Cerebral Glucose Metabolism following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review
Abstract
:1. Introduction
2. Glucose Metabolism in the Healthy Brain
2.1. Transport
2.2. Different Routes of Glucose Metabolism
3. Alterations in Plasma Glucose following TBI
3.1. Plasma Glucose Levels following TBI
3.2. Mechanisms of Hyperglycaemia in TBI
3.2.1. Stress Response
3.2.2. Diabetes
3.2.3. Inflammation
3.2.4. Iatrogenic Causes
3.3. Relationship between Plasma Glucose and Cerebral Glucose Levels
3.4. Glucose Lowering Treatments
3.4.1. Insulin
3.4.2. Glucagon-like Peptide-1 Analogues
3.5. Future Research
4. Alterations in Glucose Transport following TBI
4.1. Glucose Transporters
4.1.1. GLUT
4.1.2. Sodium-Glucose Transporters (SGLT)
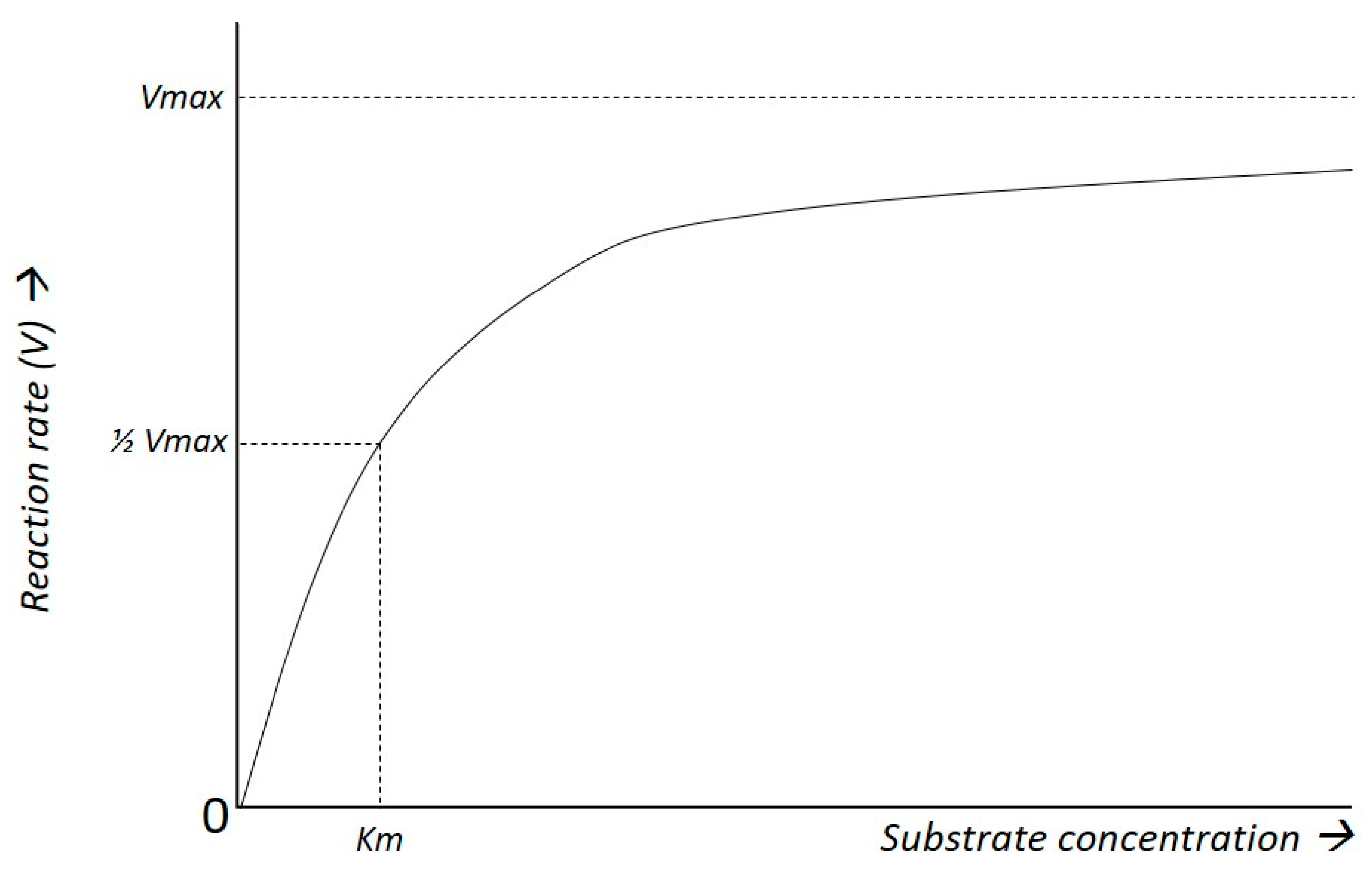
4.2. Kinetics of Glucose Transport
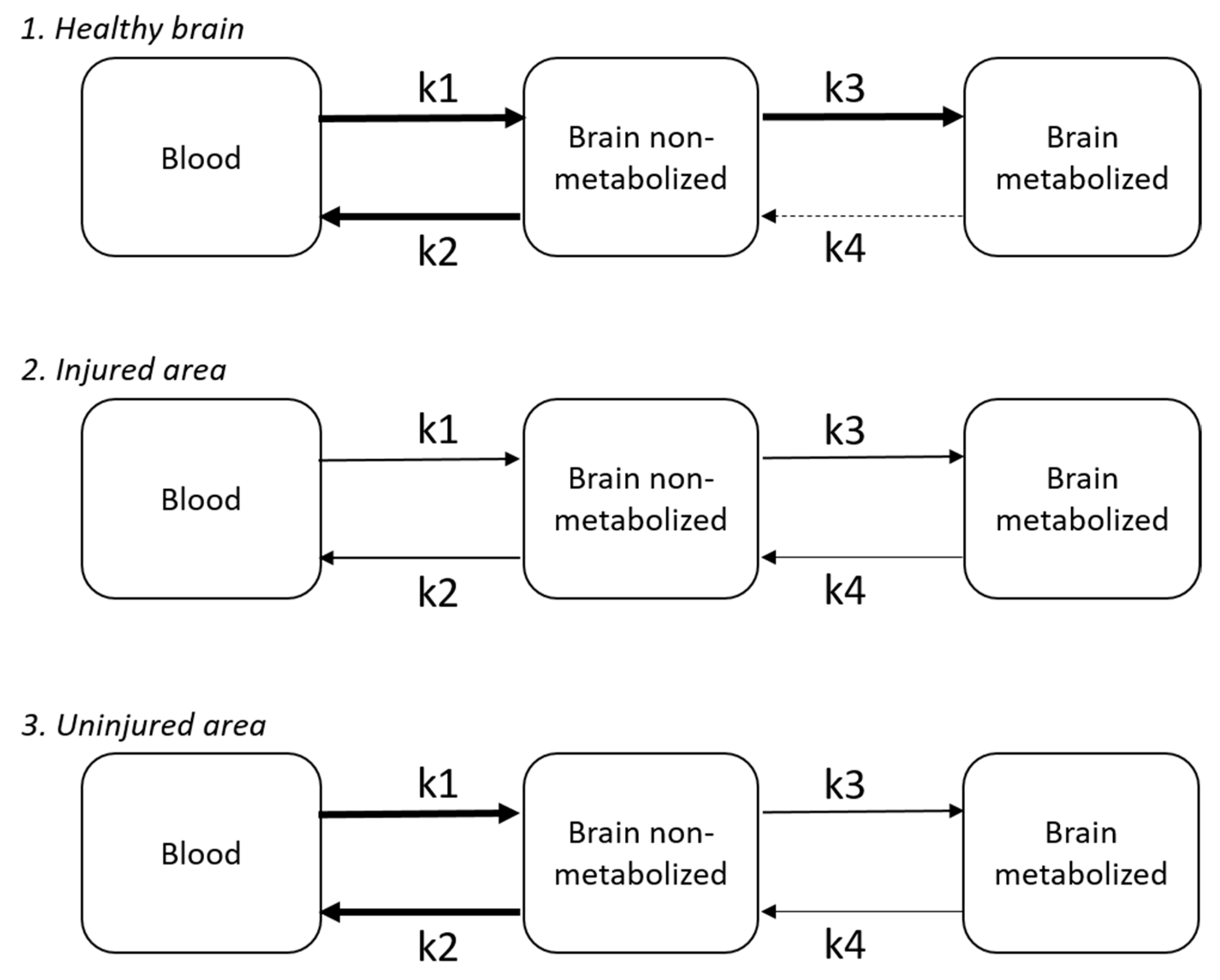
4.3. BBB Disruption
4.4. Future Research
5. Alterations in Cerebral Glucose Metabolism following TBI
5.1. Temporal Changes in Cerebral Glucose Metabolism
5.2. Changes in Alternative Pathways to Glycolysis
5.2.1. The Pentose Phosphate Pathway (PPP)
5.2.2. Lactate
5.2.3. Glycogen
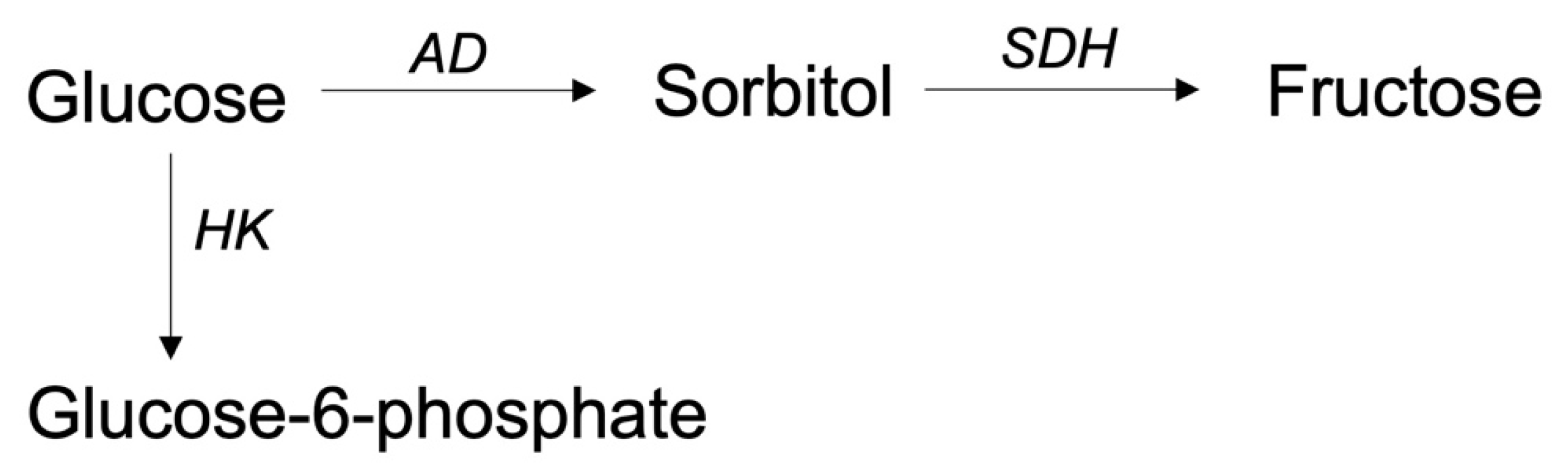
5.2.4. The Polyol Pathway
5.3. Future Research
6. Clinical Aspects of Cerebral Glucose Metabolism in TBI
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Joannides, A.; Adeleye, A.O.; Bajamal, A.H.; Bashford, T.; Biluts, H.; Budohoski, K.; Ercole, A.; Fernandez-Mendez, R.; Figaji, A.; et al. Casemix, management, and mortality of patients rreseceiving emergency neurosurgery for traumatic brain injury in the Global Neurotrauma Outcomes Study: A prospective observational cohort study. Lancet Neurol. 2022, 21, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Dijkland, S.A.; Helmrich, I.; Nieboer, D.; van der Jagt, M.; Dippel, D.W.J.; Menon, D.K.; Stocchetti, N.; Maas, A.I.R.; Lingsma, H.F.; Steyerberg, E.W.; et al. Outcome Prediction after Moderate and Severe Traumatic Brain Injury: External Validation of Two Established Prognostic Models in 1742 European Patients. J. Neurotrauma 2021, 38, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Leichtle, S.W.; Sarma, A.K.; Strein, M.; Yajnik, V.; Rivet, D.; Sima, A.; Brophy, G.M. High-Dose Intravenous Ascorbic Acid: Ready for Prime Time in Traumatic Brain Injury? Neurocrit. Care 2020, 32, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Demers-Marcil, S.; Coles, J.P. Cerebral metabolic derangements following traumatic brain injury. Curr. Opin. Anaesthesiol. 2022, 35, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dong, B.; Mao, Y.; Guan, W.; Cao, J.; Zhu, R.; Wang, S. Review: Traumatic brain injury and hyperglycemia, a potentially modifiable risk factor. Oncotarget 2016, 7, 71052–71061. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef]
