Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SCs) through the Galectin-9-Dependent Mechanism
Abstract
1. Introduction
2. Results
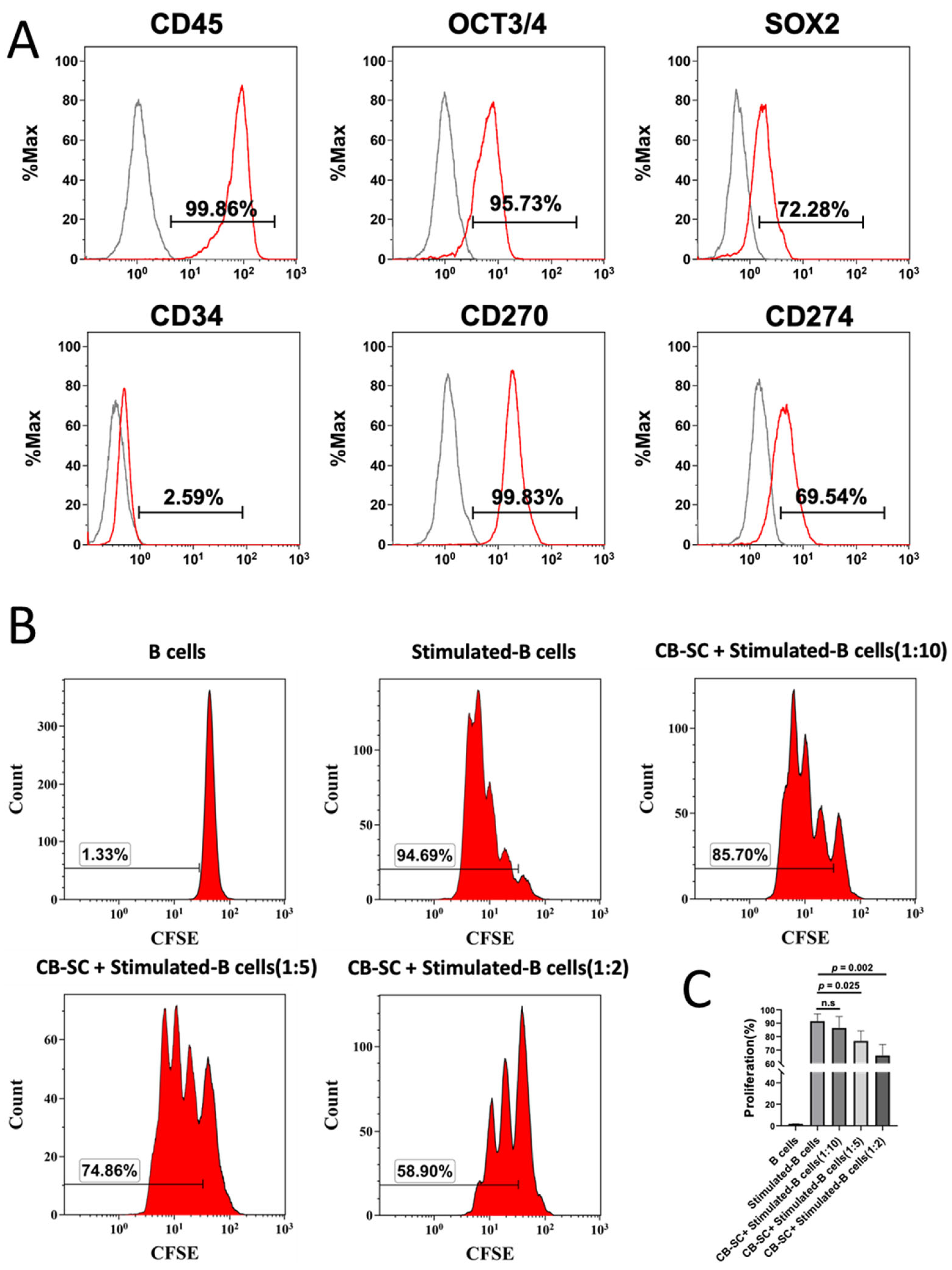
2.1. CB-SCs Suppressed the Proliferation of Activated B Cells
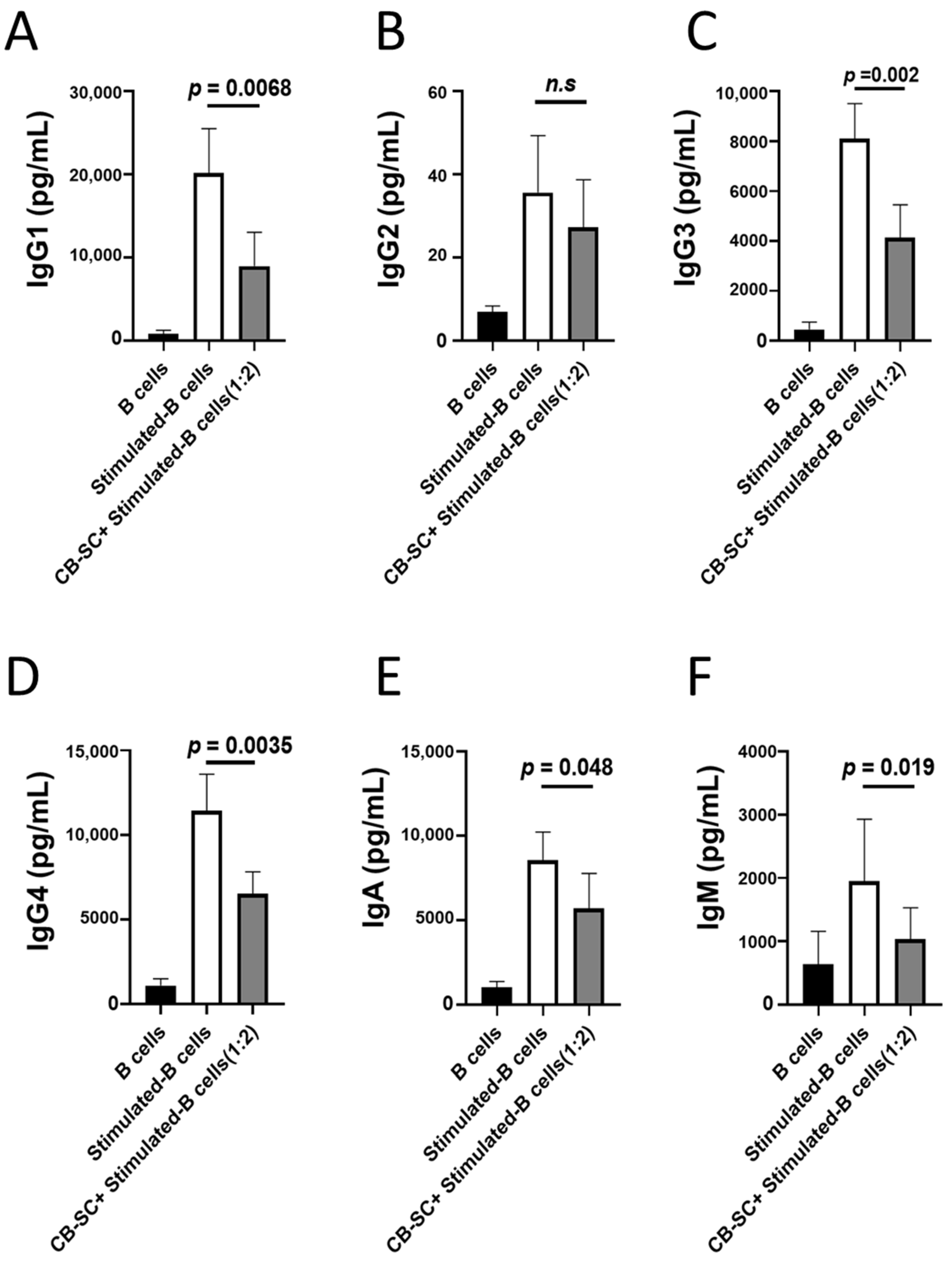
2.2. CB-SCs Inhibited Immunoglobulin Production
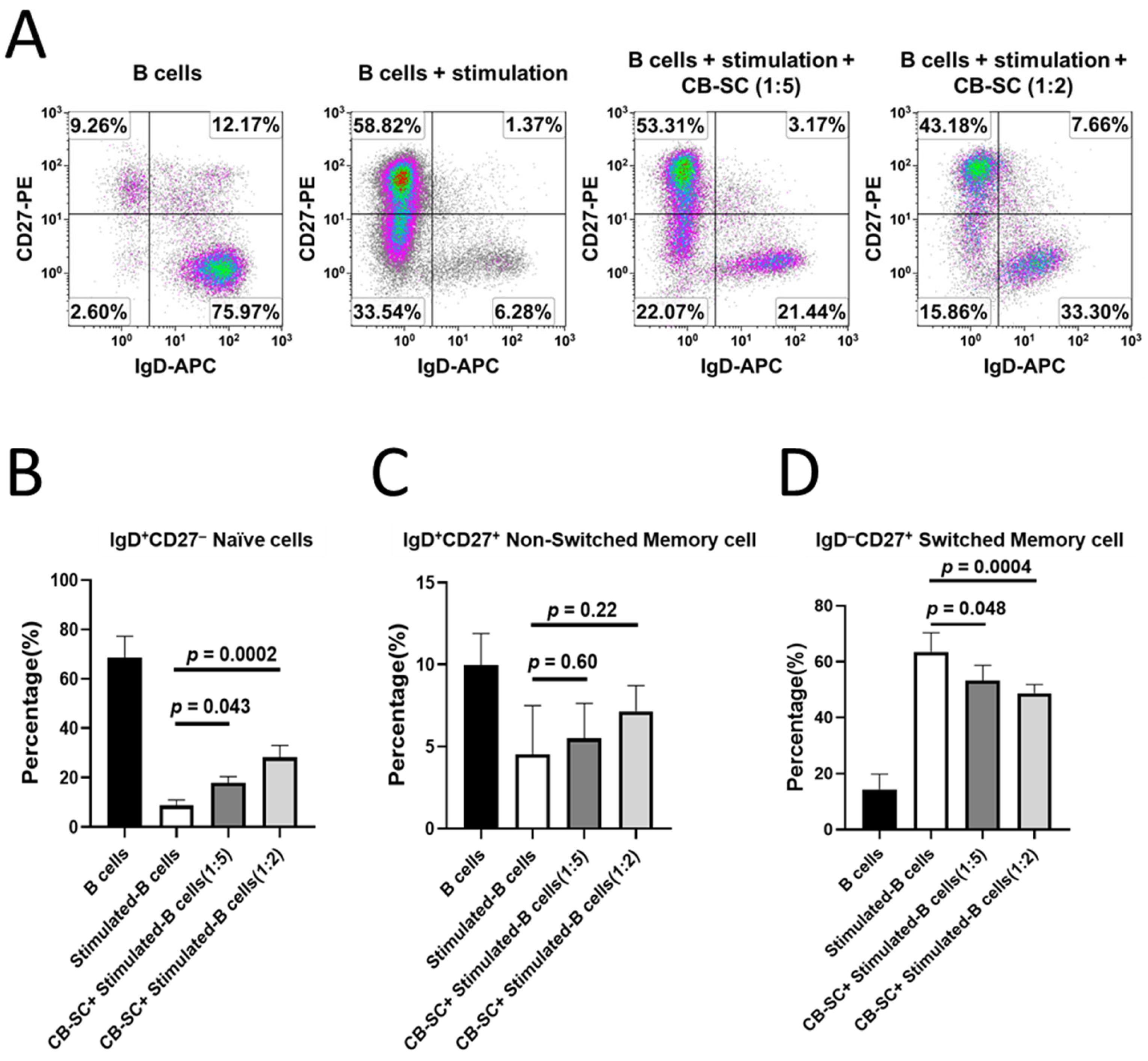
2.3. Modulation of CB-SC on Naïve and Memory B Cells
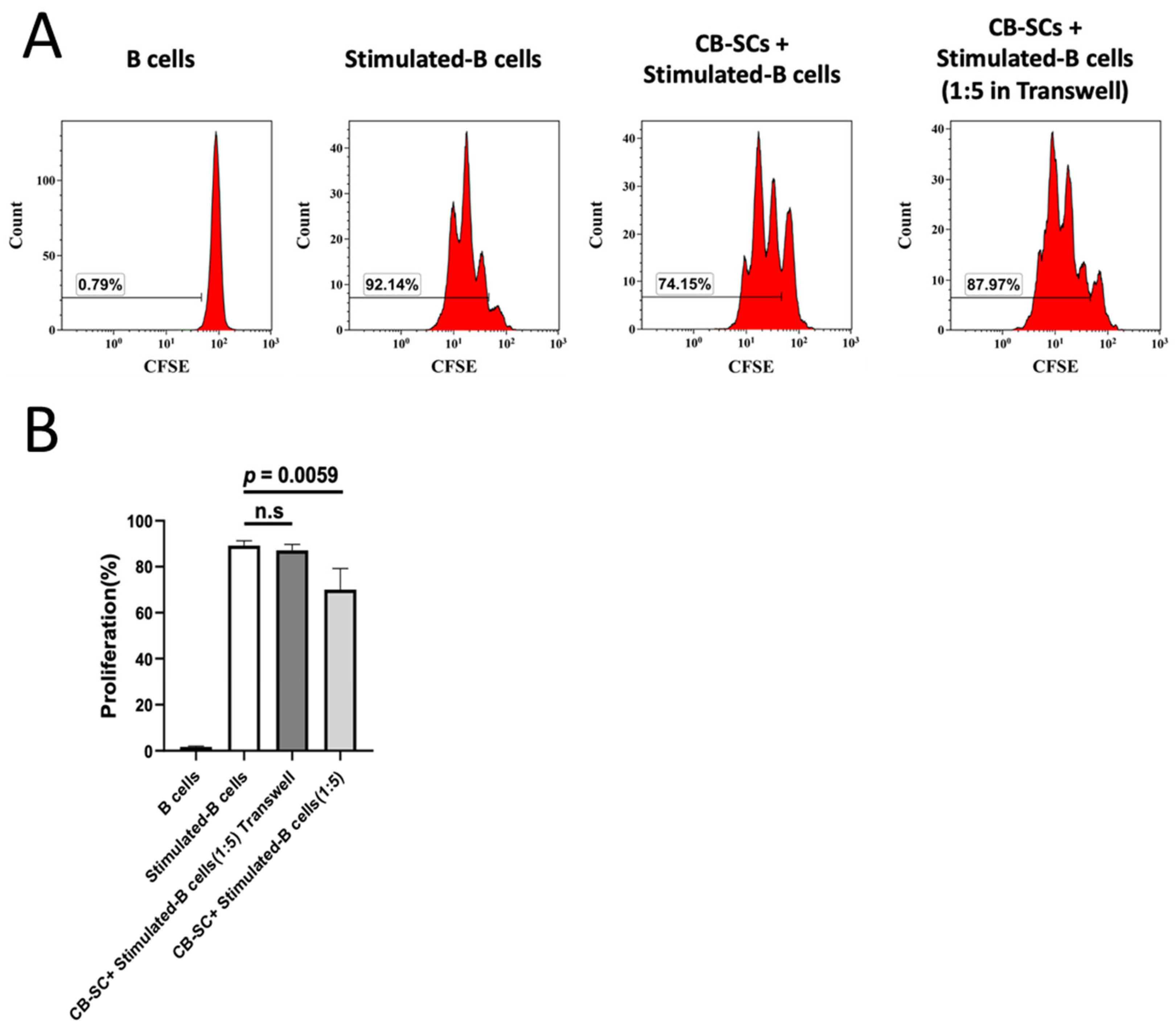
2.4. CB-SC-Mediated B Cell Suppression via the Cell–Cell Contact Regulation
2.5. The Expression of Gal-9 on CB-SCs Acts as a Key Molecule Contributing to the B-Cell Modulation
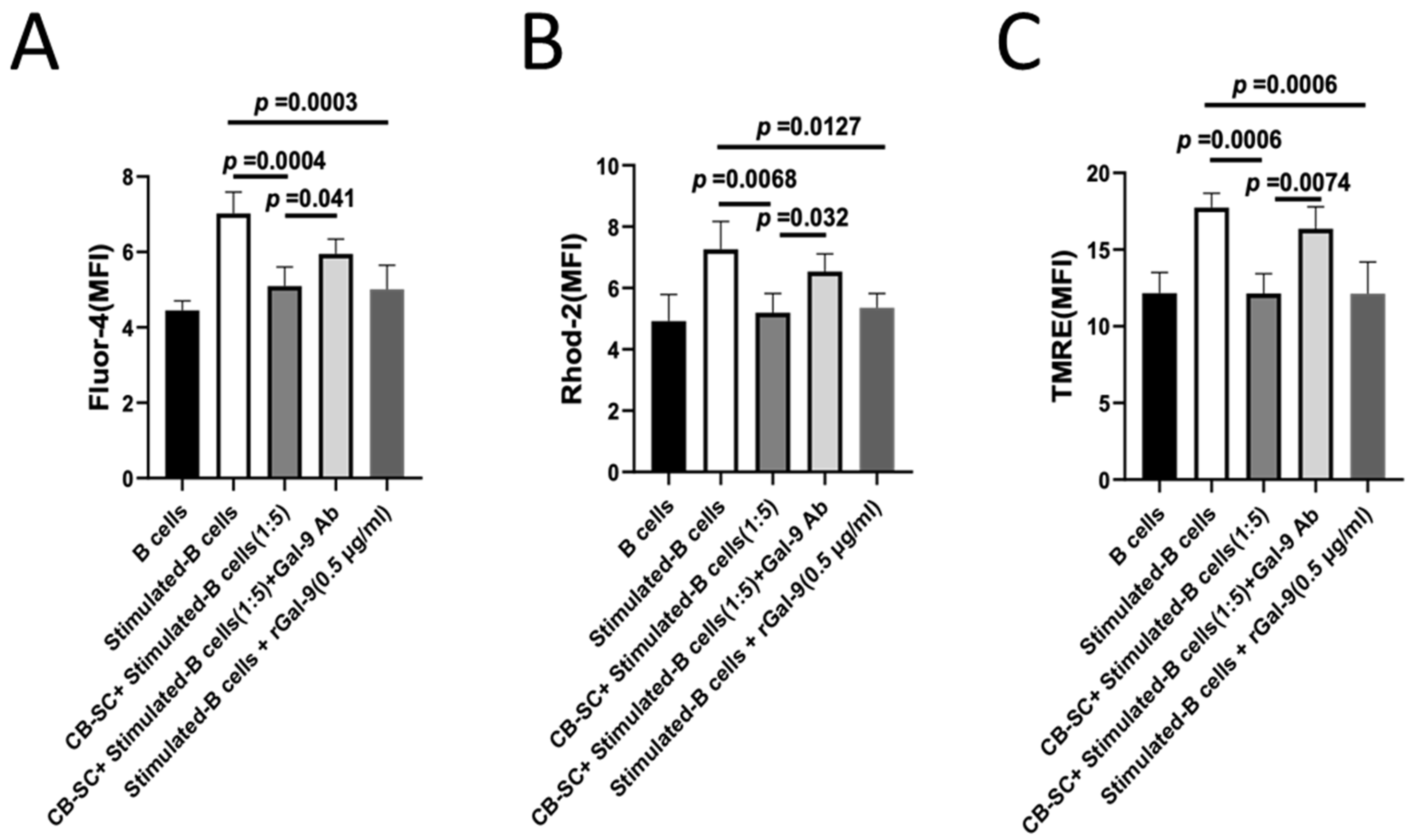
2.6. Gal-9-Dependent Suppression of Calcium Flux in B Cells by CB-SCs
3. Discussion
4. Materials and Methods
4.1. B-Cell Isolation and Culture
4.2. Proliferation Assay
4.3. Culture of CB-SC and Coculture of CB-SCs with B Cells
4.4. Quantitative Real-Time PCR Assay
4.5. Assay for Antibody Production
4.6. Blocking Experiments with Gal-9 Antibody
4.7. Flow Cytometry
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhao, Y.; Knight, C.M.; Jiang, Z.; Delgado, E.; Van Hoven, A.M.; Ghanny, S.; Zhou, Z.; Zhou, H.; Yu, H.; Hu, W.; et al. Stem Cell Educator therapy in type 1 diabetes: From the bench to clinical trials. Autoimmun. Rev. 2022, 21, 103058. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, X.; Yu, H.; Sun, J.; Zhao, Y. Released Exosomes Contribute to the Immune Modulation of Cord Blood-Derived Stem Cells. Front. Immunol. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Z.; Zhao, T.; Ye, M.; Hu, C.; Yin, Z.; Li, H.; Zhang, Y.; Diao, Y.; Li, Y.; et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Z.; Zhao, T.; Ye, M.; Hu, C.; Zhou, H.; Yin, Z.; Chen, Y.; Zhang, Y.; Wang, S.; et al. Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: Phase I/II clinical trial. BMC Med. 2013, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Häusser-Kinzel, S.; Weber, M.S. The Role of B Cells and Antibodies in Multiple Sclerosis, Neuromyelitis Optica, and Related Disorders. Front. Immunol. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Hampe, C.S. B Cell in Autoimmune Diseases. Scientifica 2012, 2012, 215308. [Google Scholar] [CrossRef]
- Wong, F.S.; Wen, L.; Tang, M.; Ramanathan, M.; Visintin, I.; Daugherty, J.; Hannum, L.G.; Janeway, C.A., Jr.; Shlomchik, M.J. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 2004, 53, 2581–2587. [Google Scholar] [CrossRef]
- Smith, M.J.; Simmons, K.M.; Cambier, J.C. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol. 2017, 13, 712–720. [Google Scholar] [CrossRef]
- Defuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef]
- Greenhill, C. Metabolism: Contribution of B cells to obesity and insulin resistance. Nat. Rev. Endocrinol. 2013, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Oleinika, K.; Slisere, B.; Catalan, D.; Rosser, E.C. B cell contribution to immunometabolic dysfunction and impaired immune responses in obesity. Clin. Exp. Immunol. 2022, 210, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Rui, K.; Wang, S.; Tian, J. Advances of Regulatory B Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 592914. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef]
