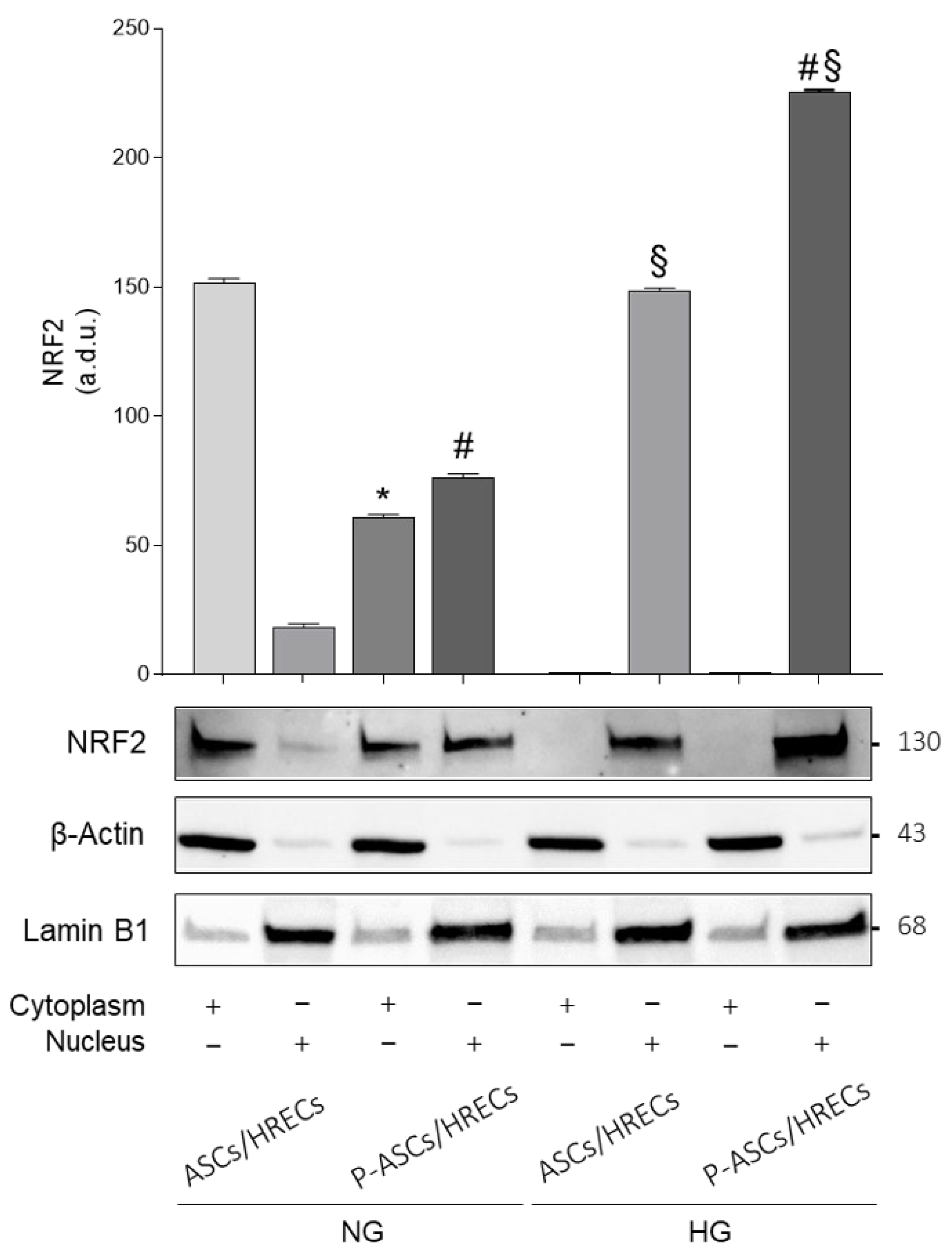
2.4. Cytoplasmic and Nuclear Nrf2 Protein Expression
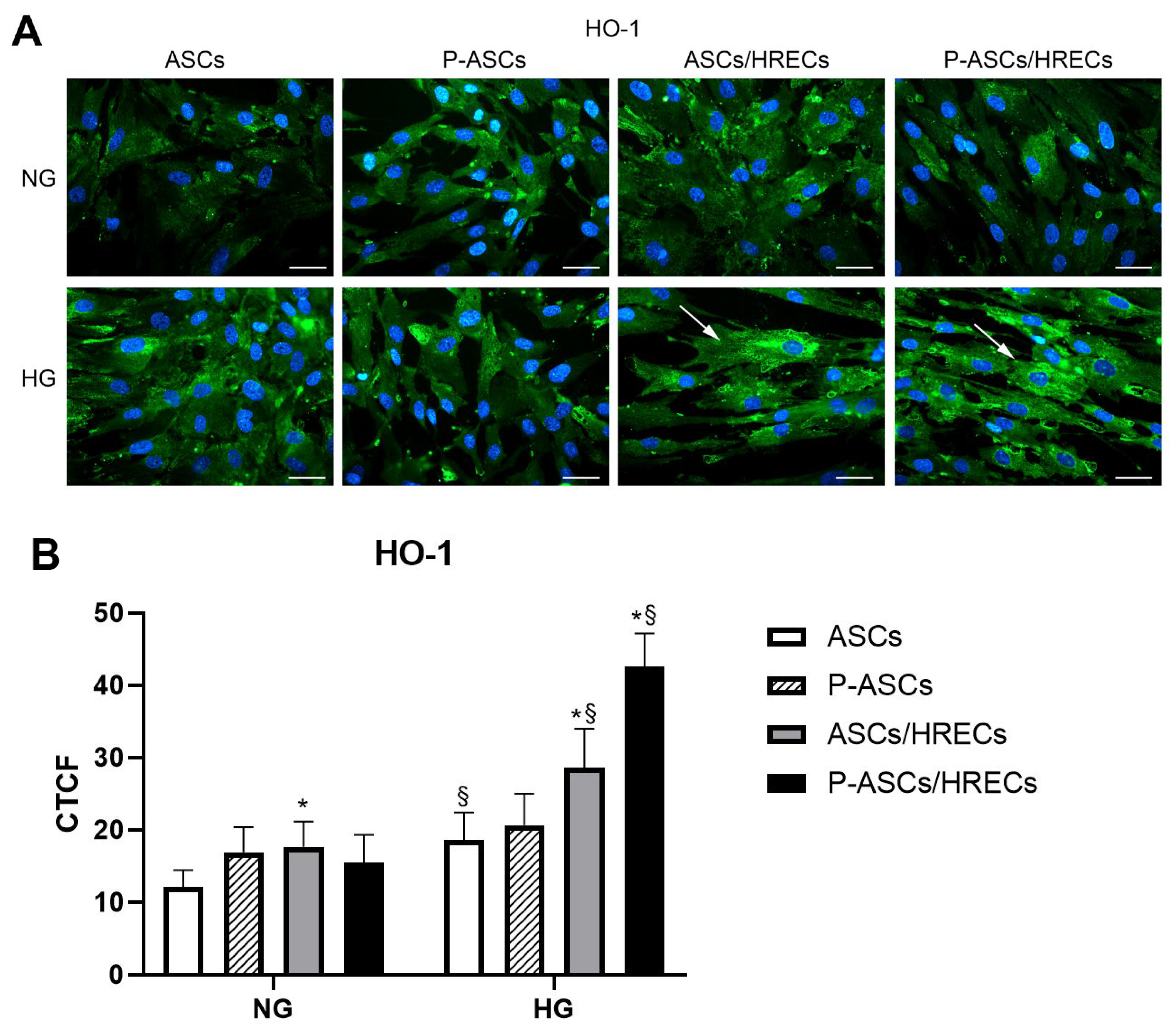
2.5. HO-1 Immunofluorescence
2.6. cPLA2 Specific Activity and HO-1 Protein Content in ASCs and P-ASCs
2.7. VEGF-A and PGE2 Levels in Media from ASC and P-ASC Monocultures and Co-Cultures with HRECs
2.8. High Glucose Effects on mRNA Levels of Pro-Inflammatory Cytokines, Interleukin-10 and Angiogenic Factors in ASCs and P-ASCs Co-Cultured with HRECs
3. Discussion
4. Materials and Methods
4.1. Human Adipose Stem Cells
4.2. Human Retinal Endothelial Cells
4.3. Cell Co-Cultures
4.4. Fluorescence Immunocytochemistry Procedures
4.5. Extraction of Total mRNA and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
4.6. Immunoblot Analyses
4.7. Subcellular Fractionation
4.8. cPLA2 Activity and HO-1 Content
4.9. VEGF and PGE2 Release
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cogan, D.G. Retinal Vascular Patterns: IV. Diabetic Retinopathy. Arch. Ophthalmol. 1961, 66, 366. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the Pathogenesis of Diabetic Retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cells Int. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Skubis, A.; Gola, J.; Sikora, B.; Hybiak, J.; Paul-Samojedny, M.; Mazurek, U.; Łos, M. Impact of Antibiotics on the Proliferation and Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2017, 18, 2522. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.; Lokmic, Z.; Peshavariya, H.; Abberton, K.M.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Hypoxic Conditioning Enhances the Angiogenic Paracrine Activity of Human Adipose-Derived Stem Cells. Stem Cells Dev. 2013, 22, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, W.; Li, L.; Peng, Y.; Chen, P.; Huang, H.; Guo, Y.; Xia, X.; Wang, Y.; Wang, H.; et al. The Relative Contribution of Paracine Effect versus Direct Differentiation on Adipose-Derived Stem Cell Transplantation Mediated Cardiac Repair. PLoS ONE 2013, 8, e59020. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Finocchio, L.; Zeppieri, M.; Gabai, A.; Spadea, L.; Salati, C. Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases. J. Clin. Med. 2023, 12, 7015. [Google Scholar] [CrossRef]
- Harrell, C.R.; Volarevic, V.; Djonov, V.; Volarevic, A. Therapeutic Potential of Exosomes Derived from Adipose Tissue-Sourced Mesenchymal Stem Cells in the Treatment of Neural and Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 4487. [Google Scholar] [CrossRef]
- Mannino, G.; Gennuso, F.; Giurdanella, G.; Conti, F.; Drago, F.; Salomone, S.; Furno, D.L.; Bucolo, C.; Giuffrida, R. Pericyte-like Differentiation of Human Adipose-Derived Mesenchymal Stem Cells: An in vitro Study. World J. Stem Cells 2020, 12, 1152–1170. [Google Scholar] [CrossRef]