- Benarroch, E.E. Brain glucose transporters: Implications for neurologic disease. Neurology 2014, 82, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Obel, L.F.; Muller, M.S.; Walls, A.B.; Sickmann, H.M.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A. Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenerg. 2012, 4, 3. [Google Scholar] [CrossRef]
- Suades, A.; Qureshi, A.; McComas, S.E.; Coincon, M.; Rudling, A.; Chatzikyriakidou, Y.; Landreh, M.; Carlsson, J.; Drew, D. Establishing mammalian GLUT kinetics and lipid composition influences in a reconstituted-liposome system. Nat. Commun. 2023, 14, 4070. [Google Scholar] [CrossRef]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007, 27, 1766–1791. [Google Scholar] [CrossRef]
- Vemula, S.; Roder, K.E.; Yang, T.; Bhat, G.J.; Thekkumkara, T.J.; Abbruscato, T.J. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J. Pharmacol. Exp. Ther. 2009, 328, 487–495. [Google Scholar] [CrossRef]
- Brooks, G.A.; Martin, N.A. Cerebral metabolism following traumatic brain injury: New discoveries with implications for treatment. Front. Neurosci. 2014, 8, 408. [Google Scholar] [CrossRef]
- Patet, C.; Suys, T.; Carteron, L.; Oddo, M. Cerebral Lactate Metabolism After Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 31. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Schonfeld, P.; Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef]
- van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Rovlias, A.; Kotsou, S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000, 46, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Lianos, G.; Fotakopoulos, G.; Michos, E.; Pachatouridis, D.; Voulgaris, S. Admission glucose and coagulopathy occurrence in patients with traumatic brain injury. Brain Inj. 2014, 28, 438–441. [Google Scholar] [CrossRef]
- Lam, A.M.; Winn, H.R.; Cullen, B.F.; Sundling, N. Hyperglycemia and neurological outcome in patients with head injury. J. Neurosurg. 1991, 75, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Ott, L.; Dempsey, R.; Haack, D.; Tibbs, P. Relationship between admission hyperglycemia and neurologic outcome of severely brain-injured patients. Ann. Surg. 1989, 210, 466–472; discussion 463–472. [Google Scholar] [CrossRef] [PubMed]
