CRISPR/Cas9-Mediated Editing of BmEcKL1 Gene Sequence Affected Silk Gland Development of Silkworms (Bombyx mori)
Abstract
:1. Introduction
2. Results
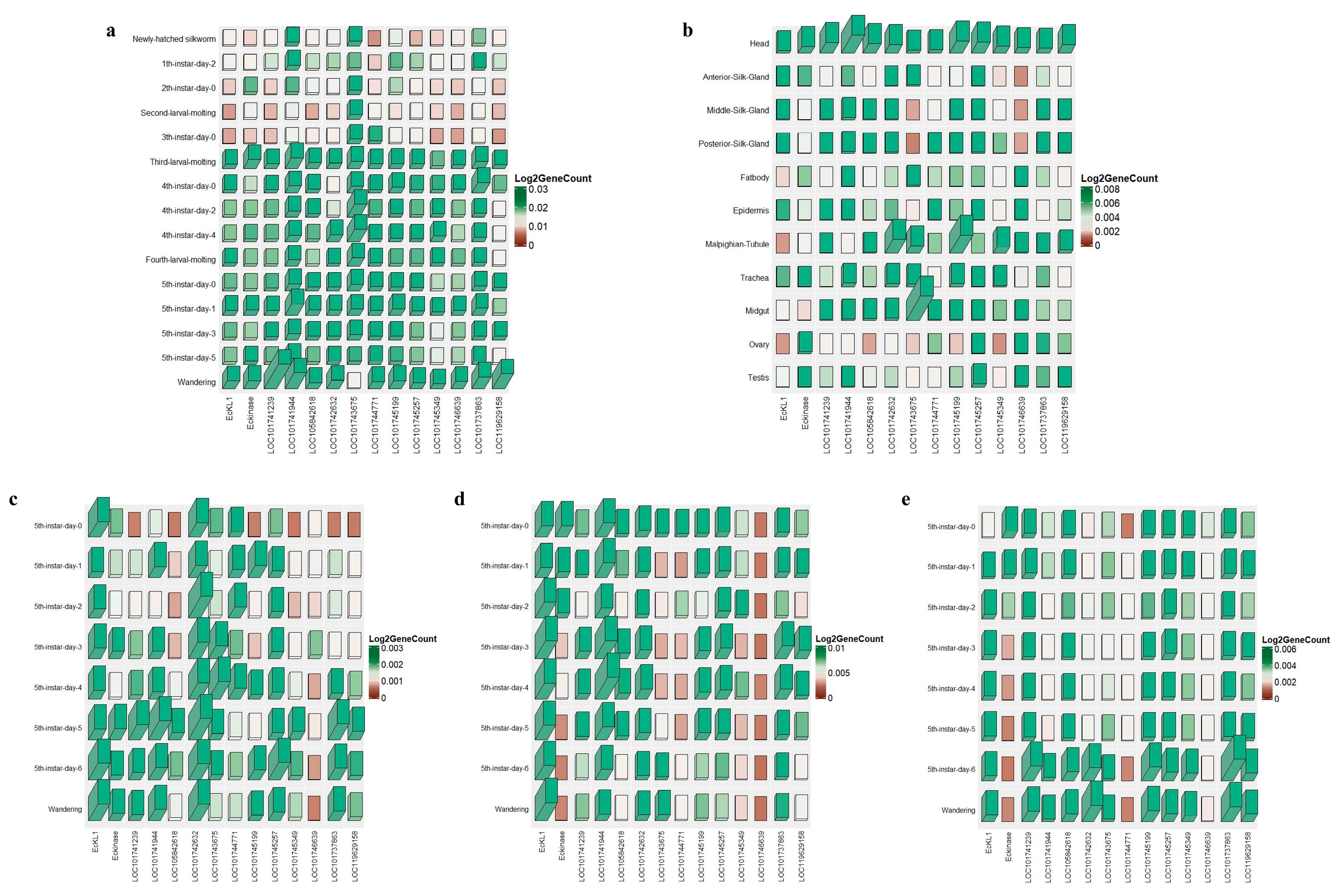
2.1. Expression Analysis of EcKL Genes in the Silkworm
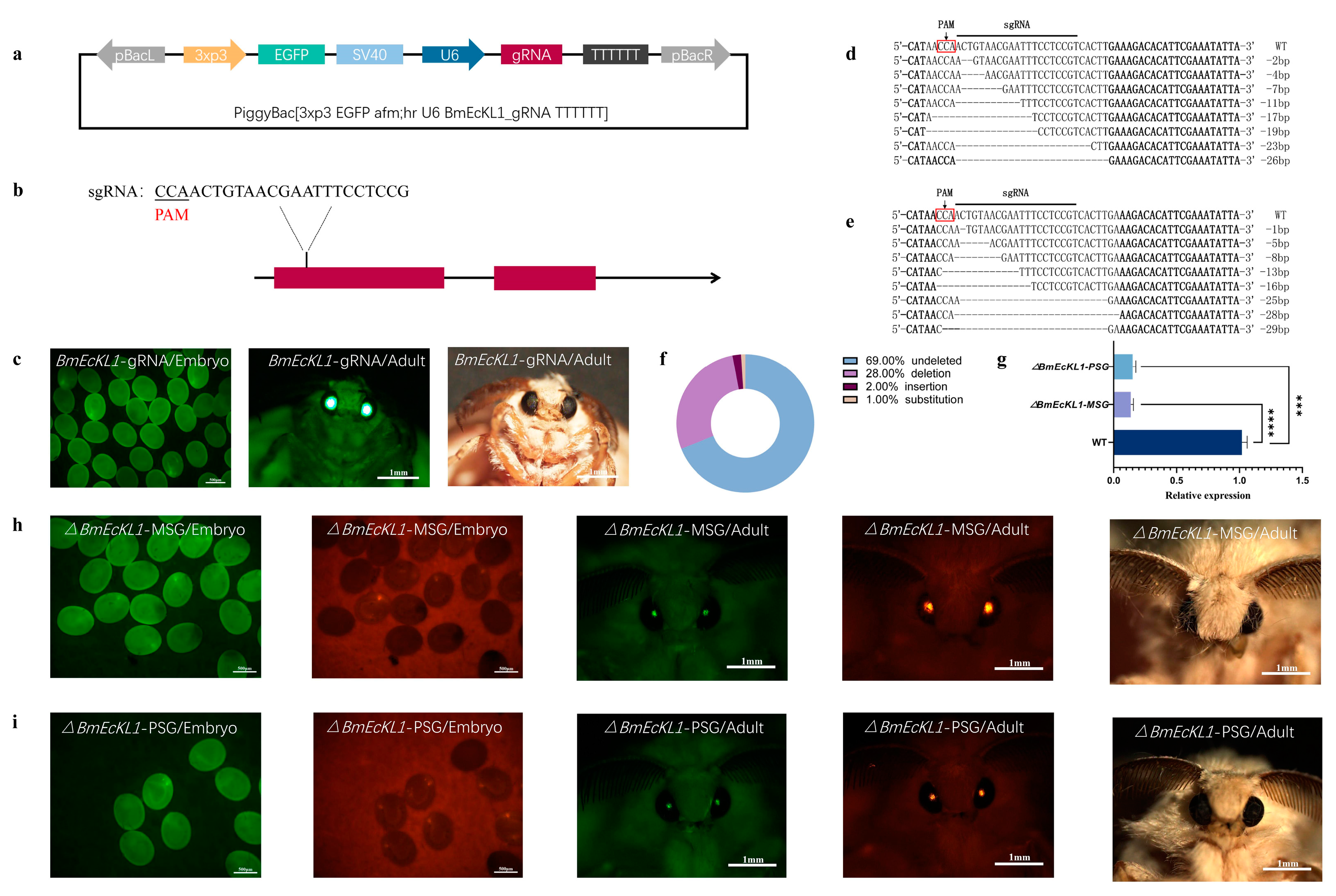
2.2. BmEcKL1 Knockout in MSG and PSG
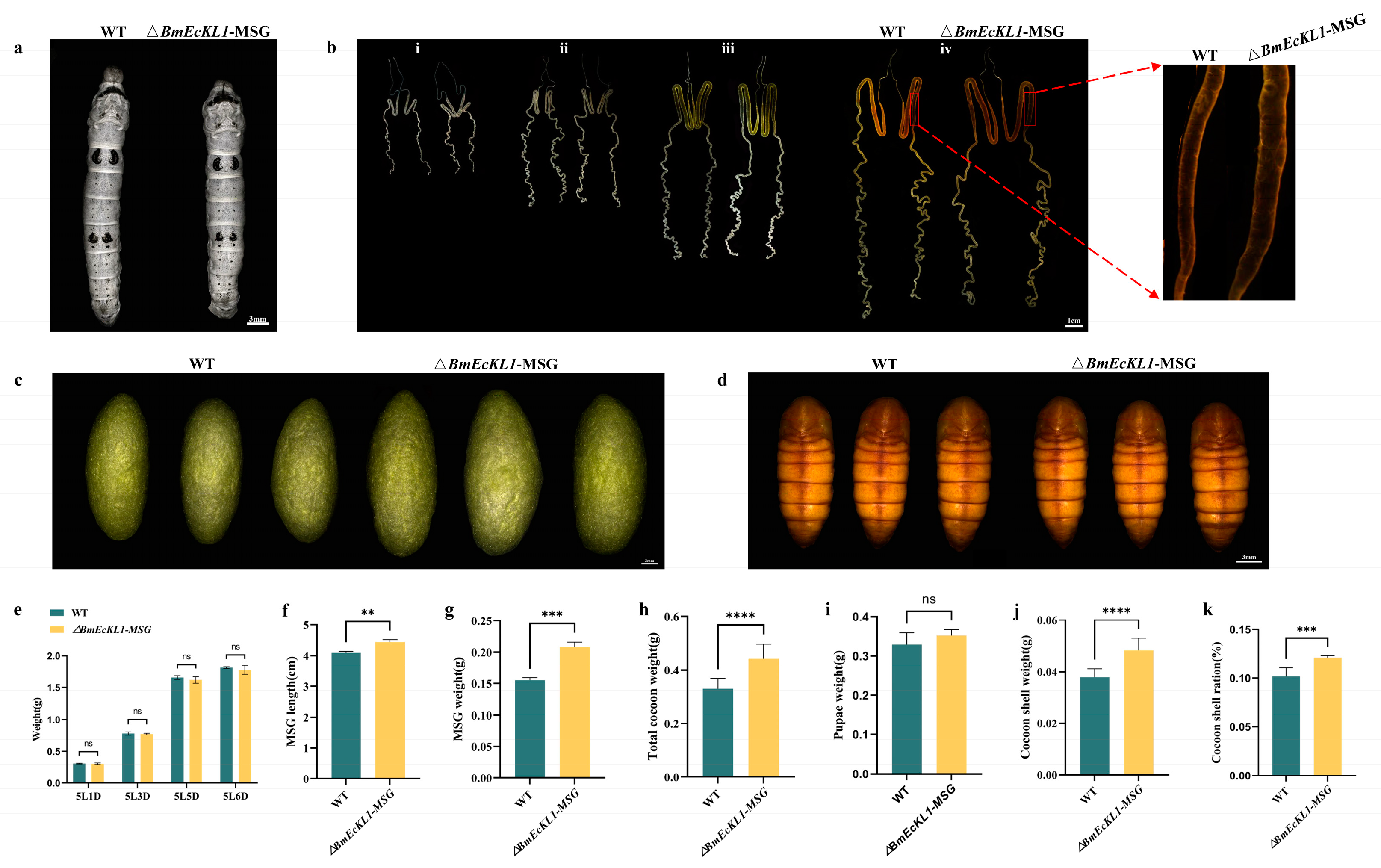
2.3. Deletion of BmEcKL1 Leads to MSG Abnormalities and Changes in Silk Protein Production
2.4. BmEcKL1 Knockout Leads to Enlarged PSG and Increased Silk Yield
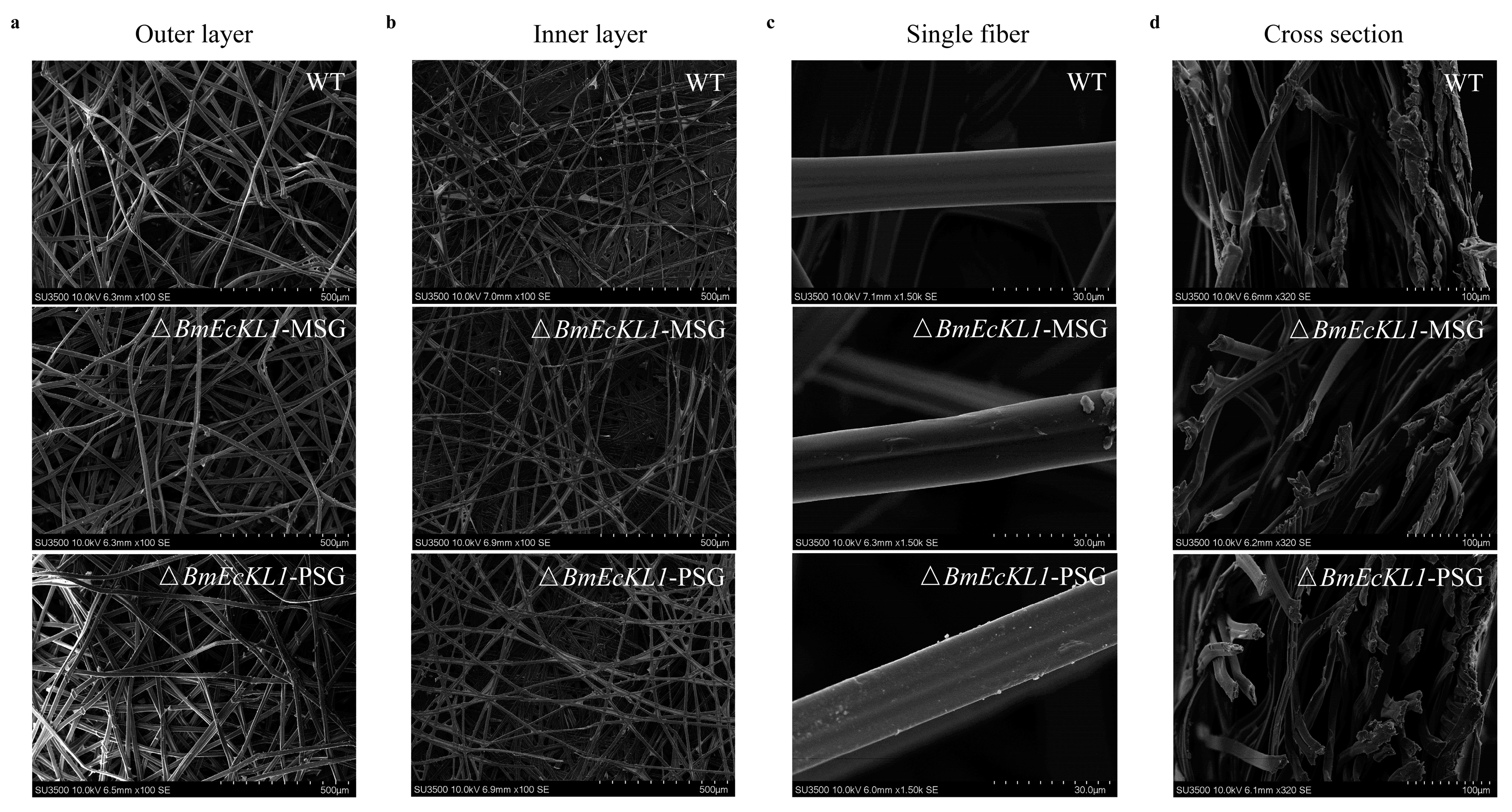
2.5. BmEcKL1 Knockout Alters Silk Protein Secretion
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Silkworm Rearing
4.3. RNA Extraction and RT-qPCR Validation
4.4. Vector Construction and Microinjection
4.5. Phenotype Statistics and Observation
4.6. Scanning Electron Microscopy
4.7. Statistics and Reproducibility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Q.Y.; Zhou, Z.Y.; Lu, C.; Cheng, D.J.; Dai, F.Y.; Li, B.; Zhao, P.; Zha, X.F.; Cheng, T.C.; Chai, C.L.; et al. A Draft Sequence for the Genome of the Domesticated Silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Wang, J.; Zhou, Z.Y.; Li, R.Q.; Fan, W.; Cheng, D.J.; Cheng, T.C.; Qin, J.J.; Duan, J.; Xu, H.F.; et al. The Genome of a Lepidopteran Model Insect, the Silkworm (Bombyx mori). Insect Biochem. Mol. Biol. 2008, 38, 1036–1045. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Guo, Y.R.; Zhang, Z.; Li, D.; Xuan, Z.L.; Li, Z.; Dai, F.Y.; Li, Y.R.; Cheng, D.J.; Li, R.Q.; et al. Complete Resequencing of 40 Genomes Reveals Domestication Events and Genes in Silkworm (Bombyx mori). Science 2009, 326, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.Y.; Cheng, D.J.; Duan, J.; Wang, G.H.; Cheng, T.C.; Zha, X.F.; Liu, C.; Zhao, P.; Dai, F.Y.; Zhang, Z.; et