The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy
Abstract
1. Introduction
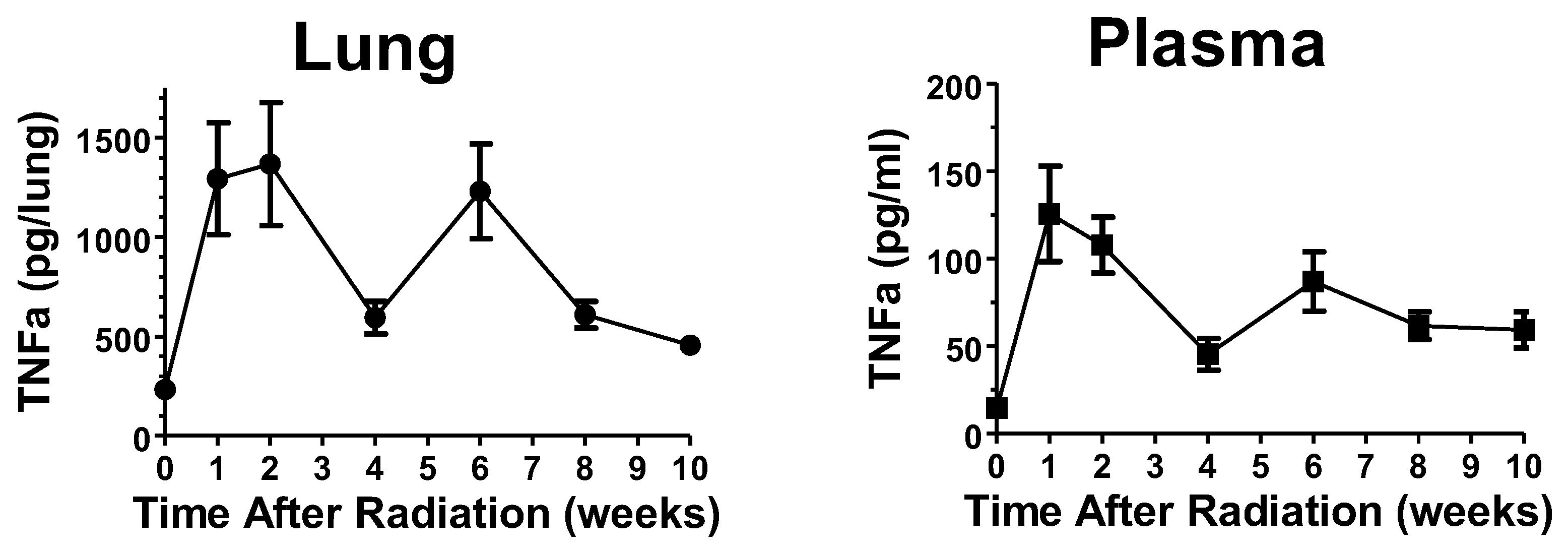
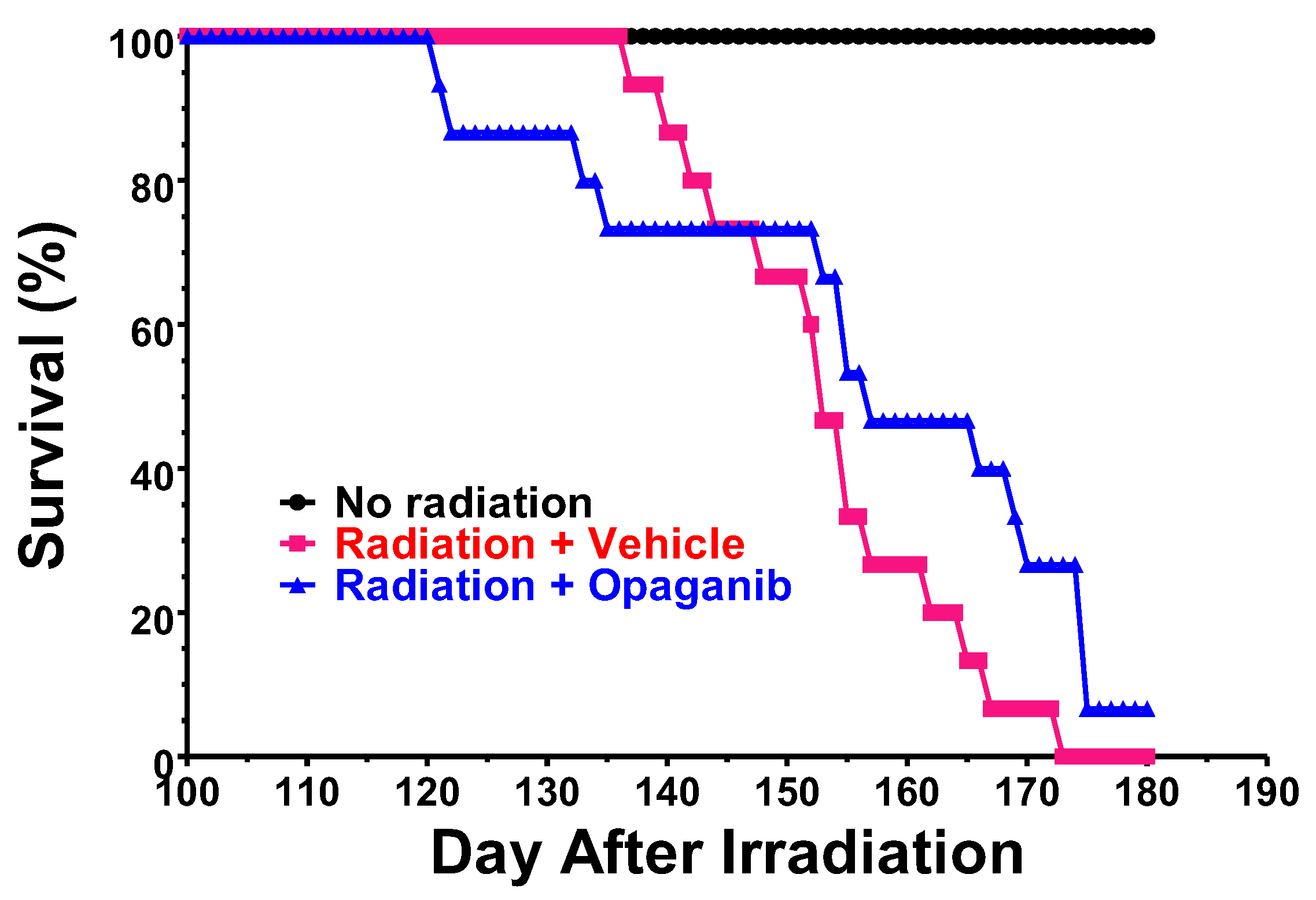
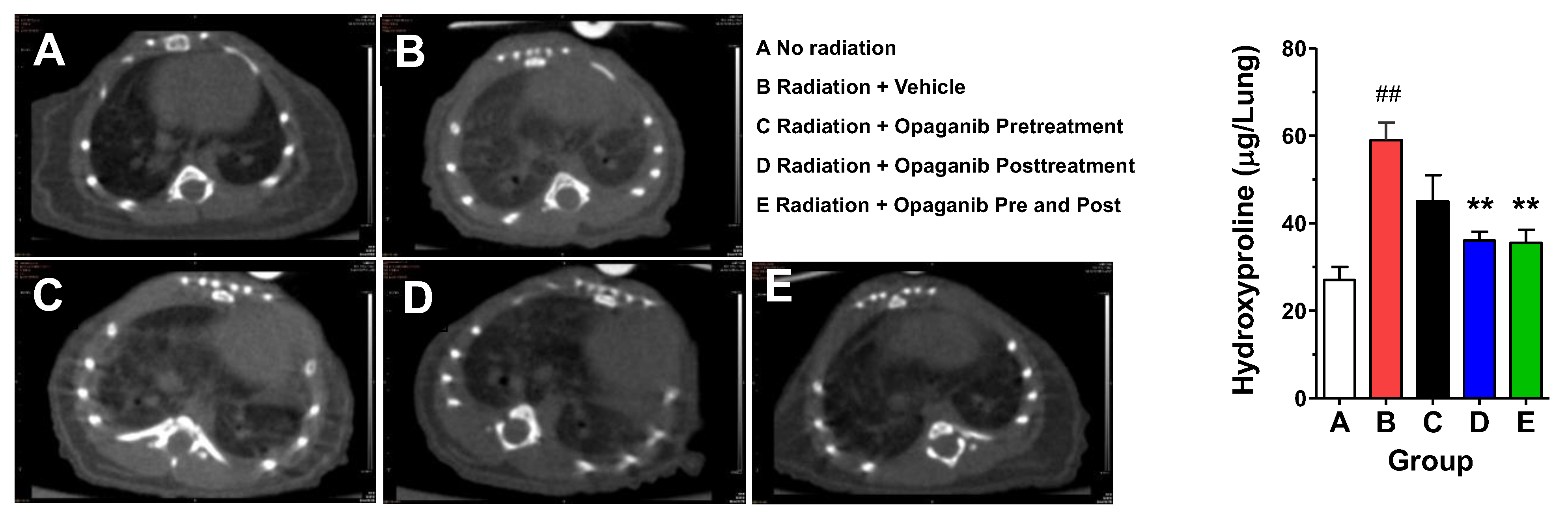
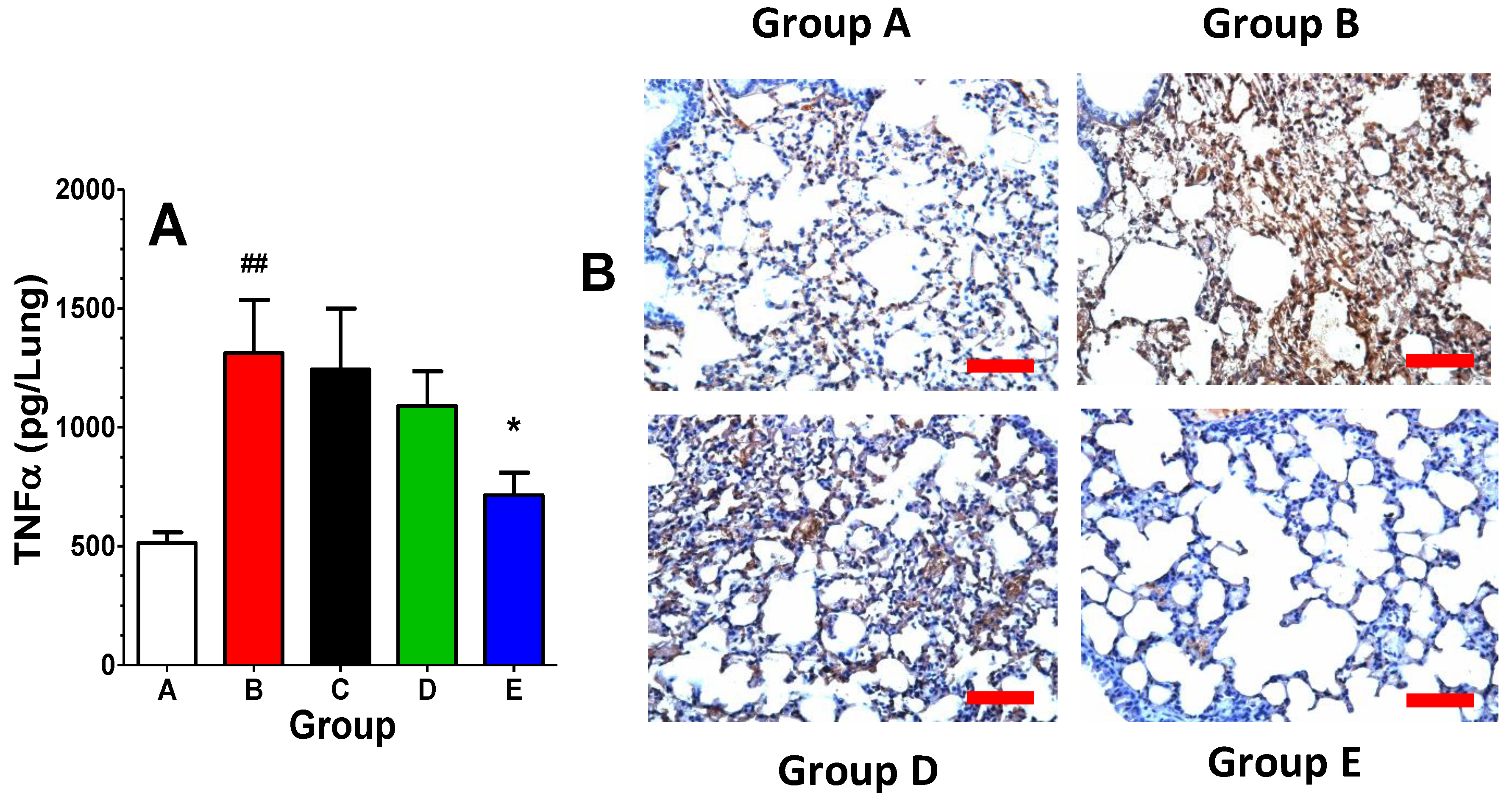
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Thoracic Irradiation Model
4.3. Total Body Irradiation with 5% Bone Marrow Shielding Model
4.4. MicroCT Imaging
4.5. Endpoint Analyses
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Cencetti, F.; Bernacchioni, C.; Vannuzzi, V.; Bruni, P. Role of sphingosine 1-phosphate signalling in tissue fibrosis. Cell. Signal. 2021, 78, 109861. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1beta or TNF-alpha Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, e0153118. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Lewis, C.S.; Voelkel-Johnson, C.; Smith, C.D. Targeting Sphingosine Kinases for the Treatment of Cancer. Adv. Cancer Res. 2018, 140, 295–325. [Google Scholar]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Agudelo, C.W.; Samaha, G.; Garcia-Arcos, I. Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 2020, 19, 122. [Google Scholar] [CrossRef]
- Jayant, G.; Kuperberg, S.; Somnay, K.; Wadgaonkar, R. The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis. Biomedicines 2023, 11, 1728. [Google Scholar] [CrossRef]
- Khan, S.A.; Goliwas, K.F.; Deshane, J.S. Sphingolipids in Lung Pathology in the Coronavirus Disease Era: A Review of Sphingolipid Involvement in the Pathogenesis of Lung Damage. Front. Physiol. 2021, 12, 760638. [Google Scholar] [CrossRef]