- Jeremitsky, E.; Omert, L.A.; Dunham, C.M.; Wilberger, J.; Rodriguez, A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma 2005, 58, 47–50. [Google Scholar] [CrossRef]
- Pin-On, P.; Saringkarinkul, A.; Punjasawadwong, Y.; Kacha, S.; Wilairat, D. Serum electrolyte imbalance and prognostic factors of postoperative death in adult traumatic brain injury patients: A prospective cohort study. Medicine 2018, 97, e13081. [Google Scholar] [CrossRef]
- Salim, A.; Hadjizacharia, P.; Dubose, J.; Brown, C.; Inaba, K.; Chan, L.S.; Margulies, D. Persistent hyperglycemia in severe traumatic brain injury: An independent predictor of outcome. Am. Surg. 2009, 75, 25–29. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Howells, T.; Ronne-Engstrom, E.; Hillered, L.; Lewen, A.; Enblad, P.; Rostami, E. High Arterial Glucose is Associated with Poor Pressure Autoregulation, High Cerebral Lactate/Pyruvate Ratio and Poor Outcome Following Traumatic Brain Injury. Neurocrit. Care 2019, 31, 526–533. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, H.; Xu, Y.; Wu, X.; Sun, Y.; Hu, J. Continuous measurement of the cumulative amplitude and duration of hyperglycemia best predicts outcome after traumatic brain injury. Neurocrit. Care 2014, 20, 69–76. [Google Scholar] [CrossRef]
- Gearhart, M.M.; Parbhoo, S.K. Hyperglycemia in the critically ill patient. AACN Clin. Issues 2006, 17, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Hacohen, N.; Golub, T.R.; Van Parijs, L.; Lodish, H.F. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: Nuclear factor-κB activation by TNF-α is obligatory. Diabetes 2002, 51, 1319–1336. [Google Scholar] [CrossRef]
- Ley, E.J.; Srour, M.K.; Clond, M.A.; Barnajian, M.; Tillou, A.; Mirocha, J.; Salim, A. Diabetic patients with traumatic brain injury: Insulin deficiency is associated with increased mortality. J. Trauma 2011, 70, 1141–1144. [Google Scholar] [CrossRef]
- Liou, D.Z.; Singer, M.B.; Barmparas, G.; Harada, M.Y.; Mirocha, J.; Bukur, M.; Salim, A.; Ley, E.J. Insulin-dependent diabetes and serious trauma. Eur. J. Trauma Emerg. Surg. 2016, 42, 491–496. [Google Scholar] [CrossRef]
- Bosarge, P.L.; Shoultz, T.H.; Griffin, R.L.; Kerby, J.D. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J. Trauma Acute Care Surg. 2015, 79, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kerby, J.D.; Griffin, R.L.; MacLennan, P.; Rue, L.W., III. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann. Surg. 2012, 256, 446–452. [Google Scholar] [CrossRef]