al. Microarray-Based Gene Expression Profiles in Multiple Tissues of the Domesticated Silkworm, Bombyx mori. Genome Biol. 2007, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhu, J.D.; Chen, Q.; Dai, F.Y.; Li, X.; Li, M.W.; Zhang, H.Y.; Zhang, G.J.; Li, D.; Dong, Y.; et al. Single Base-Resolution Methylome of the Silkworm Reveals a Sparse Epigenomic Map. Nat. Biotechnol. 2010, 28, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Daimon, T.; Kiuchi, T.; Takasu, Y. Recent Progress in Genome Engineering Techniques in the Silkworm (Bombyx mori). Dev. Growth Differ. 2014, 56, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Fujiwara, H. Electroporation-Mediated Somatic Transgenesis for Rapid Functional Analysis in Insects. Development 2013, 140, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, M.; Jouraku, A.; Toyoda, A.; Yokoi, K.; Minakuchi, Y.; Katsuma, S.; Fujiyama, A.; Kiuchi, T.; Yamamoto, K.; Shimada, T. High-Quality Genome Assembly of the Silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2019, 107, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Couble, P.; Michaille, J.J.; Garel, A.; Couble, M.L.; Prudhomme, J.C. Developmental Switches of Sericin Messenger-Rna Splicing in Individual Cells of Bombyx mori Silkgland. Dev. Biol. 1987, 124, 431–440. [Google Scholar] [CrossRef]
- Chevillard, M.; Couble, P.; Prudhomme, J.C. Complete Nucleotide-Sequence of the Gene Encoding the Bombyx mori Silk Protein-P25 and Predicted Amino-Acid-Sequence of the Protein. Nucleic Acids Res. 1986, 14, 6341–6342. [Google Scholar] [CrossRef]