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Lo Furno, D.; Giuffrida, R.; Giurdanella, G. Potential Therapeutic Applications of Mesenchymal Stem Cells for the Treatment of Eye Diseases. World J. Stem Cells 2021, 13, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Rohovsky, S.A.; D’Amore, P.A. PDGF, TGF-β, and Heterotypic Cell–Cell Interactions Mediate Endothelial Cell–Induced Recruitment of 10T1/2 Cells and Their Differentiation to a Smooth Muscle Fate. J. Cell Biol. 1998, 141, 805–814. [Google Scholar] [CrossRef]
- Giurdanella, G.; Anfuso, C.D.; Olivieri, M.; Lupo, G.; Caporarello, N.; Eandi, C.M.; Drago, F.; Bucolo, C.; Salomone, S. Aflibercept, Bevacizumab and Ranibizumab Prevent Glucose-Induced Damage in Human Retinal Pericytes in Vitro, through a PLA2/COX-2/VEGF-A Pathway. Biochem. Pharmacol. 2015, 96, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte Loss and Microaneurysm Formation in PDGF-B-Deficient Mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Feng, Y.; Wang, J.; Zhang, X.; Shen, J.; Zou, R.; Yuan, Y. Platelet-derived Growth factor-BB Promotes Proliferation and Migration of Retinal Microvascular Pericytes by Up-regulating the Expression of C-X-C Chemokine Receptor Types 4. Exp. Ther. Med. 2019, 18, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Kitahara, H.; Kajikawa, S.; Ishii, Y.; Yamamoto, S.; Hamashima, T.; Azuma, E.; Sato, H.; Matsushima, T.; Shibuya, M.; Shimada, Y.; et al. The Novel Pathogenesis of Retinopathy Mediated by Multiple RTK Signals Is Uncovered in Newly Developed Mouse Model. eBioMedicine 2018, 31, 190–201. [Google Scholar] [CrossRef]
- Glaser, K.B. Regulation of Phospholipase A2 Enzymes: Selective Inhibitors and Their Pharmacological Potential. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 32, pp. 31–66. ISBN 978-0-12-032933-5. [Google Scholar]
- Balsinde, J.; Balboa, M.A.; Insel, P.A.; Dennis, E.A. Differential Regulation of Phospholipase D and Phospholipase A2 by Protein Kinase C in P388D1 Macrophages. Biochem. J. 1997, 321, 805–810. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A.; Insel, P.A.; Dennis, E.A. Regulation and Inhibition of Phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 175–189. [Google Scholar] [CrossRef]
- Murakami, M.; Nakatani, Y.; Tanioka, T.; Kudo, I. Prostaglandin E Synthase. Prostaglandins Other Lipid Mediat. 2002, 68–69, 383–399. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic Phospholipase A2: Physiological Function and Role in Disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef]
