Protein and Polysaccharide-Based Optical Materials for Biomedical Applications
Abstract
1. Introduction
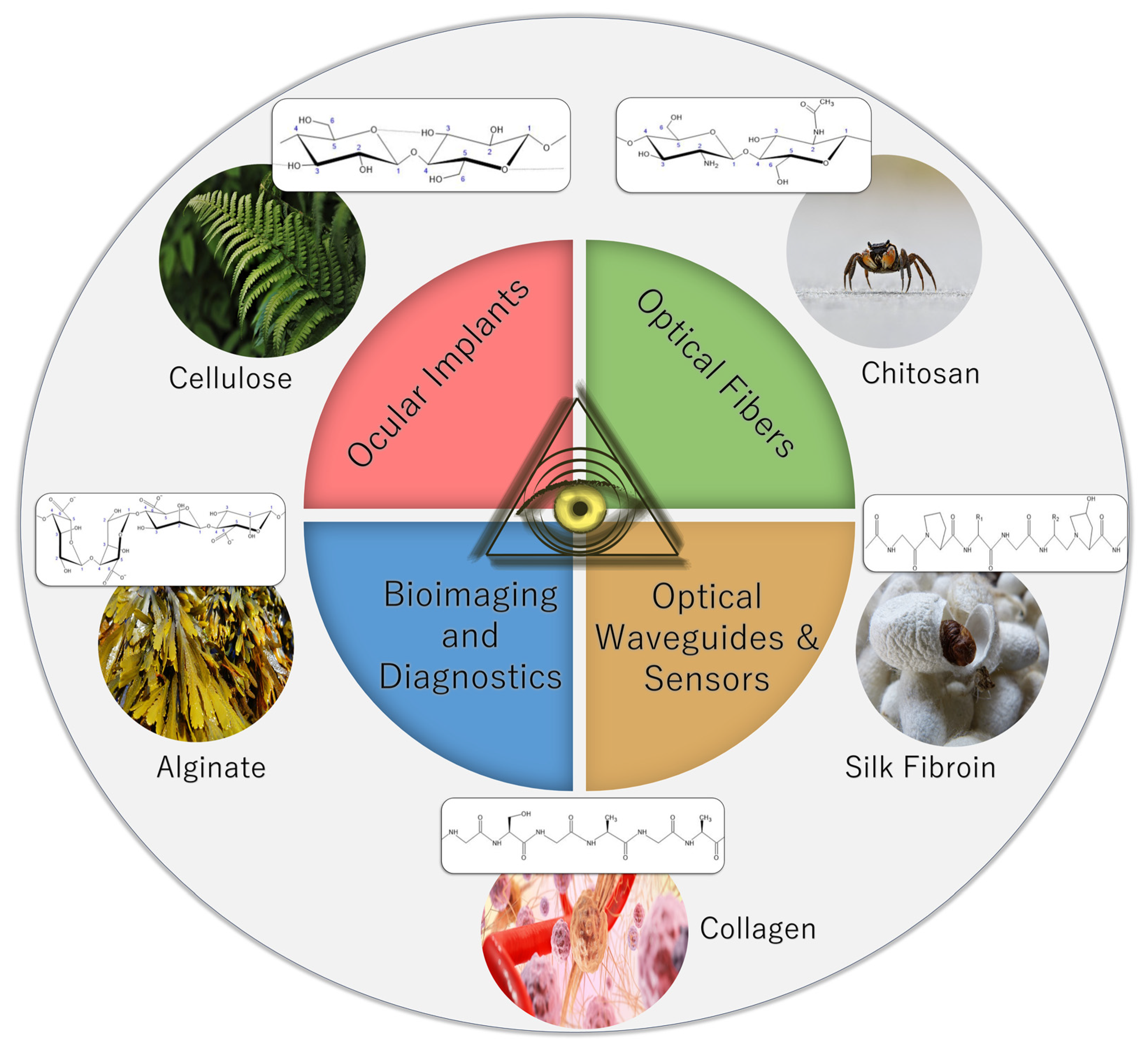
2. Optical Biopolymer Materials
2.1. Polysaccharide Biopolymers
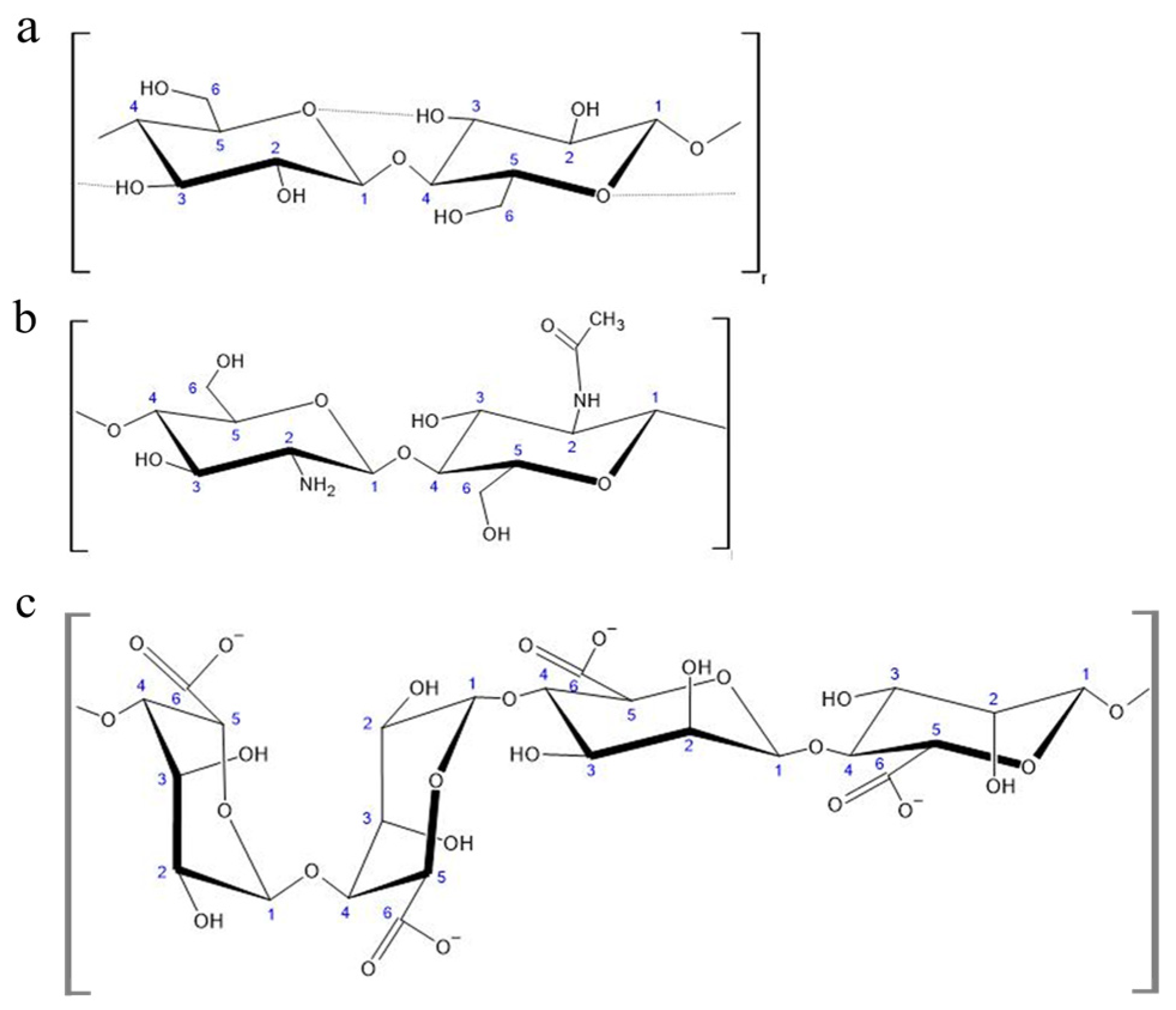
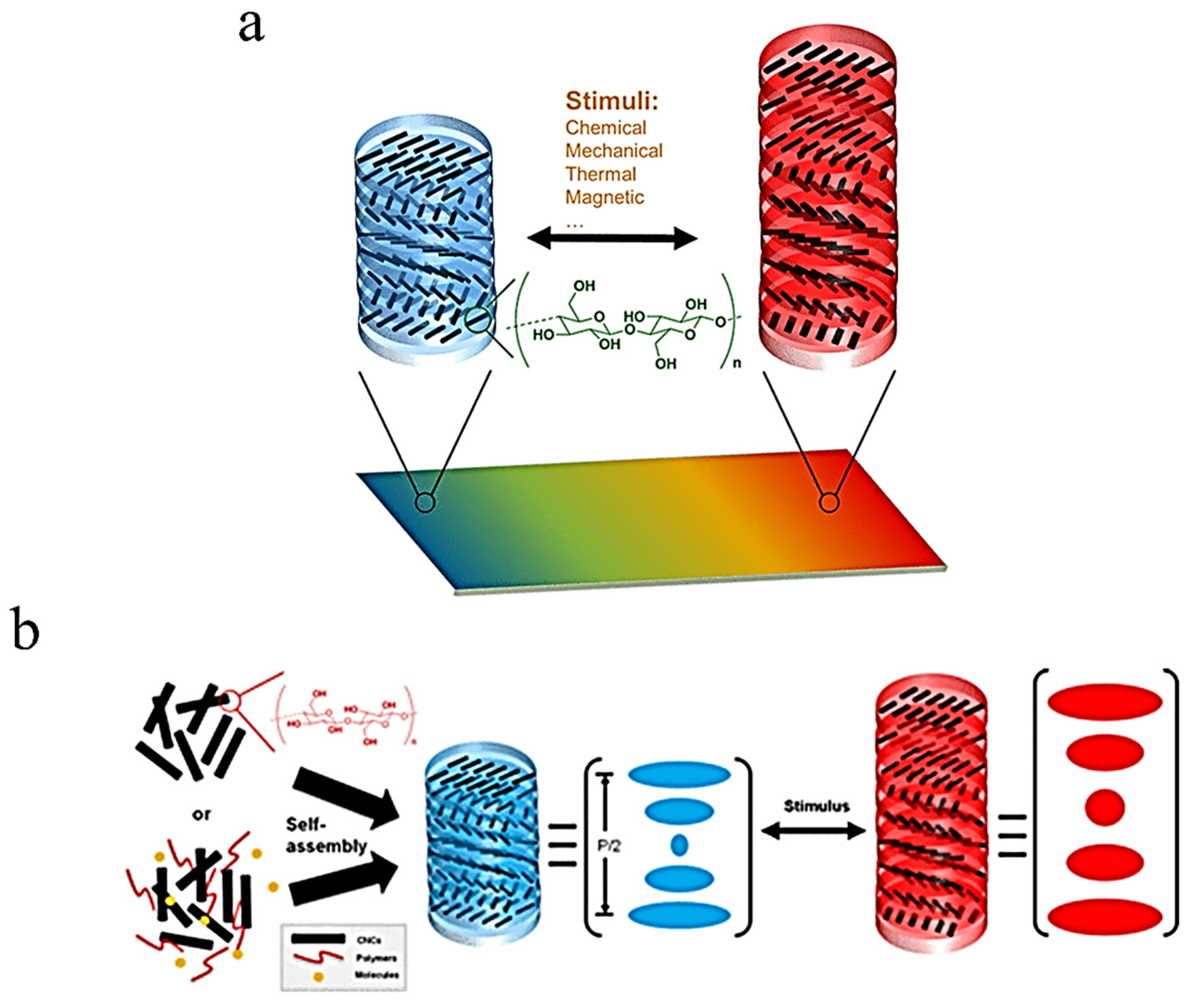
2.1.1. Cellulose
2.1.2. Chitin and Chitosan
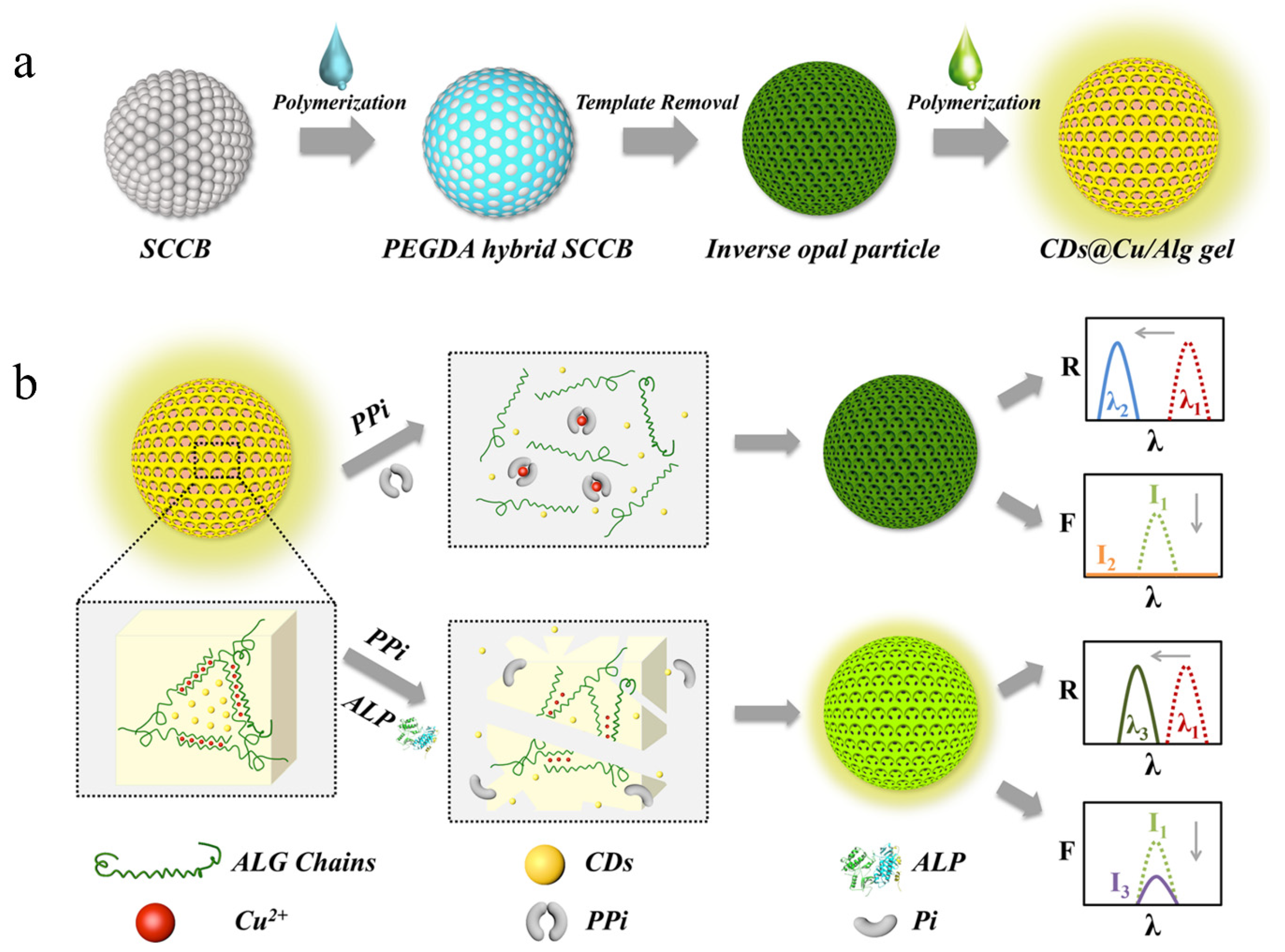
2.1.3. Alginate
2.2. Protein Biopolymers
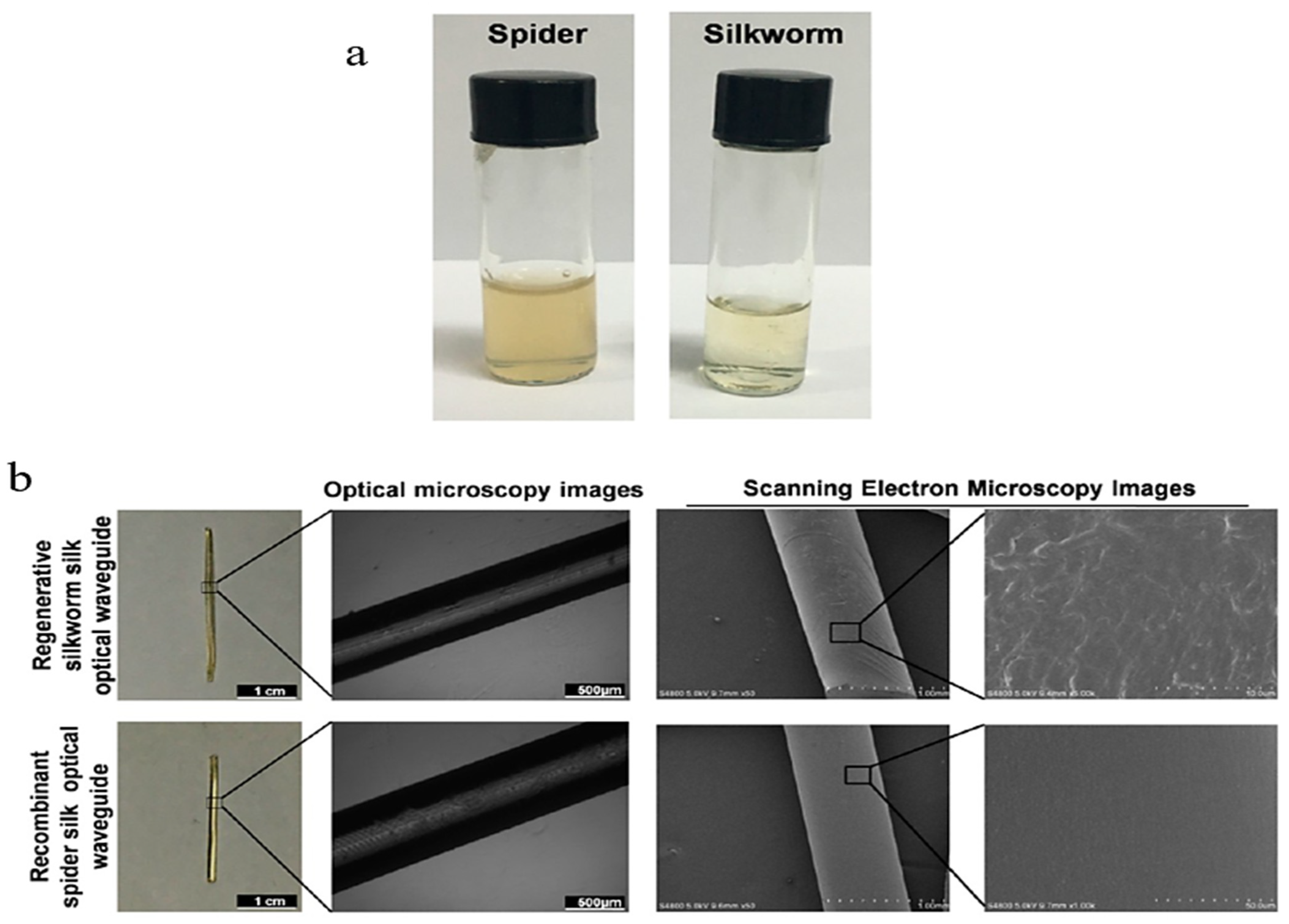
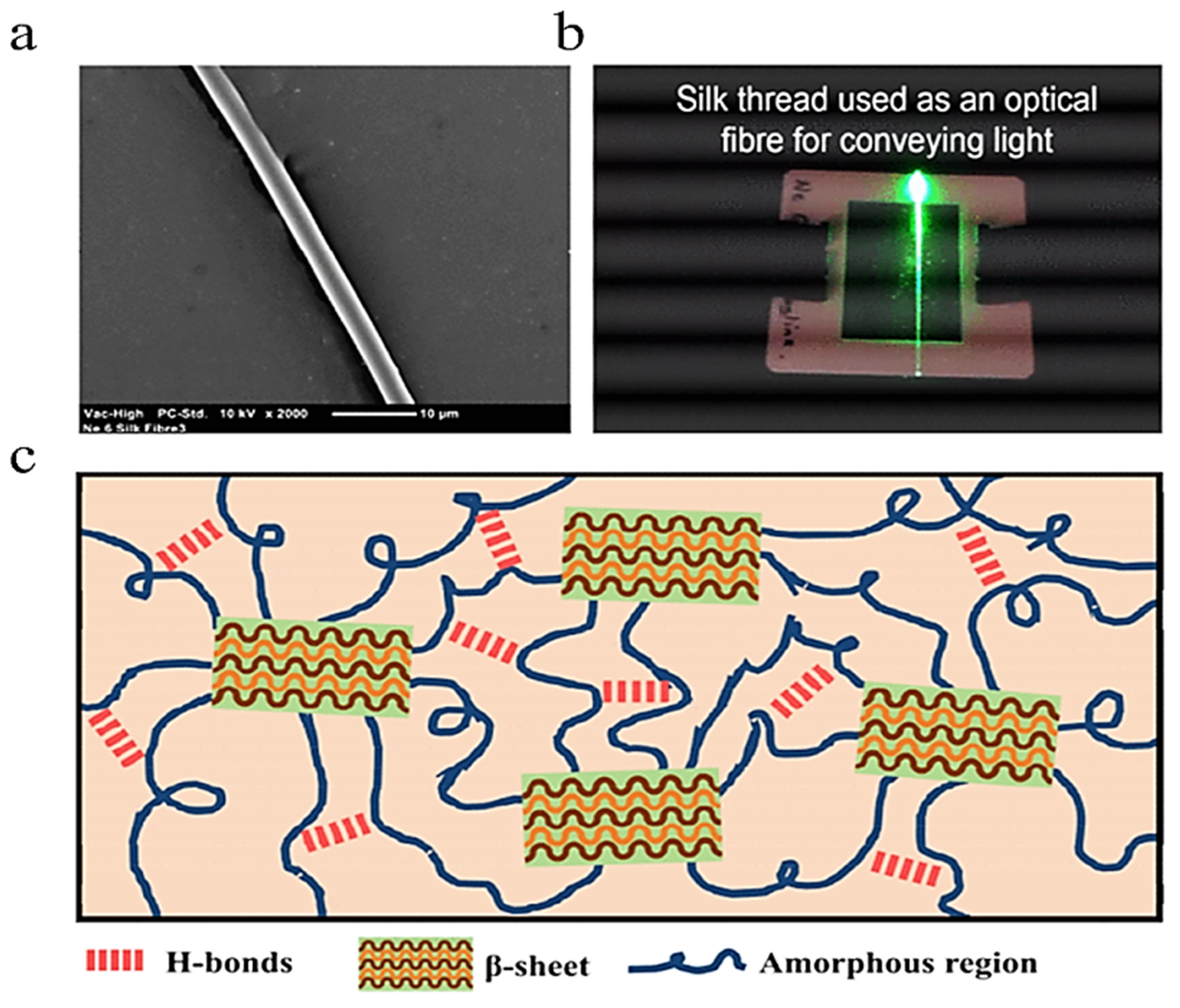
2.2.1. Silk Fibroin
2.2.2. Collagen and Gelatin
3. Optical Theory in Biomaterials Science
3.1. Refractive Index
3.2. Snell’s Law of Refraction
3.3. Optical Fiber and Waveguide Theory
4. Fabrication Methods for Optical Devices
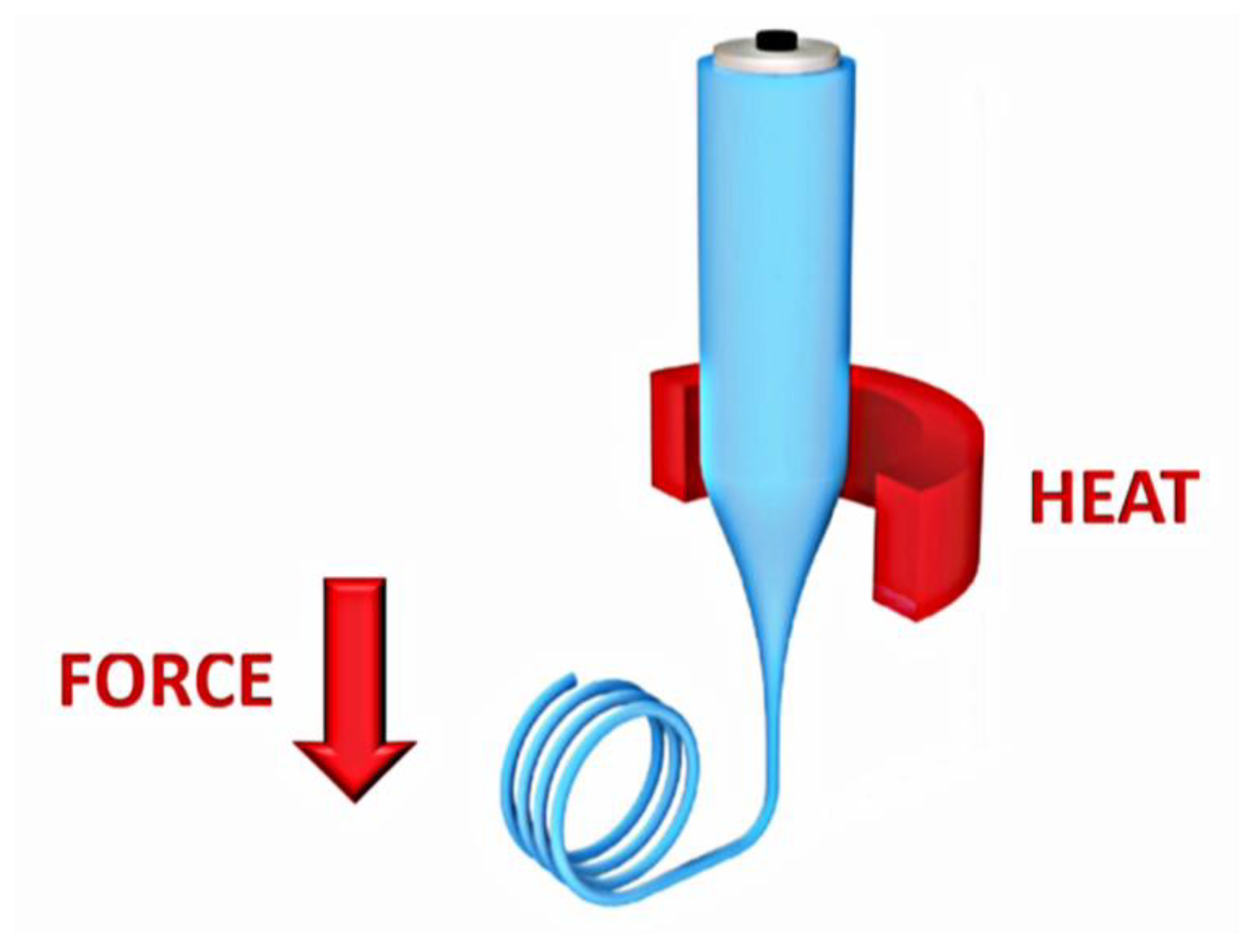
4.1. Thermal Drawing
4.2. Mold Casting
4.3. Dry-Jet Wet Spinning
4.4. Formation of Hydrogels
4.5. Extrusion and Printing
4.6. Integration of Biopolymer Nanoparticles
5. Applications
5.1. Optical Waveguides and Sensors
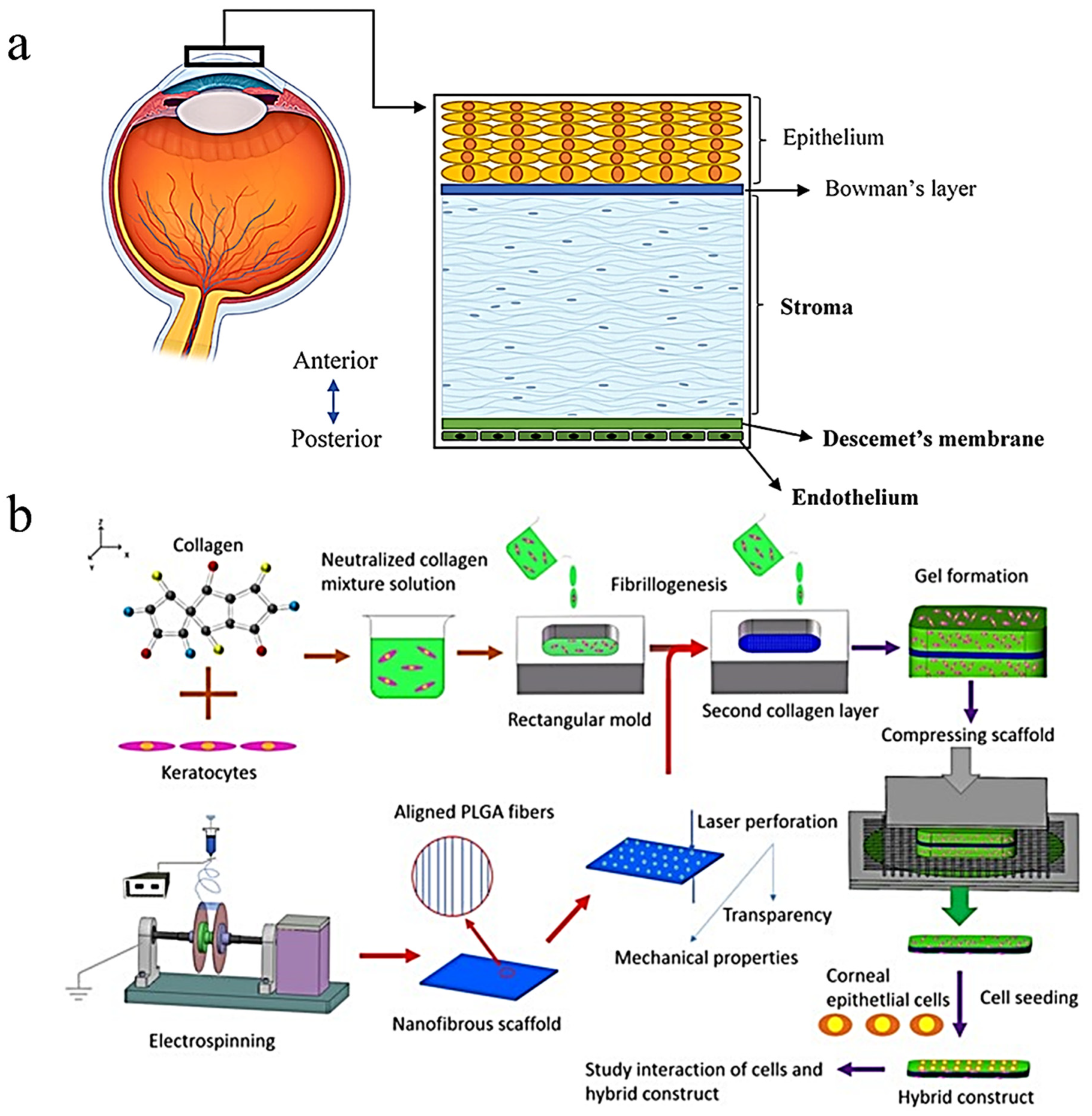
5.2. Ocular Implants
5.3. Imaging and Diagnostics
5.4. Optical Fibers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagliaro, M.; Ciriminna, R.; Morozova, S.M. Sustainable Optics? A Critical Insight into Biopolymer-Enabled Optics. Tetrahedron Green Chem. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Gierej, A.; Geernaert, T.; Van Vlierberghe, S.; Dubruel, P.; Thienpont, H.; Berghmans, F. Challenges in the Fabrication of Biodegradable and Implantable Optical Fibers for Biomedical Applications. Materials 2021, 14, 1972. [Google Scholar] [CrossRef]