- Sprague, K.U. Bombyx mori Silk Proteins—Characterization of Large Polypeptides. Biochemistry 1975, 14, 925–931. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kikuchi, Y.; Takagi, T.; Kikuchi, A.; Oyama, F.; Shimura, K.; Mizuno, S. Primary Structure of the Silk Fibroin Light Chain Determined by Cdna Sequencing and Peptide Analysis. J. Mol. Biol. 1989, 210, 127–139. [Google Scholar] [CrossRef]
- Edgar, B.A.; Orr-Weaver, T.L. Endoreplication Cell Cycles: More for Less. Cell 2001, 105, 297–306. [Google Scholar] [CrossRef]
- Perdrixgillot, S. DNA-Synthesis and Endomitoses in the Giant Nuclei of the Silkgland of Bombyx mori. Biochimie 1979, 61, 171–204. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Kakei, M.; Iwami, M.; Sakurai, S. Hormonal Regulation of the Death Commitment in Programmed Cell Death of the Silkworm Anterior Silk Glands. J. Insect Physiol. 2012, 58, 1575–1581. [Google Scholar] [CrossRef]
- Dhawan, S.; Gopinathan, K.P. Cell Cycle Events During the Development of the Silk Glands in the Mulberry Silkworm (Bombyx mori). Dev. Genes Evol. 2003, 213, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, B.; Gopinathan, K.P. Expression of Cyclin E in Endomitotic Silk-Gland Cells from Mulberry Silkworm. Gene 2000, 257, 77–85. [Google Scholar] [CrossRef]
- Kokubo, H.; Ueno, K.; Amanai, K.; Suzuki, Y. Involvement of the Bombyx mori Scr Gene in Development of the Embryonic Silk Gland. Dev. Biol. 1997, 186, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.D.; Li, F.F.; Chen, X.Y.; Huang, M.H.; Zhang, J.; Cui, H.J.; Pan, M.H.; Lu, C. DNA Replication Events During Larval Silk Gland Development in the Silkworm, Bombyx mori. J. Insect Physiol. 2012, 58, 974–978. [Google Scholar] [CrossRef]