- Suryadevara, V.; Ramchandran, R.; Kamp, D.W.; Natarajan, V. Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4257. [Google Scholar] [CrossRef]
- Huang, L.S.; Natarajan, V. Sphingolipids in pulmonary fibrosis. Adv. Biol. Regul. 2015, 57, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.R. Sphingolipids as a Novel Therapeutic Target in Radiation-Induced Lung Injury. Cell Biochem. Biophys. 2021, 79, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Bindu, A.; Viswanathan, A.; Harikumar, K.B. Sphingosine 1-phosphate signaling during infection and immunity. Prog. Lipid Res. 2023, 92, 101251. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Maines, L.W.; Keller, S.N.; Katz Ben-Yair, V.; Fathi, R.; Plasse, T.F.; Levitt, M.L. Recent Progress in the Development of Opaganib for the Treatment of Covid-19. Drug Des. Dev. Ther. 2022, 16, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Markiewicz, M.; Yamanaka, M.; Bielawska, A.; Mao, C.; Obeid, L.M.; Hannun, Y.A.; Trojanowska, M. Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J. Biol. Chem. 2003, 278, 9276–9282. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Shegogue, D.; Pei, H.; Bu, S.; Bielawska, A.; Bielawski, J.; Pettus, B.; Hannun, Y.A.; Obeid, L.; Trojanowska, M. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 up-regulation. J. Biol. Chem. 2004, 279, 53994–54001. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Kapanadze, B.; Hsu, T.; Trojanowska, M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J. Biol. Chem. 2008, 283, 19593–19602. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Nincheri, P.; Donati, C.; Bruni, P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol. Cell 2010, 21, 1111–1124. [Google Scholar] [CrossRef]