- Shabahang, S.; Kim, S.; Yun, S. Light-Guiding Biomaterials for Biomedical Applications. Adv. Funct. Mater. 2018, 28, 24. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Eldhose, M.; George, C.; John, S.; Joseph, A.; George, L. Optical Properties of Biopolymers. In Handbook of Biopolymers; Springer: Singapore, 2023; ISBN 978-981-19-0710-4. [Google Scholar]
- Lawrence, B.D.; Cronin-Golomb, M.; Georgakoudi, I.; Kaplan, D.L.; Omenetto, F.G. Bioactive Silk Protein Biomaterial Systems for Optical Devices. Bioconjug. Chem. 2008, 9, 1214–1220. [Google Scholar] [CrossRef]
- Colusso, E.; Martucci, A. An Overview of Biopolymer-Based Nanocomposites for Optics and Electronics. J. Mater. Chem. C 2021, 9, 5578–5593. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 4, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- da Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Ariel, C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinski, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [CrossRef]
- Xiong, R.; Grant, A.M.; Ma, R.; Zhang, S.; Tsukruk, V.V. Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mater. Sci. Eng. R Rep. Rev. J. 2018, 125, 1–41. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Li, X.; Yang, H.; Liu, S.; Wang, X.; Zhang, L.; Sun, Q.; Liu, X.; Chen, J. Electronic biopolymers: From molecular engineering to functional devices. Chem. Eng. J. 2020, 397, 125499. [Google Scholar] [CrossRef]
- Han, Z.; Liu, G. Sugar-based biopolymers as novel imaging agents for molecular magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1551. [Google Scholar] [CrossRef]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Stokke, B.T.; Draget, K.I.; Smidsrød, O.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Small-Angle X-ray Scattering and Rheological Characterization of Alginate Gels. 1. Ca−Alginate Gels. Macromolecules 2000, 33, 1853–1863. [Google Scholar] [CrossRef]