- Chunduri, A.R.; Rajan, R.; Lima, A.; Ramamoorthy, S.; Mamillapalli, A. Dynamics of Nuclear Matrix Attachment Regions During 5th Instar Posterior Silk Gland Development in Bombyx mori. BMC Genom. 2022, 23, 247. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.R.; Luo, C.Y.; Pu, Q.; Yin, Q.; Xu, L.L.; Peng, X.Y.; Ma, S.Y.; Xia, Q.Y.; Liu, S.P. Let-7 Microrna Is a Critical Regulator in Controlling the Growth and Function of Silk Gland in the Silkworm. RNA Biol. 2020, 17, 703–717. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Huang, Q.X.; Zhao, C.Y.; Li, X.M.; Kan, Y.C.; Li, D.D. Long Noncoding Rna Lncr17454 Regulates Metamorphosis of Silkworm through Let-7 Mirna Cluster. J. Insect Sci. 2022, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Deng, X.J.; Yang, W.Y.; Huang, Z.J.; Tettamanti, G.; Cao, Y.; Feng, Q.L. Autophagy, Apoptosis, and Ecdysis-Related Gene Expression in the Silk Gland of the Silkworm (Bombyx mori) during Metamorphosis. Can. J. Zool. 2010, 88, 1169–1178. [Google Scholar] [CrossRef]
- Yi, H.Y.; Yang, W.Y.; Wu, W.M.; Li, X.X.; Deng, X.J.; Li, Q.R.; Cao, Y.; Zhong, Y.J.; Huang, Y.D. Bmcalpains Are Involved in Autophagy and Apoptosis During Metamorphosis and after Starvation in Bombyx mori. Insect Sci. 2018, 25, 379–388. [Google Scholar] [CrossRef]
- Li, G.L.; Li, Y.Z.; He, C.H.; Wei, Y.T.; Cai, K.P.; Lu, Q.Y.; Liu, X.B.; Zhu, Y.Z.; Xu, K.Z. The Promoting Effects of Pyriproxyfen on Autophagy and Apoptosis in Silk Glands of Non-Target Insect Silkworm, Bombyx mori. Pestic. Biochem. Physiol. 2023, 196, 105586. [Google Scholar] [CrossRef]
- Scanlan, J.L.; Gledhill-Smith, R.S.; Battlay, P.; Robin, C. Genomic and Transcriptomic Analyses in Drosophila Suggest That the Ecdysteroid Kinase-Like (Eckl) Gene Family Encodes the ‘Detoxification-by-Phosphorylation’ Enzymes of Insects. Insect Biochem. Mol. Biol. 2020, 123, 103429. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, J.L.; Battlay, P.; Robin, C. Ecdysteroid Kinase-Like (Eckl) Paralogs Confer Developmental Tolerance to Caffeine in Drosophila melanogaster. Curr. Res. Insect Sci. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.L.; Zheng, Y.; Xiong, E.J.; Li, J.J.; Yuan, L.L.; Yu, X.Q.; Wang, Y.F. Wolbachia-Induced Paternal Defect in Drosophila Is Likely by Interaction with the Juvenile Hormone Pathway. Insect Biochem. Mol. Biol. 2014, 49, 49–58. [Google Scholar] [CrossRef]
- Nozawa, M.; Minakuchi, Y.; Satomura, K.; Kondo, S.; Toyoda, A.; Tamura, K. Shared Evolutionary Trajectories of Three Independent Neo-Sex Chromosomes in Drosophila. Genome Res. 2021, 31, 2069–2079. [Google Scholar] [CrossRef]
- Sonobe, H.; Ohira, T.; Ieki, K.; Maeda, S.; Ito, Y.; Ajimura, M.; Mita, K.; Matsumoto, H.; Wilder, M.N. Purification, Kinetic Characterization, and Molecular Cloning of a Novel Enzyme, Ecdysteroid 22-Kinase. J. Biol. Chem. 2006, 281, 29513–29524. [Google Scholar] [CrossRef]
- Fujinaga, D.; Gu, J.J.; Kawahara, H.; Ogihara, M.H.; Kojima, I.; Takeshima, M.; Kataoka, H. Twenty-Hydroxyecdysone Produced by Dephosphorylation and Ecdysteroidogenesis Regulates Early Embryonic Development in the Silkmoth, Bombyx mori. Insect Biochem. Mol. Biol. 2020, 127, 103491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Zhang, Z.; Sun, W. Ecdysone Oxidase and 3-Dehydroecdysone-3β-Reductase Contribute to the Synthesis of Ecdysone During Early Embryonic Development of the Silkworm. Int. J. Biol. Sci. 2018, 14, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.P.; Zeng, W.H.; Tan, T.T.; Chen, T.; Luo, Q.; Qu, D.W.; Tang, Y.Y.; Long, D.P.; Xu, H.F. Insights into the Regulatory Characteristics of Silkworm Fibroin Gene Promoters Using a Modified Gal4/Uas System. Transgenic Res. 2019, 28, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Schoenfelder, S.; Fraser, P. Long-Range Enhancer-Promoter Contacts in Gene Expression Control. Nat. Rev. Genet. 