- Yang, L.; Chang, N.; Liu, X.; Han, Z.; Zhu, T.; Li, C.; Yang, L.; Li, L. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am. J. Pathol. 2012, 181, 85–97. [Google Scholar] [CrossRef]
- Gencer, S.; Oleinik, N.; Kim, J.; Panneer Selvam, S.; De Palma, R.; Dany, M.; Nganga, R.; Thomas, R.J.; Senkal, C.E.; Howe, P.H.; et al. TGF-beta receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci. Signal. 2017, 10, eaam7464. [Google Scholar] [CrossRef]
- Nicholas, S.E.; Rowsey, T.G.; Priyadarsini, S.; Mandal, N.A.; Karamichos, D. Unravelling the interplay of sphingolipids and TGF-beta signaling in the human corneal stroma. PLoS ONE 2017, 12, e0182390. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, S.E.; Choi, A.J.; Lam, T.N.; Basu, S.K.; Mandal, N.; Karamichos, D. Potentiation of Sphingolipids and TGF-beta in the human corneal stroma reveals intricate signaling pathway crosstalks. Exp. Eye Res. 2023, 231, 109487. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Peterson, Y.K.; Smith, R.A.; Smith, C.D. Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS ONE 2012, 7, e44543. [Google Scholar] [CrossRef] [PubMed]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Sundaramoorthy, P.; Gasparetto, C.; Feinberg, D.; Fan, S.; Long, G.; Sellars, E.; Garrett, A.; Tuchman, S.A.; Reeves, B.N.; et al. Phase I study of opaganib, an oral sphingosine kinase 2-specific inhibitor, in relapsed and/or refractory multiple myeloma. Ann. Hematol. 2023, 102, 369–383. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Skolnick, A.W.; Rafiq, A.M.; Beegle, S.H.; Suszanski, J.; Koehne, G.; Barnett-Griness, O.; Bibliowicz, A.; Fathi, R.; Anderson, P.; et al. Opaganib in Coronavirus Disease 2019 Pneumonia: Results of a Randomized, Placebo-Controlled Phase 2a Trial. Open Forum Infect. Dis. 2022, 9, ofac232. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Berdyshev, E.V.; Bronova, I.A.; Liu, Y.; Tiruppathi, C.; Komarova, Y.; Benevolenskaya, E.V.; Suryadevara, V.; Ha, A.W.; Harijith, A.; et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax 2019, 74, 579–591. [Google Scholar] [CrossRef]
- Snider, A.J.; Ruiz, P.; Obeid, L.M.; Oates, J.C. Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS ONE 2013, 8, e53521. [Google Scholar] [CrossRef][Green Version]
- Shin, S.H.; Cho, K.A.; Hahn, S.; Lee, Y.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H.; Park, W.J.; Park, J.W. Inhibiting Sphingosine Kinase 2 Derived-sphingosine-1-phosphate Ameliorates Psoriasis-like Skin Disease via Blocking Th17 Differentiation of Naive CD4 T Lymphocytes in Mice. Acta Derm. Venereol. 2019, 99, 594–601. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, D.; Cao, K.; Ru, D.; Ren, J.; Rao, Z.; Chen, Y.; You, Q.; Dai, C.; Liu, L.; et al. Sphingosine kinase 2 cooperating with Fyn promotes kidney fibroblast activation and fibrosis via STAT3 and AKT. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3824–3836. [Google Scholar] [CrossRef]
- Maines, L.W.; Schrecengost, R.S.; Zhuang, Y.; Keller, S.N.; Smith, R.A.; Green, C.L.; Smith, C.D. Opaganib Protects against Radiation Toxicity: Implications for Homeland Security and Antitumor Radiotherapy. Int. J. Mol. Sci. 2022, 23, 13191. [Google Scholar] [CrossRef]