- Karrar, S.; Cunninghame Graham, D.S. Abnormal B Cell Development in Systemic Lupus Erythematosus: What the Genetics Tell Us. Arthritis Rheumatol. 2018, 70, 496–507. [Google Scholar] [CrossRef]
- Wilson, C.L.; Hine, D.W.; Pradipta, A.; Pearson, J.P.; van Eden, W.; Robinson, J.H.; Knight, A.M. Presentation of the candidate rheumatoid arthritis autoantigen aggrecan by antigen-specific B cells induces enhanced CD4+ T helper type 1 subset differentiation. Immunology 2012, 135, 344–354. [Google Scholar] [CrossRef]
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Chen, H.Y.; Fermin, A.; Vardhana, S.; Weng, I.C.; Lo, K.F.; Chang, E.Y.; Maverakis, E.; Yang, R.Y.; Hsu, D.K.; Dustin, M.L.; et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 14496–14501. [Google Scholar] [CrossRef]
- Modenutti, C.P.; Capurro, J.I.B.; Di Lella, S.; Marti, M.A. The Structural Biology of Galectin-Ligand Recognition: Current Advances in Modeling Tools, Protein Engineering, and Inhibitor Design. Front. Chem. 2019, 7, 823. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Gilson, R.C.; Gunasinghe, S.D.; Johannes, L.; Gaus, K. Galectin-3 modulation of T-cell activation: Mechanisms of membrane remodelling. Prog. Lipid Res. 2019, 76, 101010. [Google Scholar] [CrossRef]
- Lhuillier, C.; Barjon, C.; Niki, T.; Gelin, A.; Praz, F.; Morales, O.; Souquere, S.; Hirashima, M.; Wei, M.; Dellis, O.; et al. Impact of Exogenous Galectin-9 on Human T Cells: Contribution of the T cell receptor complex to antigen-independent activation but not to apoptosis induction. J. Biol. Chem. 2015, 290, 16797–16811. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, N.; Smith, L.K.; Treanor, B.; Dimitroff, C.J. Galectin-Glycan Interactions as Regulators of B Cell Immunity. Front. Immunol. 