- Askarova, S.; Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.-M. Role of Aβ-Receptor for Advanced Glycation Endproducts Interaction in Oxidative Stress and Cytosolic Phospholipase A2 Activation in Astrocytes and Cerebral Endothelial Cells. Neuroscience 2011, 199, 375–385. [Google Scholar] [CrossRef]
- Nicotra, A.; Lupo, G.; Giurdanella, G.; Anfuso, C.D.; Ragusa, N.; Tirolo, C.; Marchetti, B.; Alberghina, M. MAPKs Mediate the Activation of Cytosolic Phospholipase A2 by Amyloid Beta(25-35) Peptide in Bovine Retina Pericytes. Biochim. Biophys. Acta 2005, 1733, 172–186. [Google Scholar] [CrossRef]
- Peña, L.; Meana, C.; Astudillo, A.M.; Lordén, G.; Valdearcos, M.; Sato, H.; Murakami, M.; Balsinde, J.; Balboa, M.A. Critical Role for Cytosolic Group IVA Phospholipase A2 in Early Adipocyte Differentiation and Obesity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1083–1095. [Google Scholar] [CrossRef]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Yan, H. Roles of Elevated Intravitreal IL-1β and IL-10 Levels in Proliferative Diabetic Retinopathy. Indian J. Ophthalmol. 2014, 62, 699. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.-T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 Upregulation Correlates with Increased Levels of MMP-9, VEGF, EPO and TGFβ1 in Diabetic Eyes Undergoing Vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Pittalà, V.; Pascale, A.; Marchesi, N.; Anfuso, C.D.; Lupo, G.; Cristaldi, M.; Olivieri, M.; Lazzara, F.; Di Paola, L.; et al. Novel Indole Derivatives Targeting HuR-mRNA Complex to Counteract High Glucose Damage in Retinal Endothelial Cells. Biochem. Pharmacol. 2020, 175, 113908. [Google Scholar] [CrossRef]
- Mahdy, R.A.; Nada, W.M.; Hadhoud, K.M.; El-Tarhony, S.A. The Role of Vascular Endothelial Growth Factor in the Progression of Diabetic Vascular Complications. Eye 2010, 24, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The Vascular Endothelial Growth Factor (VEGF)/VEGF Receptor System and Its Role under Physiological and Pathological Conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Stringer, S.E. The Splice Variants of Vascular Endothelial Growth Factor (VEGF) and Their Receptors. J. Cell Sci. 2001, 114, 853–865. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, Y.; Zhang, J.; Shi, J.; Zheng, P.; Yang, C.; Chen, Y. Celastrol Inhibits Pathologic Neovascularization in Oxygen-Induced Retinopathy by Targeting the miR-17-5p/HIF-1α/VEGF Pathway. Cell Cycle 2022, 21, 2091–2108. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.; Wraight, C.; Werther, G. The Role of Growth Hormone, Insulin-Like Growth Factor and Somatostatin in Diabetic Retinopathy. Curr. Med. Chem. 2006, 13, 3307–3317. [Google Scholar] [CrossRef]