- Rehm, B.H.A.; Valla, S. Bacterial alginates: Biosynthesis and applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Heah, W.Y.; Yamagishi, H.; Fujita, K.; Sumitani, M.; Mikami, Y.; Yoshioka, H.; Oki, Y.; Yamamoto, Y. Silk fibroin microspheres as optical resonators for wide-range humidity sensing and biodegradable lasers. Mater. Chem. Front. 2021, 5, 5653–5657. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Cao, Y.; Marelli, B.; Xia, X.; Tao, T.H. Engineering the Future of Silk Materials through Advanced Manufacturing. Adv. Mater. 2018, 30, e1706983. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Weiss, V.; Amitai, Y.; Friesem, A.A.; Millul, E. Silver Halide Sensitized Gelatin Holographic Recording Materials. Proc. SPIE Int. Soc. Opt. Eng. 1989, 1038, 110–115. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Dresselhaus, M.S. Solid State Physics Part II Optical Properties of Solids; Course 6.732 Solid State Physics, Archived (PDF) from the original on 2015-07-24. Retrieved 2015-01-05; MIT: Cambridge, MA, USA, 1999. [Google Scholar]
- Fonn, D. Targeting contact lens induced dryness and discomfort: What properties will make lenses more comfortable. Optom. Vision. Sci. 2007, 84, 279–285. [Google Scholar] [CrossRef]
- Se-yuen, M. A closer look at Fermat’s principle. Phys. Educ. 1986, 21, 365. [Google Scholar] [CrossRef]