- Plummer, M.P.; Finnis, M.E.; Horsfall, M.; Ly, M.; Kar, P.; Abdelhamid, Y.A.; Deane, A.M. Prior exposure to hyperglycaemia attenuates the relationship between glycaemic variability during critical illness and mortality. Crit. Care Resusc. 2016, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kajbaf, F.; Mojtahedzadeh, M.; Abdollahi, M. Mechanisms underlying stress-induced hyperglycemia in critically ill patients. Clin. Pract. 2007, 4, 97. [Google Scholar] [CrossRef]
- Duggan, E.W.; Carlson, K.; Umpierrez, G.E. Perioperative Hyperglycemia Management: An Update. Anesthesiology 2017, 126, 547–560. [Google Scholar] [CrossRef]
- Rehman, H.U.; Mohammed, K. Perioperative management of diabetic patients. Curr. Surg. 2003, 60, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; He, Y.; Zhou, C.; Zheng, Q.; Chen, C.; Liang, P. Impact of total intravenous anesthesia and total inhalation anesthesia as the anesthesia maintenance approaches on blood glucose level and postoperative complications in patients with type 2 diabetes mellitus: A double-blind, randomized controlled trial. BMC Anesthesiol. 2023, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.L.; Morrison, D.; Llano, A.; Sainsbury, C.A.R.; Jones, G.C. Practical Guide to Glucocorticoid Induced Hyperglycaemia and Diabetes. Diabetes Ther. 2023, 14, 937–945. [Google Scholar] [CrossRef]
- Nigrovic, L.E.; Kimia, A.A.; Shah, S.S.; Neuman, M.I. Relationship between cerebrospinal fluid glucose and serum glucose. N. Engl. J. Med. 2012, 366, 576–578. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Leen, W.G.; Willemsen, M.A.; Slats, D.; Claassen, J.A. Hourly analysis of cerebrospinal fluid glucose shows large diurnal fluctuations. J. Cereb. Blood Flow Metab. 2016, 36, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Parejo, P.; Stahl, N.; Xu, W.; Reinstrup, P.; Ungerstedt, U.; Nordstrom, C.H. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med. 2003, 29, 544–550. [Google Scholar] [CrossRef]
- Magnoni, S.; Tedesco, C.; Carbonara, M.; Pluderi, M.; Colombo, A.; Stocchetti, N. Relationship between systemic glucose and cerebral glucose is preserved in patients with severe traumatic brain injury, but glucose delivery to the brain may become limited when oxidative metabolism is impaired: Implications for glycemic control. Crit. Care Med. 2012, 40, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Bellander, B.M. Monitoring of glucose in brain, adipose tissue, and peripheral blood in patients with traumatic brain injury: A microdialysis study. J. Diabetes Sci. Technol. 2011, 5, 596–604. [Google Scholar] [CrossRef]