- Jo, D.H.; Yun, J.-H.; Cho, C.S.; Kim, J.H.; Kim, J.H.; Cho, C.-H. Interaction between Microglia and Retinal Pigment Epithelial Cells Determines the Integrity of Outer Blood-Retinal Barrier in Diabetic Retinopathy. Glia 2019, 67, 321–331. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of Retinal Vasculature Is Mediated by Hypoxia-Induced Vascular Endothelial Growth Factor (VEGF) Expression by Neuroglia. J. Neurosci. 1995, 15, 4738–4747. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the Human Retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Grigsby, J.; Allen, D.; Ferrigno, A.; Vellanki, S.; Pouw, C.; Hejny, W.; Tsin, A. Autocrine and Paracrine Secretion of Vascular Endothelial Growth Factor in the Pre-Hypoxic Diabetic Retina. Curr. Diabetes Rev. 2017, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Giurdanella, G.; Lupo, G.; Gennuso, F.; Conti, F.; Furno, D.L.; Mannino, G.; Anfuso, C.D.; Drago, F.; Salomone, S.; Bucolo, C. Activation of the VEGF-A/ERK/PLA2 Axis Mediates Early Retinal Endothelial Cell Damage Induced by High Glucose: New Insight from an In Vitro Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 7528. [Google Scholar] [CrossRef] [PubMed]
- Uludag, G.; Hassan, M.; Matsumiya, W.; Pham, B.H.; Chea, S.; Trong Tuong Than, N.; Doan, H.L.; Akhavanrezayat, A.; Halim, M.S.; Do, D.V.; et al. Efficacy and Safety of Intravitreal Anti-VEGF Therapy in Diabetic Retinopathy: What We Have Learned and What Should We Learn Further? Expert. Opin. Biol. Ther. 2022, 22, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, G.; Jiang, T.; Qin, Y. Pharmacologic Induction of Heme Oxygenase-1 Plays a Protective Role in Diabetic Retinopathy in Rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6541. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Lian, L.; Zhou, J.; Xiang, S.; Zhai, Y.; Chen, Y.; Ma, X.; Wu, W.; Hou, L. High Dose Expression of Heme Oxigenase-1 Induces Retinal Degeneration through ER Stress-Related DDIT3. Mol. Neurodegener. 2021, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to Heme Oxygenase-1 and Its Anti-Inflammatory Therapeutic Potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Mackern-Oberti, J.P.; Obreque, J.; Méndez, G.P.; Llanos, C.; Kalergis, A.M. Carbon Monoxide Inhibits T Cell Activation in Target Organs during Systemic Lupus Erythematosus. Clin. Exp. Immunol. 2015, 182, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Meng, G.; Jiang, X. Influence of Heme Oxygenase-1 on Rats with Diabetic Retinopathy through ERK1/2 Signaling Pathway. Cell. Mol. Biol. 2022, 68, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Prawan, A.; Kundu, J.K.; Surh, Y.