- Maines, L.W.; Fitzpatrick, L.R.; French, K.J.; Zhuang, Y.; Xia, Z.; Keller, S.N.; Upson, J.J.; Smith, C.D. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 2008, 53, 997–1012. [Google Scholar] [CrossRef]
- Liu, Q.; Rehman, H.; Shi, Y.; Krishnasamy, Y.; Lemasters, J.J.; Smith, C.D.; Zhong, Z. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS ONE 2012, 7, e41834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebenezer, D.L.; Fu, P.; Suryadevara, V.; Zhao, Y.; Natarajan, V. Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase. Adv. Biol. Regul. 2017, 63, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, D.L.; Fu, P.; Krishnan, Y.; Maienschein-Cline, M.; Hu, H.; Jung, S.; Madduri, R.; Arbieva, Z.; Harijith, A.; Natarajan, V. Genetic deletion of Sphk2 confers protection against Pseudomonas aeruginosa mediated differential expression of genes related to virulent infection and inflammation in mouse lung. BMC Genom. 2019, 20, 984. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Munoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [PubMed]
- Pyne, N.J.; Adams, D.R.; Pyne, S. Sphingosine Kinase 2 in Autoimmune/Inflammatory Disease and the Development of Sphingosine Kinase 2 Inhibitors. Trends Pharmacol. Sci. 2017, 38, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N. A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury. Int. J. Mol. Sci. 2022, 23, 4010. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gamble, J.R.; Rye, K.A.; Wang, L.; Hii, C.S.; Cockerill, P.; Khew-Goodall, Y.; Bert, A.G.; Barter, P.J.; Vadas, M.A. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 14196–14201. [Google Scholar] [CrossRef]