- Hermanides, J.; Hong, Y.T.; Trivedi, M.; Outtrim, J.; Aigbirhio, F.; Nestor, P.J.; Guilfoyle, M.; Winzeck, S.; Newcombe, V.F.J.; Das, T.; et al. Metabolic derangements are associated with impaired glucose delivery following traumatic brain injury. Brain 2021, 144, 3492–3504. [Google Scholar] [CrossRef]
- Nice-Sugar Study Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group; Canadian Critical Care Trials Group. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: Long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med. 2015, 41, 1037–1047. [Google Scholar] [CrossRef]
- Gunst, J.; Debaveye, Y.; Guiza, F.; Dubois, J.; De Bruyn, A.; Dauwe, D.; De Troy, E.; Casaer, M.P.; De Vlieger, G.; Haghedooren, R.; et al. Tight Blood-Glucose Control without Early Parenteral Nutrition in the ICU. N. Engl. J. Med. 2023, 389, 1180–1190. [Google Scholar] [CrossRef]
- Hermanides, J.; Plummer, M.P.; Finnis, M.; Deane, A.M.; Coles, J.P.; Menon, D.K. Glycaemic control targets after traumatic brain injury: A systematic review and meta-analysis. Crit Care 2018, 22, 11. [Google Scholar] [CrossRef]
- Plummer, M.P.; Notkina, N.; Timofeev, I.; Hutchinson, P.J.; Finnis, M.E.; Gupta, A.K. Cerebral metabolic effects of strict versus conventional glycaemic targets following severe traumatic brain injury. Crit. Care 2018, 22, 16. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Drucker, D.J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 262–269. [Google Scholar] [CrossRef]
- Li, Y.; Bader, M.; Tamargo, I.; Rubovitch, V.; Tweedie, D.; Pick, C.G.; Greig, N.H. Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J. Neurochem. 2015, 135, 1203–1217. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, T.; Cheng, S.; Zhang, S. Glucagon-like peptide-1 receptor agonist Exendin-4 improves neurological outcomes by attenuating TBI-induced inflammatory responses and MAPK activation in rats. Int. Immunopharmacol. 2020, 86, 106715. [Google Scholar] [CrossRef] [PubMed]
- Glotfelty, E.J.; Delgado, T.; Tovar, Y.R.L.B.; Luo, Y.; Hoffer, B.; Olson, L.; Karlsson, T.; Mattson, M.P.; Harvey, B.; Tweedie, D.; et al. Incretin Mimetics as Rational Candidates for the Treatment of Traumatic Brain Injury. ACS Pharmacol. Transl. Sci. 2019, 2, 66–91. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Hakon, J.; Ruscher, K.; Romner, B.; Tomasevic, G. Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury. PLoS ONE 2015, 10, e0120074. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wang, J.; Zhu, M.; Yao, Y.; Liu, J. Hypoxia preconditioning protects neuronal cells against traumatic brain injury through stimulation of glucose transport mediated by HIF-1α/GLUTs signaling pathway in rat. Neurosurg. Rev. 2021, 44, 411–422. [Google Scholar] [CrossRef]