-J. Molecular Basis of Heme Oxygenase-1 Induction: Implications for Chemoprevention and Chemoprotection. Antioxid. Redox Signal. 2005, 7, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Lu, H.; Matsukura, M.; Zhao, J.; Shinohara, M. Silencing Heme Oxygenase-1 Gene Expression in Retinal Pigment Epithelial Cells Inhibits Proliferation, Migration and Tube Formation of Cocultured Endothelial Cells. Biochem. Biophys. Res. Commun. 2013, 434, 492–497. [Google Scholar] [CrossRef]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE Signaling Pathway: An Update on Its Regulation and Possible Role in Cancer Prevention and Treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by P300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell. Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Theodore, M.; Kawai, Y.; Yang, J.; Kleshchenko, Y.; Reddy, S.P.; Villalta, F.; Arinze, I.J. Multiple Nuclear Localization Signals Function in the Nuclear Import of the Transcription Factor Nrf2. J. Biol. Chem. 2008, 283, 8984–8994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE Pathway Protects Endothelial Cells from Oxidant Injury and Inhibits Inflammatory Gene Expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.; Agafonova, A.; Cosentino, A.; Giurdanella, G.; Mannino, G.; Lo Furno, D.; Romano, I.R.; Giuffrida, R.; D’Angeli, F.; Anfuso, C.D. Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy. Int. J. Mol. Sci. 2023, 24, 913. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic Roles of Pericytes in the Blood–Retinal Barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Et. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Mannino, G.; Longo, A.; Gennuso, F.; Anfuso, C.D.; Lupo, G.; Giurdanella, G.; Giuffrida, R.; Lo Furno, D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4604. [Google Scholar] [CrossRef]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from Hypoxia-Treated Human Adipose-Derived Mesenchymal Stem Cells Enhance Angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Kida, T.; Oku, H.; Osuka, S.; Horie, T.; Ikeda, T. Hyperglycemia-Induced VEGF and ROS Production in Retinal Cells Is Inhibited by the mTOR Inhibitor, Rapamycin. Sci. Rep. 2021, 11, 1885. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kim, H.-S.; Shin, T.-H.; Kang, I.; Lee, J.Y.; Kim, J.-J.; Kang, H.K.; Seo, Y.; Lee, S.; Yu, K.-R.; et al. PGE2 Maintains Self-Renewal of Human Adult Stem Cells via EP2-Mediated Autocrine Signaling and Its Production Is Regulated by Cell-to-Cell Contact. Sci. Rep. 2016, 6, 26298. [Google Scholar] [CrossRef]