- Weik, M.H. (Ed.) Refractive-Index Contrast. In Computer Science and Communications Dictionary; Springer: Boston, MA, USA, 2001; p. 1451. [Google Scholar]
- Dupuis, A.; Guo, N.; Gao, Y.; Skorobogata, O.; Gauvreau, B.; Dubois, C.; Skorobogatiy, M. Fabrication strategies and potential applications of the “green” microstructured optical fibers. J. Biomed. Opt. 2008, 13, 054003. [Google Scholar] [CrossRef]
- Qiao, X.; Qian, Z.; Li, J.; Sun, H.; Han, Y.; Xia, X.; Zhou, J.; Wang, C.; Wang, Y.; Wang, C. Synthetic Engineering of Spider Silk Fiber as Implantable Optical Waveguides for Low-Loss Light Guiding. ACS Appl. Mater. Interfaces 2017, 9, 14665–14676. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Anjum, N.; Atthoff, B.; Hilborn, J. Preparation of poly(lactic acid) fiber by dry-jet-wet-spinning. I. Influence of draw ratio on fiber properties. J. Appl. Polym. Sci. 2006, 100, 1239–1246. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef]
- Choi, M.; Humar, M.; Kim, S.; Yun, S.H. Step-Index Optical Fiber Made of Biocompatible Hydrogels. Adv. Mater. 2015, 27, 4081–4086. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Ahmed, R.; Marques, A.P.; Reis, R.L.; Demirci, U. Engineering Hydrogel-Based Biomedical Photonics: Design, Fabrication, and Applications. Adv. Mater. 2021, 33, e2006582. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Parker, S.T.; Domachuk, P.; Amsden, J.; Bressner, J.; Lewis, J.A.; Kaplan, D.L.; Omenetto, F.G. Biocompatible Silk Printed Optical Waveguides. Adv. Mater. 2009, 21, 2411–2415. [Google Scholar] [CrossRef]