- Zhou, J.; Burns, M.P.; Huynh, L.; Villapol, S.; Taub, D.D.; Saavedra, J.M.; Blackman, M.R. Temporal Changes in Cortical and Hippocampal Expression of Genes Important for Brain Glucose Metabolism Following Controlled Cortical Impact Injury in Mice. Front. Endocrinol. 2017, 8, 231. [Google Scholar] [CrossRef]
- Hamlin, G.P.; Cernak, I.; Wixey, J.A.; Vink, R. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J. Neurotrauma 2001, 18, 1011–1018. [Google Scholar] [CrossRef]
- Cornford, E.M.; Hyman, S.; Cornford, M.E.; Caron, M.J. Glut1 glucose transporter activity in human brain injury. J. Neurotrauma 1996, 13, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflug. Arch. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Oerter, S.; Forster, C.; Bohnert, M. Validation of sodium/glucose cotransporter proteins in human brain as a potential marker for temporal narrowing of the trauma formation. Int. J. Legal Med. 2019, 133, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Huang, S.C.; Wu, H.M.; Liao, W.; Glenn, T.C.; Vespa, P.M.; Phelps, M.E.; Hovda, D.A.; Bergsneider, M. Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J. Nucl. Med. 2004, 45, 775–783. [Google Scholar] [PubMed]
- Gruetter, R.; Ugurbil, K.; Seaquist, E.R. Steady-state cerebral glucose concentrations and transport in the human brain. J. Neurochem. 1998, 70, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Rodriguez-Grande, B.; Ichkova, A.; Lemarchant, S.; Badaut, J. Early to Long-Term Alterations of CNS Barriers After Traumatic Brain Injury: Considerations for Drug Development. AAPS J. 2017, 19, 1615–1625. [Google Scholar] [CrossRef]
- Hudak, A.M.; Peng, L.; Marquez de la Plata, C.; Thottakara, J.; Moore, C.; Harper, C.; McColl, R.; Babcock, E.; Diaz-Arrastia, R. Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: Assessment with FLAIR and DWI imaging. Brain Inj. 2014, 28, 1602–1609. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Song, J.; Zhao, J.; Huang, T.; Zhang, M.; Zhao, Y. Hyperglycemia Aggravates Blood-Brain Barrier Disruption Following Diffuse Axonal Injury by Increasing the Levels of Inflammatory Mediators through the PPARgamma/Caveolin-1/TLR4 Pathway. Inflammation 2023, 46, 129–145. [Google Scholar] [CrossRef]