- Lo Furno, D.; Graziano, A.C.E.; Avola, R.; Giuffrida, R.; Perciavalle, V.; Bonina, F.; Mannino, G.; Cardile, V. A Citrus Bergamia Extract Decreases Adipogenesis and Increases Lipolysis by Modulating PPAR Levels in Mesenchymal Stem Cells from Human Adipose Tissue. PPAR Res. 2016, 2016, 4563815. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Lo Furno, D.; Giuffrida, R.; Rusciano, D. ARPE-19 Conditioned Medium Promotes Neural Differentiation of Adipose-Derived Mesenchymal Stem Cells. World J. Stem Cells 2021, 13, 1783–1796. [Google Scholar] [CrossRef]
- Geevarghese, A.; Herman, I.M. Pericyte-Endothelial Crosstalk: Implications and Opportunities for Advanced Cellular Therapies. Transl. Res. 2014, 163, 296–306. [Google Scholar] [CrossRef]
- Papetti, M.; Shujath, J.; Riley, K.N.; Herman, I.M. FGF-2 Antagonizes the TGF-Beta1-Mediated Induction of Pericyte Alpha-Smooth Muscle Actin Expression: A Role for Myf-5 and Smad-Mediated Signaling Pathways. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4994–5005. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Aalderink, M.; Scotter, E.L.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Graham, E.S.; Faull, R.L.M.; Curtis, M.A.; Park, T.I.-H.; et al. TGF-Beta1 Regulates Human Brain Pericyte Inflammatory Processes Involved in Neurovasculature Function. J. Neuroinflamm. 2016, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Crivellato, E. The Role of Pericytes in Angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative Therapeutic Potential of Adipose Stromal Cells in Early Stage Diabetic Retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.-U.; Lee, H.-N.; Kim, S.H.; Kim, C.; Cha, Y.-N.; Joe, Y.; Chung, H.T.; Jang, J.; Kim, K.; et al. Taurine Chloramine Stimulates Efferocytosis Through Upregulation of Nrf2-Mediated Heme Oxygenase-1 Expression in Murine Macrophages: Possible Involvement of Carbon Monoxide. Antioxid. Redox Signal. 2015, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jin, X.; Wang, Q.-S.; Wei, S.-H.; Hou, B.-K.; Li, H.-Y.; Zhang, M.-N.; Li, Z.-H. The Involvement of High Mobility Group 1 Cytokine and Phospholipases A2 in Diabetic Retinopathy. Lipids Health Dis. 2014, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Hughes, J.M.; Vogels, I.M.C.; Schalkwijk, C.G.; Van Noorden, C.J.F.; Schlingemann, R.O. Altered Expression of Genes Related to Blood–Retina Barrier Disruption in Streptozotocin-Induced Diabetes. Exp. Eye Res. 2009, 89, 4–15. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular Endothelial Growth Factor in Eye Disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J.; Sheng, J.; Rezaei, K.A.; Lalezary, M.; Cherney, E. Increased Prostaglandin E2 (PGE2) Levels in Proliferative Diabetic Retinopathy, and Correlation with VEGF and Inflammatory Cytokines. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5906. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Xie, T.; Zhan, P.; Zou, J.; Nie, X.; Shao, J.; Zhuang, M.; Tan, C.; Tan, J.; et al. Prostaglandin E2/EP2 Receptor Signalling Pathway Promotes Diabetic Retinopathy in a Rat Model of Diabetes. Diabetologia 2019, 62, 335–348. [Google Scholar] [CrossRef]