2019, 20, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Bellés, X. When Inordinate Tissue Growth Is Beneficial: Improving Silk Production by Increasing Silk Gland Size. Cell Res. 2011, 21, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, H.F.; Zhu, J.Q.; Ma, S.Y.; Liu, Y.; Jiang, R.J.; Xia, Q.Y.; Li, S. Ras1 (Ca) Overexpression in the Posterior Silk Gland Improves Silk Yield. Cell Res. 2011, 21, 934–943. [Google Scholar] [CrossRef]
- Zhang, P.L.; Liu, S.M.; Song, H.S.; Zhang, G.Z.; Jia, Q.Q.; Li, S. Yorkie (Ca) Overexpression in the Posterior Silk Gland Improves Silk Yield in Bombyx mori. J. Insect Physiol. 2017, 100, 93–99. [Google Scholar] [CrossRef]
- Brenner, S. Phosphotransferase Sequence Homology. Nature 1987, 329, 21. [Google Scholar] [CrossRef]
- Bairoch, A.; Claverie, J.M. Sequence Patterns in Protein-Kinases. Nature 1988, 331, 22. [Google Scholar] [CrossRef]
- Hanks, S.K.; Hunter, T. Protein Kinases. 6. The Eukaryotic Protein-Kinase Superfamily—Kinase (Catalytic) Domain-Structure and Classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [CrossRef]
- Peisach, D.; Gee, P.; Kent, C.; Xu, Z.H. The Crystal Structure of Choline Kinase Reveals a Eukaryotic Protein Kinase Fold. Structure 2003, 11, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Kent, C. Identification of Critical Residues of Choline Kinase A2 from Caenorhabditis Elegans. J. Biol. Chem. 2004, 279, 17801–17809. [Google Scholar] [CrossRef] [PubMed]
- Burk, D.L.; Hon, W.C.; Leung, A.K.W.; Berghuis, A.M. Structural Analyses of Nucleotide Binding to an Aminoglycoside Phosphotransferase. Biochemistry 2001, 40, 8756–8764. [Google Scholar] [CrossRef] [PubMed]