- Bergsneider, M.; Hovda, D.A.; Shalmon, E.; Kelly, D.F.; Vespa, P.M.; Martin, N.A.; Phelps, M.E.; McArthur, D.L.; Caron, M.J.; Kraus, J.F.; et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. J. Neurosurg. 1997, 86, 241–251. [Google Scholar] [CrossRef]
- Zhou, T.; Kalanuria, A. Cerebral Microdialysis in Neurocritical Care. Curr. Neurol. Neurosci. Rep. 2018, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, B.L.; Lee, S.M.; Hovda, D.A.; Sutton, R.L. The fate of glucose during the period of decreased metabolism after fluid percussion injury: A 13C NMR study. J. Neurotrauma 2007, 24, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Bergsneider, M.; Hovda, D.A.; Lee, S.M.; Kelly, D.F.; McArthur, D.L.; Vespa, P.M.; Lee, J.H.; Huang, S.C.; Martin, N.A.; Phelps, M.E.; et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J. Neurotrauma 2000, 17, 389–401. [Google Scholar] [CrossRef]
- Sharma, H.; McGinnis, J.P.; Kabotyanski, K.E.; Gopinath, S.P.; Goodman, J.C.; Robertson, C.; Cruz Navarro, J. Cerebral microdialysis and glucopenia in traumatic brain injury: A review. Front. Neurol. 2023, 14, 1017290. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; McArthur, D.; O’Phelan, K.; Glenn, T.; Etchepare, M.; Kelly, D.; Bergsneider, M.; Martin, N.A.; Hovda, D.A. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: A microdialysis study. J. Cereb. Blood Flow Metab. 2003, 23, 865–877. [Google Scholar] [CrossRef]
- Blanco, M.M.B.; Prashant, G.N.; Vespa, P.M. Cerebral Metabolism and the Role of Glucose Control in Acute Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 453–463. [Google Scholar] [CrossRef]
- Wu, H.M.; Huang, S.C.; Hattori, N.; Glenn, T.C.; Vespa, P.M.; Yu, C.L.; Hovda, D.A.; Phelps, M.E.; Bergsneider, M. Selective metabolic reduction in gray matter acutely following human traumatic brain injury. J. Neurotrauma 2004, 21, 149–161. [Google Scholar] [CrossRef]
- Hattori, N.; Huang, S.C.; Wu, H.M.; Yeh, E.; Glenn, T.C.; Vespa, P.M.; McArthur, D.; Phelps, M.E.; Hovda, D.A.; Bergsneider, M. Correlation of regional metabolic rates of glucose with glasgow coma scale after traumatic brain injury. J. Nucl. Med. 2003, 44, 1709–1716. [Google Scholar]
- Kaisti, K.K.; Metsahonkala, L.; Teras, M.; Oikonen, V.; Aalto, S.; Jaaskelainen, S.; Hinkka, S.; Scheinin, H. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 2002, 96, 1358–1370. [Google Scholar] [CrossRef]