2018, 9, 2839. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Mishra, R. Galectin-9: From cell biology to complex disease dynamics. J. Biosci. 2016, 41, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schuttrumpf, J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Yasinska, I.M.; Sakhnevych, S.S.; Pavlova, L.; Teo Hansen Selnø, A.; Teuscher Abeleira, A.M.; Benlaouer, O.; Gonçalves Silva, I.; Mosimann, M.; Varani, L.; Bardelli, M.; et al. The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front. Immunol. 2019, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, N.; Liang, J.; Antonopoulos, A.; Geddes Sweeney, J.; King, S.L.; Pochebit, S.M.; Bhattacharyya, N.; Lee, G.S.; Dell, A.; Widlund, H.R.; et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 2018, 9, 3287. [Google Scholar] [CrossRef]
- Cao, A.; Alluqmani, N.; Buhari, F.H.M.; Wasim, L.; Smith, L.K.; Quaile, A.T.; Shannon, M.; Hakim, Z.; Furmli, H.; Owen, D.M.; et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 2018, 9, 3288. [Google Scholar] [CrossRef]
- Delgado, E.; Perez-Basterrechea, M.; Suarez-Alvarez, B.; Zhou, H.; Revuelta, E.M.; Garcia-Gala, J.M.; Perez, S.; Alvarez-Viejo, M.; Menendez, E.; Lopez-Larrea, C.; et al. Modulation of Autoimmune T-Cell Memory by Stem Cell Educator Therapy: Phase 1/2 Clinical Trial. EBioMedicine 2015, 2, 2024–2036. [Google Scholar] [CrossRef]