- Beydon, P.; Girault, J.P.; Lafont, R. Ecdysone Metabolism in Pieris-Brassicae During the Feeding Last Larval Instar. Arch. Insect Biochem. Physiol. 1987, 4, 139–149. [Google Scholar] [CrossRef]
- Hou, S.H.; Sun, Y.; Wu, Y.C.; Cheng, T.C.; Liu, C. Bmsage Is Involved in the Determination of Cell Number in the Silk Gland of Bombyx mori. Insect Biochem. Mol. Biol. 2019, 113, 103205. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk Protein, Sericin, Inhibits Lipid Peroxidation and Tyrosinase Activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q. Applications of Natural Silk Protein Sericin in Biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-Chain, L-Chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, Z.Q.; Xu, J.; Zeng, B.S.; Ling, L.; You, L.; Chen, Y.Z.; Huang, Y.P.; Tan, A.J. The Crispr/Cas System Mediates Efficient Genome Engineering in Bombyx mori. Cell Res. 2013, 23, 1414–1416. [Google Scholar] [CrossRef]
- Takasu, Y.; Kobayashi, I.; Beumer, K.; Uchino, K.; Sezutsu, H.; Sajwan, S.; Carroll, D.; Tamura, T.; Zurovec, M. Targeted Mutagenesis in the Silkworm (Bombyx mori) Using Zinc Finger Nuclease Mrna Injection. Insect Biochem. Mol. Biol. 2010, 40, 759–765. [Google Scholar] [CrossRef]
- Gudmunds, E.; Wheat, C.W.; Khila, A.; Husby, A. Functional Genomic Tools for Emerging Model Species. Trends Ecol. Evol. 2022, 37, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Expasy. Available online: https://www.expasy.org/ (accessed on 10 February 2023).
- Interpro. Available online: http://www.ebi.ac.uk/interpro/ (accessed on 15 February 2023).
- Smart. Available online: http://smart.embl-heidelberg.de/ (accessed on 21 February 2023).