- Igarashi, J.; Bernier, S.G.; Michel, T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001, 276, 12420–12426. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Michel, T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J. Biol. Chem. 2001, 276, 36281–36288. [Google Scholar] [CrossRef]
- Pettus, B.J.; Bielawski, J.; Porcelli, A.M.; Reames, D.L.; Johnson, K.R.; Morrow, J.; Chalfant, C.E.; Obeid, L.M.; Hannun, Y.A. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003, 17, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Hauser, C.J. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J. Biol. Chem. 2003, 278, 27540–27547. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Buckley, A.; Chilvers, E.R.; Rossi, A.G.; Haslett, C.; Sethi, T. Sphingosine kinase: A point of convergence in the action of diverse neutrophil priming agents. J. Immunol. 2002, 169, 6394–6400. [Google Scholar] [CrossRef]
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2020, 154, 104170. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Furuya, H.; Ng, M.L.; Friedemann, M.; Menschikowski, M.; Tarasov, V.V.; Chubarev, V.N.; Klochkov, S.G.; Neganova, M.E.; Mangoni, A.A.; et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020, 207, 107464. [Google Scholar] [CrossRef]
- Wang, J.; Goren, I.; Yang, B.; Lin, S.; Li, J.; Elias, M.; Fiocchi, C.; Rieder, F. Review article: The sphingosine 1 phosphate/sphingosine 1 phosphate receptor axis—A unique therapeutic target in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2022, 55, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Wilfert, F.; Palm, J.; Konig, J.; Burdak-Rothkamm, S.; Liu, L.; Schuck, A.; Willich, N.; Rube, C. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlenther. Onkol. 2004, 180, 442–448. [Google Scholar] [CrossRef]