- Nevalainen, T.; Autio, A.; Kummola, L.; Salomaa, T.; Junttila, I.; Jylhä, M.; Hurme, M. CD27- IgD- B cell memory subset associates with inflammation and frailty in elderly individuals but only in males. Immun. Ageing 2019, 16, 19. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Qi, M.; Lazzarini, P.; Mazzone, T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol. Lett. 2007, 108, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Fawaz, K.; Treanor, B. Galectin-9 regulates the threshold of B cell activation and autoimmunity. eLife 2021, 10, e64557. [Google Scholar] [CrossRef]
- De Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. 2019, 97, 605–618. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.K. Understanding B cell activation: From single molecule tracking, through Tolls, to stalking memory in malaria. Immunol. Res. 2009, 43, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Kurosaki, T. Role of Calcium Signaling in B Cell Activation and Biology. Curr. Top. Microbiol. Immunol. 2016, 393, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Musette, P.; Bouaziz, J.D. B Cell Modulation Strategies in Autoimmune Diseases: New Concepts. Front. Immunol. 2018, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Rodriguez-Pinto, D.; Du, W.; Ahuja, A.; Henegariu, O.; Wong, F.S.; Shlomchik, M.J.; Wen, L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Investig. 2007, 117, 3857–3867. [Google Scholar] [CrossRef]
- Hu, C.; Ding, H.; Zhang, X.; Wong, F.S.; Wen, L. Combination treatment with anti-CD20 and oral anti-CD3 prevents and reverses autoimmune diabetes. Diabetes 2013, 62, 2849–2858. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009, 361, 2143–2152. [Google Scholar] [CrossRef]