- Itoh, K.; Yamamoto, M. Regulatory Role of the COX-2 Pathway in the Nrf2-Mediated Anti-Inflammatory Response. J. Clin. Biochem. Nutr. 2005, 37, 9–18. [Google Scholar] [CrossRef]
- Silvestre, J.-S.; Mallat, Z.; Duriez, M.; Tamarat, R.; Bureau, M.F.; Scherman, D.; Duverger, N.; Branellec, D.; Tedgui, A.; Levy, B.I. Antiangiogenic Effect of Interleukin-10 in Ischemia-Induced Angiogenesis in Mice Hindlimb. Circ. Res. 2000, 87, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Tan, W. Increased Vitreal Levels of Interleukin-10 in Diabetic Retinopathy: A Meta-Analysis. Int. J. Ophthalmol. 2020, 13, 1477–1483. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, W.; Kwon, O.H.; Kim, J.-H.; Kwon, O.W.; Kim, K.H.; Lim, J.-B. Cytokine Profile of Peripheral Blood in Type 2 Diabetes Mellitus Patients with Diabetic Retinopathy. Ann. Clin. Lab. Sci. 2008, 38, 361–367. [Google Scholar] [PubMed]
- Dong, H.; Li, Q.; Wang, M.; Wan, G. Association Between IL-10 Gene Polymorphism and Diabetic Retinopathy. Med. Sci. Monit. 2015, 21, 3203–3208. [Google Scholar] [CrossRef][Green Version]
- Kowluru, R.A.; Mishra, M. Regulation of Matrix Metalloproteinase in the Pathogenesis of Diabetic Retinopathy. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 148, pp. 67–85. ISBN 978-0-12-812776-6. [Google Scholar]
- Ünal, A.; Baykal, O.; Öztürk, N. Comparison of Matrix Metalloproteinase 9 and 14 Levels in Vitreous Samples in Diabetic and Non-Diabetic Patients: A Case Control Study. Int. J. Retin. Vitr. 2022, 8, 44. [Google Scholar] [CrossRef]
- Lo Furno, D.; Tamburino, S.; Mannino, G.; Gili, E.; Lombardo, G.; Tarico, M.S.; Vancheri, C.; Giuffrida, R.; Perrotta, R.E. Nanofat 2.0: Experimental Evidence for a Fat Grafting Rich in Mesenchymal Stem Cells. Physiol. Res. 2017, 66, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.; Motta, C.; Salmeri, M.; Spina-Purrello, V.; Alberghina, M.; Anfuso, C.D. An In Vitro Retinoblastoma Human Triple Culture Model of Angiogenesis: A Modulatory Effect of TGF-β. Cancer Lett. 2014, 354, 181–188. [Google Scholar] [CrossRef] [PubMed]

| Mono-/Co-Cultures | cPLA2 Activity (pmol/min/mg) | HO-1 (ng/mL) |
|---|---|---|
| Normal Glucose | ||
| ASCs | 19.3 ± 1.01 | 0.72 ± 0.05 |
| P-ASCs | 18.4 ± 1.3 | 0.80 ± 0.06 |
| ASCs/HRECs | 20.1 ± 1.24 | 0.83 ± 0.04 |
| P-ASCs/HRECs | 22.3 ± 1.37 | 0.78 ± 0.05 |
| Mannitol | ||
| ASCs | 17.8 ± 1.9 | 0.68 ± 0.07 |