- Fleckenstein, K.; Zgonjanin, L.; Chen, L.; Rabbani, Z.; Jackson, I.L.; Thrasher, B.; Kirkpatrick, J.; Foster, W.M.; Vujaskovic, Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 196–204. [Google Scholar] [CrossRef]
- Carvalho Neuenschwander, F.; Barnett-Griness, O.; Piconi, S.; Maor, Y.; Sprinz, E.; Assy, N.; Khmelnitskiy, O.; Lomakin, N.; Goloshchekin, B.M.; Nahorecka, E.; et al. Effect of Opaganib on Supplemental Oxygen and Mortality in Patients with Severe SARS-CoV-2 Pneumonia. medRxiv 2022. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R. Novel Pharmacological Approaches for Inflammatory Bowel Disease: Targeting Key Intracellular Pathways and the IL-23/IL-17 Axis. Int. J. Inflam. 2012, 2012, 389404. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed]

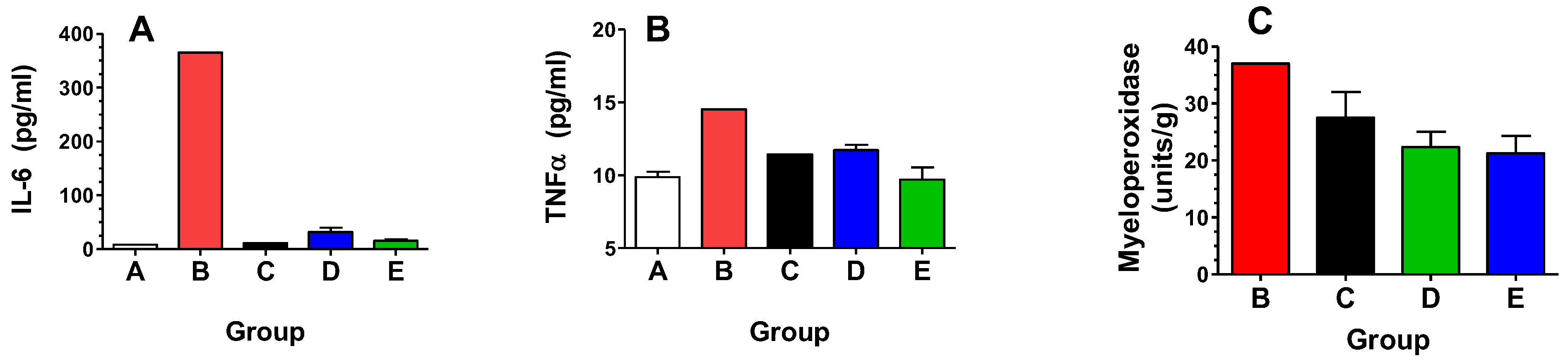
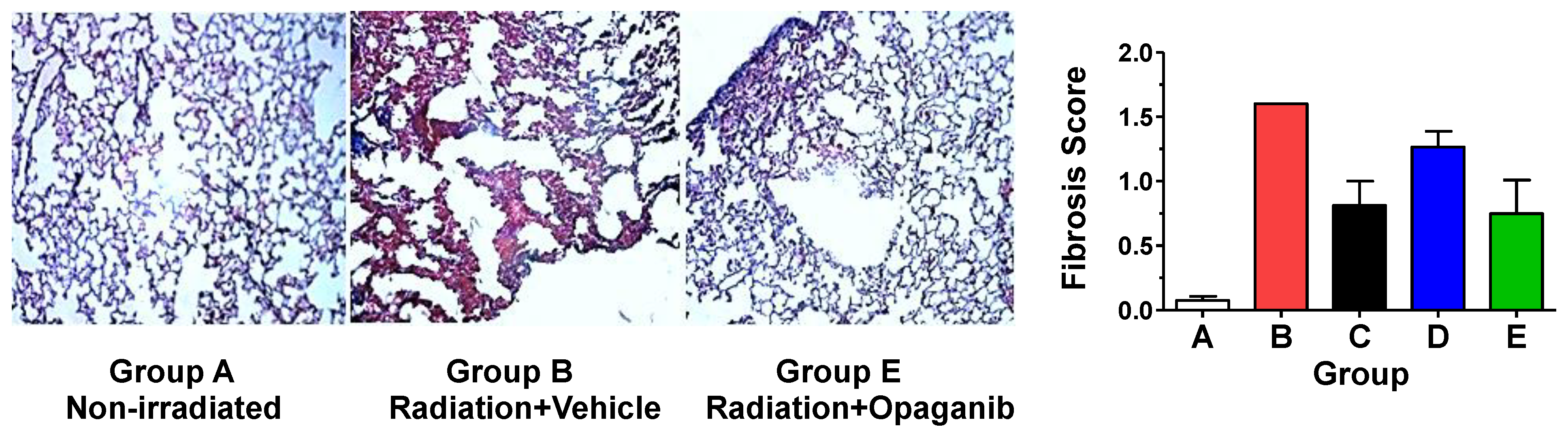
| Group | Radiation (Gy) | Opaganib (mg/kg) | Treatment Schedule |
|---|---|---|---|
| A | 0 | 0 | Vehicle at 2 h before radiation |
| B | 15 | 0 | Vehicle at 2 h before radiation |
| C | 15 | 100 | Single dose at 2 h before radiation |
| D | 15 | 100 | Daily doses on Days 14–18 and 21, 22, and 24 |
| E | 15 | 100 | Single dose at 2 h before radiation, followed by daily doses on Day 14–18 and 21, 22, and 24 |
| Group | Radiation (Gy) | Opaganib (mg/kg) | Treatment Schedule |
|---|---|---|---|
| A | 0 | 0 | Vehicle at 24 h after radiation |
| B | 14 | 0 | Vehicle at 24 h after radiation |
| C | 14 | 100 | Single dose at 24 h after radiation |