- Iwata, S.; Tanaka, Y. B-cell subsets, signaling and their roles in secretion of autoantibodies. Lupus 2016, 25, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kubo, S.; Iwata, S.; Yoshikawa, M.; Nakayamada, S. B cell phenotypes, signaling and their roles in secretion of antibodies in systemic lupus erythematosus. Clin. Immunol. 2018, 186, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lloyd, K.A.; Melas, I.; Zhou, D.; Thyagarajan, R.; Lindqvist, J.; Hansson, M.; Svärd, A.; Mathsson-Alm, L.; Kastbom, A.; et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci. Rep. 2019, 9, 19995. [Google Scholar] [CrossRef] [PubMed]
- Metzger, T.C.; Khan, I.S.; Gardner, J.M.; Mouchess, M.L.; Johannes, K.P.; Krawisz, A.K.; Skrzypczynska, K.M.; Anderson, M.S. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep. 2013, 5, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Z.; Delgado, E.; Li, H.; Zhou, H.; Hu, W.; Perez-Basterrechea, M.; Janostakova, A.; Tan, Q.; Wang, J.; et al. Platelet-Derived Mitochondria Display Embryonic Stem Cell Markers and Improve Pancreatic Islet beta-cell Function in Humans. Stem Cells Transl. Med. 2017, 6, 1684–1697. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.R.; Ahsan, F.M.; Wolf, D.M.; Shirihai, O.; Teitell, M.A. Initial B Cell Activation Induces Metabolic Reprogramming and Mitochondrial Remodeling. iScience 2018, 5, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-D.; Huang, Q.; Huang, A.-F. Emerging role of galectin family in inflammatory autoimmune diseases. Autoimmun. Rev. 2021, 20, 102847. [Google Scholar] [CrossRef]