- Coelho, F.; do Vale Braido, G.V.; Cavicchioli, M.; Mendes, L.S.; Specian, S.S.; Franchi, L.P.; Lima Ribeiro, S.J.; Messaddeq, Y.; Scarel-Caminaga, R.M.; Capote, T.S.O. Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency. Contact Lens Anterior Eye 2019, 42, 512–519. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Kogelnik, H. Theory of Optical Waveguides. In Guided-Wave Optoelectronics; Tamir, T., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 7–88. [Google Scholar]
- Wang, J.; Dong, J. Optical Waveguides and Integrated Optical Devices for Medical Diagnosis, Health Monitoring and Light Therapies. Sensors 2020, 20, 3981. [Google Scholar] [CrossRef]
- Prajzler, V.; Arif, S.; Min, K.; Kim, S.; Nekvindova, P. All-polymer silk-fibroin optical planar waveguides. Opt. Mater. 2021, 114, 110932. [Google Scholar] [CrossRef]
- Amin, A.; Ahmed, E.H.; Wickleder, C.; Adlung, M.; Hashem, A.I.; Ayoub, M.M.H.; Battisha, I.K. Phosphosilicate–polyamidoamine hyperbranched polymer–Er3+ nanocomposite toward planar optical waveguide applications. Polym. Compos. 2019, 40, 2029–2038. [Google Scholar] [CrossRef]
- Ahmed, E.H.; Hashem, A.I.; Adlung, M.; Wickleder, C.; Ayoub, M.M.H.; Battisha, I.K.; Amin, A. Tailoring Chitosan Nanocomposites for Planar Optical Waveguide Applications. Polym. Sci. Ser. A 2022, 64, 342–353. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, Q.; Rogin, P.; de Oliveira, P.W.; del Campo, A. Printed Soft Optical Waveguides of PLA Copolymers for Guiding Light into Tissue. ACS Appl. Mater. Interfaces 2020, 12, 20287–20294. [Google Scholar] [CrossRef]