| D | 14 | 100 | Once daily for 5 days, beginning 24 h after radiation |
| E | 14 | 100 | Three times per week for 6 weeks, beginning 24 h after radiation |
| Group | Radiation (Gy) | Opaganib (mg/kg) | Treatment Schedule |
|---|---|---|---|
| A | 0 | 0 | Vehicle |
| B | 15.25 | 0 | Vehicle |
| C | 15.25 | 100 | Two times daily for 3 days, beginning at 24 h after radiation |
| D | 15.25 | 100 | Two times daily for 3 days, beginning at 24 h after radiation, and then once daily on Day 31–45. |
| E | 15.25 | 100 | Once daily on Day 31–45 after radiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maines, L.W.; Keller, S.N.; Smith, R.A.; Green, C.L.; Smith, C.D. The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy. Int. J. Mol. Sci. 2024, 25, 2322. https://doi.org/10.3390/ijms25042322
Maines LW, Keller SN, Smith RA, Green CL, Smith CD. The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy. International Journal of Molecular Sciences. 2024; 25(4):2322. https://doi.org/10.3390/ijms25042322
Chicago/Turabian StyleMaines, Lynn W., Staci N. Keller, Ryan A. Smith, Cecelia L. Green, and Charles D. Smith. 2024. "The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy" International Journal of Molecular Sciences 25, no. 4: 2322. https://doi.org/10.3390/ijms25042322
APA StyleMaines, L. W., Keller, S. N., Smith, R. A., Green, C. L., & Smith, C. D. (2024). The Sphingolipid-Modulating Drug Opaganib Protects against Radiation-Induced Lung Inflammation and Fibrosis: Potential Uses as a Medical Countermeasure and in Cancer Radiotherapy. International Journal of Molecular Sciences, 25(4), 2322. https://doi.org/10.3390/ijms25042322