- Kanzaki, M.; Wada, J.; Sugiyama, K.; Nakatsuka, A.; Teshigawara, S.; Murakami, K.; Inoue, K.; Terami, T.; Katayama, A.; Eguchi, J.; et al. Galectin-9 and T Cell Immunoglobulin Mucin-3 Pathway Is a Therapeutic Target for Type 1 Diabetes. Endocrinology 2012, 153, 612–620. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, D.; Wang, H.; Jin, W.; Li, X.; Hu, Y.; Qin, Y.; Kong, D.; Li, G.; Ellen, A.; et al. Galectin-9 Mediates the Therapeutic Effect of Mesenchymal Stem Cells on Experimental Endotoxemia. Front. Cell Dev. Biol. 2022, 10, 700702. [Google Scholar] [CrossRef]
- Maity, P.C.; Blount, A.; Jumaa, H.; Ronneberger, O.; Lillemeier, B.F.; Reth, M. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci. Signal. 2015, 8, ra93. [Google Scholar] [CrossRef]
- Mattila, P.K.; Feest, C.; Depoil, D.; Treanor, B.; Montaner, B.; Otipoby, K.L.; Carter, R.; Justement, L.B.; Bruckbauer, A.; Batista, F.D. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 2013, 38, 461–474. [Google Scholar] [CrossRef]
- Berry, C.T.; Liu, X.; Myles, A.; Nandi, S.; Chen, Y.H.; Hershberg, U.; Brodsky, I.E.; Cancro, M.P.; Lengner, C.J.; May, M.J.; et al. BCR-Induced Ca2+ Signals Dynamically Tune Survival, Metabolic Reprogramming, and Proliferation of Naive B Cells. Cell Rep. 2020, 31, 107474. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Hulsmans, S.; Griffioen, A.W. The galectin profile of the endothelium: Altered expression and localization in activated and tumor endothelial cells. Am. J. Pathol. 2008, 172, 545–553. [Google Scholar] [CrossRef]

| Forward Primer (5′–3′) | Reverse Primer (5′–3′) | |
|---|---|---|
| Galectin 1 | TGCAACAGCAAGGACGGC | CACCTCTGCAACACTTCCA |
| Galectin 2 | GATGGCACTGATGGCTTTG | AGACAATGGTGGATTCGCT |
| Galectin 3 | CAGAATTGCTTTAGATTTCCAA | TTATCCAGCTTTGTATTGCAA |
| Galectin 4 | CGAGGAGAAGAAGATCACCC | CTCTGGAAGGCCGAGAGG |