- Fu, R.; Luo, W.; Nazempour, R.; Tan, D.; Ding, H.; Zhang, K.; Yin, L.; Guan, J.; Sheng, X. Implantable and Biodegradable Poly(l-lactic acid) Fibers for Optical Neural Interfaces. Adv. Opt. Mater. 2018, 6, 1700941. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, Q.; Tai, Y.A.; Wang, Y. Applications of Cellulose Nanomaterials in Stimuli-Responsive Optics. J. Agric. Food Chem. 2020, 68, 12940–12955. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Song, Y.; Overmass, M.; Fan, J.; Hodge, C.; Sutton, G.; Lovicu, F.J.; You, J. Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues. Front. Surg. 2021, 8, 639500. [Google Scholar] [CrossRef]
- Mi, S.; Connon, C.J. The Formation of a Tissue-Engineered Cornea Using Plastically Compressed Collagen Scaffolds and Limbal Stem Cells. In Corneal Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2013; pp. 143–155. [Google Scholar] [CrossRef]
- Hayes, S.; Lewis, P.; Islam, M.M.; Doutch, J.; Sorensen, T.; White, T.; Griffith, M.; Meek, K.M. The structural and optical properties of type III human collagen biosynthetic corneal substitutes. Acta Biomater. 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Inyushi, M.; Meshalkina, D.; Zueva, L.; Zayas-Santiago, A. Tissue Transparency In Vivo. Molecules 2019, 24, 2388. [Google Scholar] [CrossRef]
- Mangalath, A.; Raj, V.; Santhosh, R.; Thekkuveettil, A. Alginate Gel Immobilization of Caenorhabditis elegans for Optical Calcium Imaging of Neurons. Bio Protoc. 2023, 13, e4697. [Google Scholar] [CrossRef]
- Brichacek, A.L.; Brown, C.M. Alkaline phosphatase: A potential biomarker for stroke and implications for treatment. Metab. Brain Dis. 2019, 34, 3–19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Zhang, H.; Shang, L.; Zhao, Y. Responsive photonic alginate hydrogel particles for the quantitative detection of alkaline phosphatase. NPG Asia Mater. 