- Wang, G.H.; Xia, Q.Y.; Cheng, D.J.; Duan, J.; Zhao, P.; Chen, J.; Zhu, L. Reference Genes Identified in the Silkworm Bombyx mori During Metamorphism Based on Oligonucleotide Microarray and Confirmed by Qrt-Pcr. Insect Sci. 2008, 15, 405–413. [Google Scholar] [CrossRef]
- Cctop. Available online: https://www.cos.uni-heidelberg.de/en (accessed on 16 May 2022).
- Yusa, K. Piggybac Transposon. Microbiol. Spectr. 2015, 3, MDNA3-0028-2014. [Google Scholar] [CrossRef]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real Time Quantitative Pcr. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.; Jung, C.K.S.; Shackel, I.; Williams, P.M. Development and Validation of Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction for Monitoring Gene Expression in Cardiac Myocytes In Vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Lao, J.; Sun, Y.; Hua, X.; Lin, P.; Wang, F.; Shen, G.; Zhao, P.; Xia, Q. CRISPR/Cas9-Mediated Editing of BmEcKL1 Gene Sequence Affected Silk Gland Development of Silkworms (Bombyx mori). Int. J. Mol. Sci. 2024, 25, 1907. https://doi.org/10.3390/ijms25031907
Li S, Lao J, Sun Y, Hua X, Lin P, Wang F, Shen G, Zhao P, Xia Q. CRISPR/Cas9-Mediated Editing of BmEcKL1 Gene Sequence Affected Silk Gland Development of Silkworms (Bombyx mori). International Journal of Molecular Sciences. 2024; 25(3):1907. https://doi.org/10.3390/ijms25031907
Chicago/Turabian StyleLi, Shimin, Junjie Lao, Yue Sun, Xiaoting Hua, Ping Lin, Feng Wang, Guanwang Shen, Ping Zhao, and Qingyou Xia. 2024. "CRISPR/Cas9-Mediated Editing of BmEcKL1 Gene Sequence Affected Silk Gland Development of Silkworms (Bombyx mori)" International Journal of Molecular Sciences 25, no. 3: 1907. https://doi.org/10.3390/ijms25031907