| Galectin 7 | CAGCAAGGAGCAAGGCTC | AAGTGGTGGTACTGGGCG |
| Galectin 8 | CTTAGGCTGCCATTCGCT | AAGCTTTTGGCATTTGCA |
| Galectin 9 | CTTTCATCACCACCATTCTG | ATGTGGAACCTCTGAGCACTG |
| Galectin 10 | AGTGTGCTTTGGTCGTCGT | ATGCTCAGTTCAAATTCTTGG |
| Galectin 12 | TGTGAGCCTGAGGGACCA | GCTGAGATCAGTTTCTTCTGC |
| Galectin 13 | CTTTACCCGTGCCATACAA | GTGGGTCATTGATAAAAGAGTG |
| Galectin 14 | CCTTGATGATTGTGGTACCAT | GTGGGTCCTTGACAAAAGTG |
| Beta actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Song, X.; Yu, H.; Fan, S.; Shi, A.; Sun, J.; Wang, H.; Zhao, L.; Zhao, Y. Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SCs) through the Galectin-9-Dependent Mechanism. Int. J. Mol. Sci. 2024, 25, 1830. https://doi.org/10.3390/ijms25031830
Hu W, Song X, Yu H, Fan S, Shi A, Sun J, Wang H, Zhao L, Zhao Y. Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SCs) through the Galectin-9-Dependent Mechanism. International Journal of Molecular Sciences. 2024; 25(3):1830. https://doi.org/10.3390/ijms25031830
Chicago/Turabian StyleHu, Wei, Xiang Song, Haibo Yu, Sophia Fan, Andrew Shi, Jingyu Sun, Hongjun Wang, Laura Zhao, and Yong Zhao. 2024. "Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SCs) through the Galectin-9-Dependent Mechanism" International Journal of Molecular Sciences 25, no. 3: 1830. https://doi.org/10.3390/ijms25031830
APA StyleHu, W., Song, X., Yu, H., Fan, S., Shi, A., Sun, J., Wang, H., Zhao, L., & Zhao, Y. (2024). Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SCs) through the Galectin-9-Dependent Mechanism. International Journal of Molecular Sciences, 25(3), 1830. https://doi.org/10.3390/ijms25031830