2022, 14, 54. [Google Scholar] [CrossRef]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and Polysaccharide-Based Magnetic Composite Materials for Medical Applications. Int. J. Mol. Sci. 2020, 21, 186. [Google Scholar] [CrossRef]
- Funovics, M.; Weissleder, R.; Tung, C. Protease sensors for bioimaging. Anal. Bioanal. Chem. 2003, 377, 956–963. [Google Scholar] [CrossRef]
- Peters, K. Polymer optical fiber sensors—A review. Smart Mater. Struct. 2011, 20, 013002. [Google Scholar] [CrossRef]
- Reimer, M.; Van Opdenbosch, D.; Zollfrank, C. Fabrication of Cellulose-Based Biopolymer Optical Fibers and Their Theoretical Attenuation Limit. Biomacromolecules 2021, 22, 3297–3312. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Hey Tow, K.; Chow, D.M.; Vollrath, F.; Dicaire, I.; Gheysens, T.; Thevenaz, L. Exploring the Use of Native Spider Silk as an Optical Fiber for Chemical Sensing. J. Light. Technol. 2018, 36, 1138–1144. [Google Scholar] [CrossRef]
- Rivera-Galletti, A.; Gough, C.R.; Kaleem, F.; Burch, M.; Ratcliffe, C.; Lu, P.; Salas-de la Cruz, D.; Hu, X. Silk-Cellulose Acetate Biocomposite Materials Regenerated from Ionic Liquid. Polymers 2021, 13, 2911. [Google Scholar] [CrossRef]
- Xue, Y.; Jackson, K.; Page, N.; Mou, X.; Lofland, S.; Hu, X. Water-annealing regulated protein-based magnetic nanofiber materials: Tuning silk structure and properties to enhance cell response under magnetic fields. Mater. Today Chem. 2021, 22, 100570. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Xue, Y.; Callaway, K.; Gough, C.; Moore, R.; Bessette, K.; Mou, X.; Hu, X. Air-jet spinning corn zein protein nanofibers for drug delivery: Effect of biomaterial structure and shape on release properties. Mater. Sci. Eng. C 2021, 118, 111419. [Google Scholar] [CrossRef]