| P-ASCs | 19.8 ± 1.6 | 0.84 ± 0.09 |
| ASCs/HRECs | 21.4 ± 2.11 | 0.75 ± 0.08 |
| P-ASCs/HRECs | 24.7 ± 2.25 | 0.86 ± 0.07 |
| High Glucose | ||
| ASCs | 67.5 ± 4.03 * | 0.91 ± 0.06 * |
| P-ASCs | 39.7 ± 2.3 *§ | 0.95 ± 0.09 * |
| ASCs/HRECs | 48.2 ± 3.16 *‡ | 1.6 ± 0.1 *‡ |
| P-ASCs/HRECs | 28.0 ± 2.23 *‡† | 3.1 ± 0.4 *‡† |
| Mono/Co-Cultures | VEGFA (pg/mL) | PGE2 (pg/mL) |
|---|---|---|
| Normal Glucose | ||
| HRECs | 36 ± 1.8 | 84 ± 6.1 |
| ASCs | 91 ± 7.2 | 125 ± 9.8 |
| P-ASCs | 60 ± 4.2 § | 90 ± 5.3 § |
| ASCs/HRECs | 142 ± 12.0 | 214 ± 11.2 |
| P-ASCs/HRECs | 109 ± 10.1 † | 181 ± 8.9 † |
| Mannitol | ||
| HRECs | 41 ± 2.9 | 91 ± 8.0 |
| ASCs | 87 ± 6.4 | 132 ± 10.2 |
| P-ASCs | 69 ± 5.1 § | 91 ± 7.6 § |
| ASCs/HRECs | 126 ± 10.7 | 222 ± 10.9 |
| P-ASCs/HRECs | 98 ± 8.4 † | 177 ± 9.5 † |
| High Glucose | ||
| HRECs | 71 ± 5.1 * | 133 ± 9.1 * |
| ASCs | 142 ± 8.4 * | 234 ± 10.8 * |
| P-ASCs | 85 ± 5.3 *§ | 104 ± 7.9 § |
| ASCs/HRECs | 191 ± 16.4 * | 417 ± 22.8 * |
| P-ASCs/HRECs | 112 ± 10.8 † | 216 ± 16.6 *† |
| Gene | Sequence (5′-3′) |
|---|---|
| IL10 | Fw: GACTTTAAGGGTTACCTGGGTTG |
| Rv: TCACATGCGCCTTGATGTCTG | |
| IL-1β | Fw: AGCTACGAATCTCCGACCAC |
| Rv: CGTTATCCCATGTGTCGAAGAA | |
| TNF-α | Fw: AGCCCATGTTGTAGCAAACC |
| Rv: TGAGGTACAGGCCCTCTGAT | |
| MMP9 | Fw: CACTGTCCACCCCTCAGAGC |
| Rv: GCCAACTTGTCGGCGATAAGG | |
| ANG2 | Fw: CTCGAATACGATGACTCGGTG |
| Rv: TCATTAGCCACTGAGTGTTGTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agafonova, A.; Cosentino, A.; Romano, I.R.; Giurdanella, G.; D’Angeli, F.; Giuffrida, R.; Lo Furno, D.; Anfuso, C.D.; Mannino, G.; Lupo, G. Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2024, 25, 1774. https://doi.org/10.3390/ijms25031774
Agafonova A, Cosentino A, Romano IR, Giurdanella G, D’Angeli F, Giuffrida R, Lo Furno D, Anfuso CD, Mannino G, Lupo G. Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy. International Journal of Molecular Sciences. 2024; 25(3):1774. https://doi.org/10.3390/ijms25031774
Chicago/Turabian StyleAgafonova, Aleksandra, Alessia Cosentino, Ivana Roberta Romano, Giovanni Giurdanella, Floriana D’Angeli, Rosario Giuffrida, Debora Lo Furno, Carmelina Daniela Anfuso, Giuliana Mannino, and Gabriella Lupo. 2024. "Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy" International Journal of Molecular Sciences 25, no. 3: 1774. https://doi.org/10.3390/ijms25031774
APA StyleAgafonova, A., Cosentino, A., Romano, I. R., Giurdanella, G., D’Angeli, F., Giuffrida, R., Lo Furno, D., Anfuso, C. D., Mannino, G., & Lupo, G. (2024). Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy. International Journal of Molecular Sciences, 25(3), 1774. https://doi.org/10.3390/ijms25031774