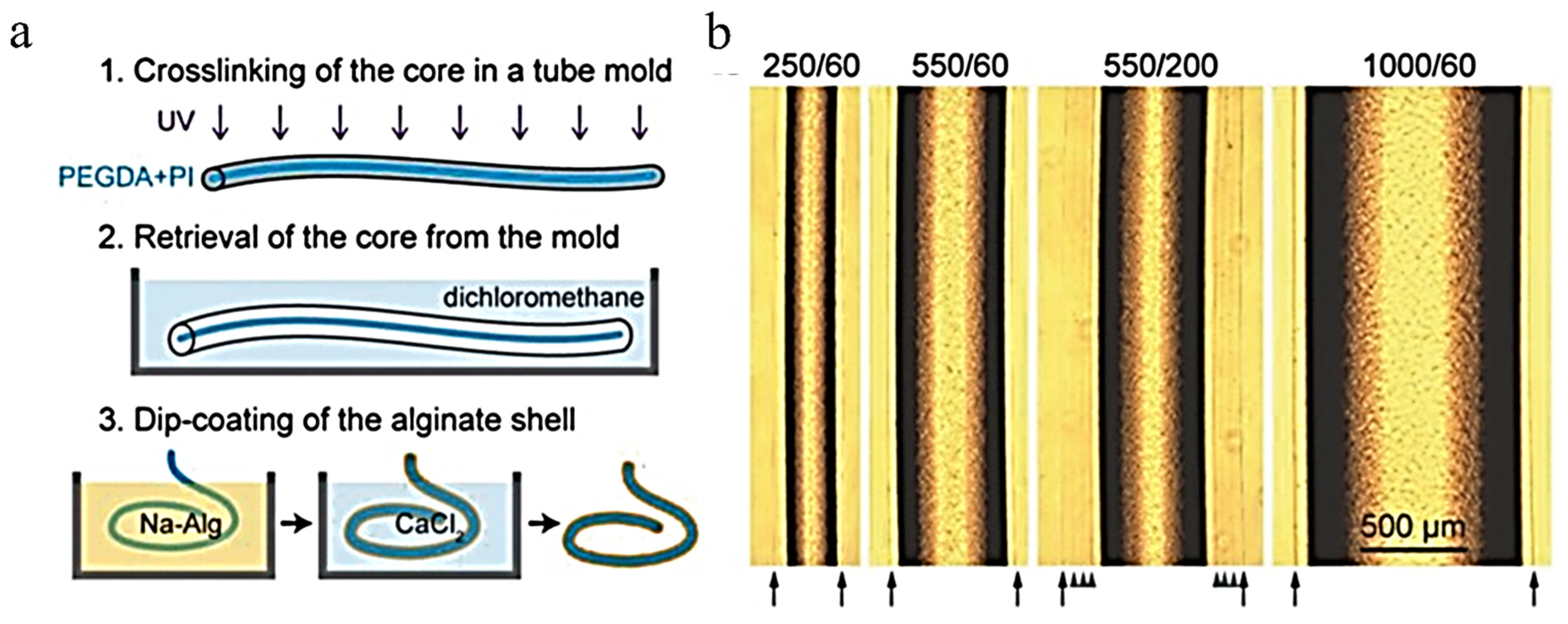
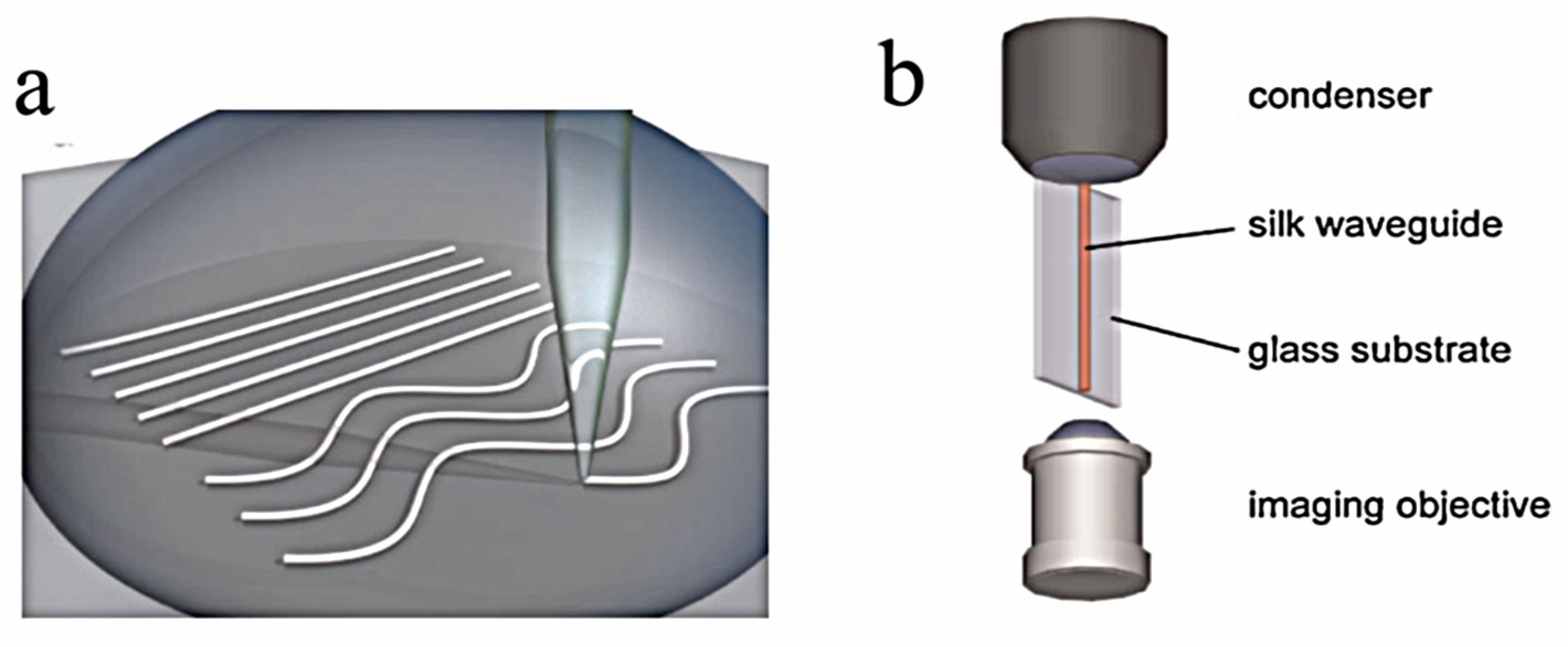
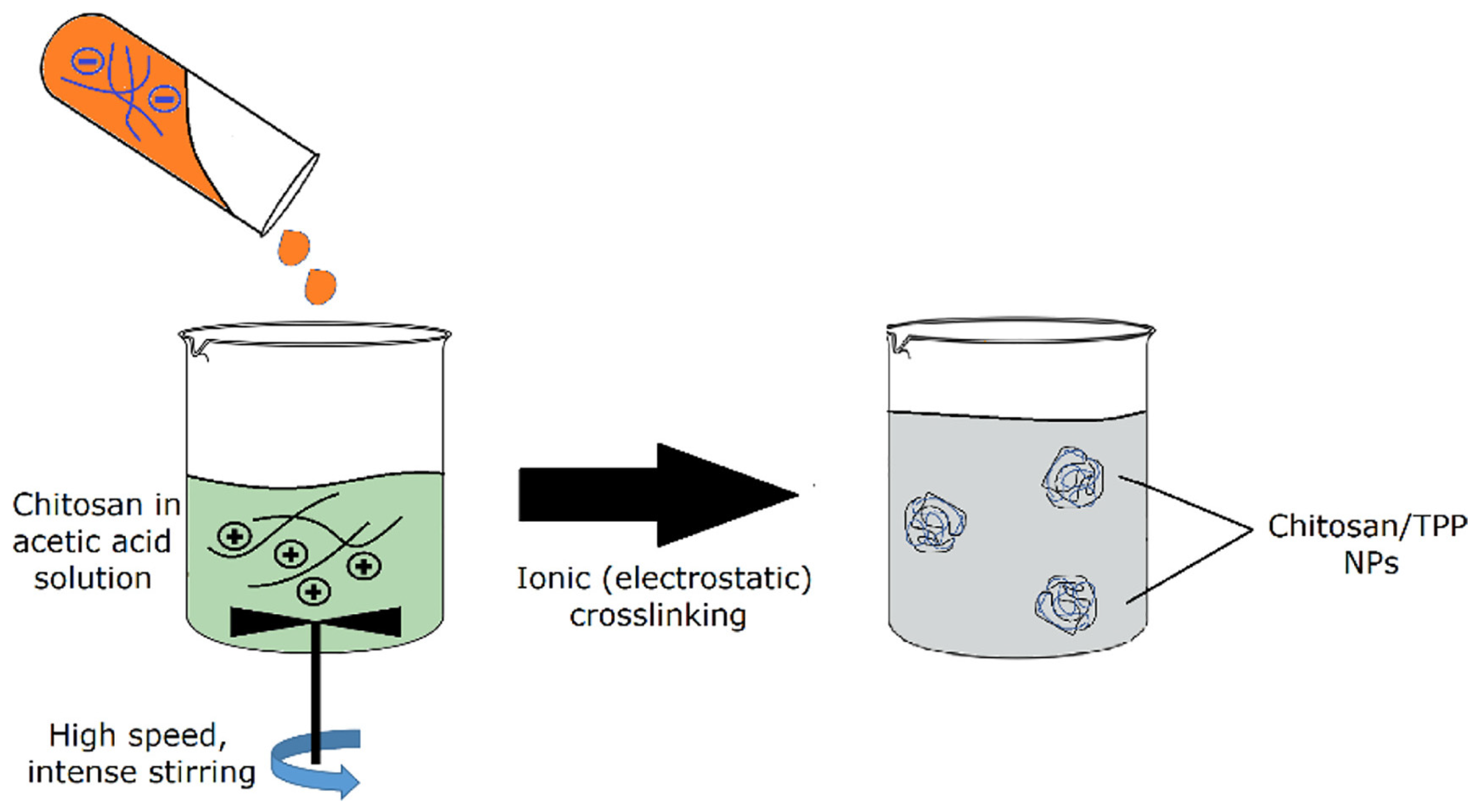
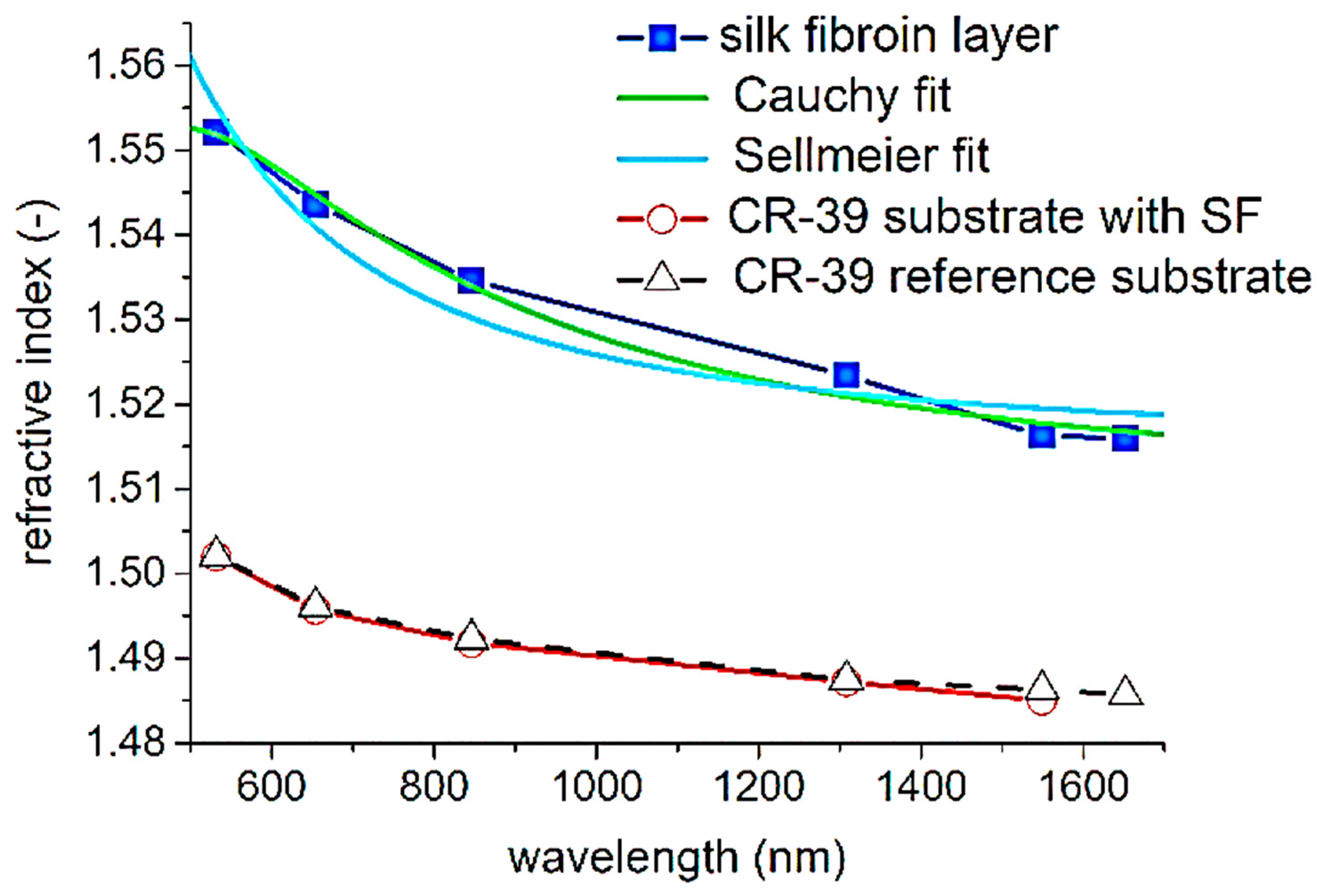

| Method | Materials | Description | Benefits | Disadvantages |
|---|---|---|---|---|
| Thermal Drawing |
|
| ||
| Mold Casting |
|
| ||
| Dry-Jet Wet Spinning |
|
| ||
| Hydrogels |
|
| ||
| Extrusion and Printing |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riviello, G.; Connor, B.; McBrearty, J.; Rodriguez, G.; Hu, X. Protein and Polysaccharide-Based Optical Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 1861. https://doi.org/10.3390/ijms25031861
Riviello G, Connor B, McBrearty J, Rodriguez G, Hu X. Protein and Polysaccharide-Based Optical Materials for Biomedical Applications. International Journal of Molecular Sciences. 2024; 25(3):1861. https://doi.org/10.3390/ijms25031861
Chicago/Turabian StyleRiviello, Gianna, Brendan Connor, Jake McBrearty, Gianna Rodriguez, and Xiao Hu. 2024. "Protein and Polysaccharide-Based Optical Materials for Biomedical Applications" International Journal of Molecular Sciences 25, no. 3: 1861. https://doi.org/10.3390/ijms25031861
APA StyleRiviello, G., Connor, B., McBrearty, J., Rodriguez, G., & Hu, X. (2024). Protein and Polysaccharide-Based Optical Materials for Biomedical Applications. International Journal of Molecular Sciences, 25(3), 1861. https://doi.org/10.3390/ijms25031861