- Schlunzen, L.; Juul, N.; Hansen, K.V.; Cold, G.E. Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol. Scand. 2012, 56, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; O’Connell, M.T.; Seal, A.; Nortje, J.; Timofeev, I.; Al-Rawi, P.G.; Coles, J.P.; Fryer, T.D.; Menon, D.K.; Pickard, J.D.; et al. A combined microdialysis and FDG-PET study of glucose metabolism in head injury. Acta Neurochir. 2009, 151, 51–61; discussion 61. [Google Scholar] [CrossRef] [PubMed]
- Dusick, J.R.; Glenn, T.C.; Lee, W.N.; Vespa, P.M.; Kelly, D.F.; Lee, S.M.; Hovda, D.A.; Martin, N.A. Increased pentose phosphate pathway flux after clinical traumatic brain injury: A [1,2-13C2]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 2007, 27, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, I.; Carpenter, K.L.; Grice, P.; Howe, D.J.; Mason, A.; Gallagher, C.N.; Helmy, A.; Murphy, M.P.; Menon, D.K.; Carpenter, T.A.; et al. Glycolysis and the pentose phosphate pathway after human traumatic brain injury: Microdialysis studies using 1,2-(13)C2 glucose. J. Cereb. Blood Flow Metab. 2015, 35, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, B.L.; Sutton, R.L.; Fukushima, M.; Harris, N.G.; Hovda, D.A.; Lee, S.M. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma 2005, 22, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Brooks, G.A. Lactate: Brain fuel in human traumatic brain injury: A comparison with normal healthy control subjects. J. Neurotrauma 2015, 32, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.N.; Carpenter, K.L.; Grice, P.; Howe, D.J.; Mason, A.; Timofeev, I.; Menon, D.K.; Kirkpatrick, P.J.; Pickard, J.D.; Sutherland, G.R.; et al. The human brain utilizes lactate via the tricarboxylic acid cycle: A 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009, 132, 2839–2849. [Google Scholar] [CrossRef]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.M.; Huang, S.C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef]
- Glenn, T.C.; Kelly, D.F.; Boscardin, W.J.; McArthur, D.L.; Vespa, P.; Oertel, M.; Hovda, D.A.; Bergsneider, M.; Hillered, L.; Martin, N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: Indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003, 23, 1239–1250. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Johnson, M.L.; Horning, M.A.; Brooks, G.A. Endogenous Nutritive Support after Traumatic Brain Injury: Peripheral Lactate Production for Glucose Supply via Gluconeogenesis. J. Neurotrauma 2015, 32, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Otori, T.; Friedland, J.C.; Sinson, G.; McIntosh, T.K.; Raghupathi, R.; Welsh, F.A. Traumatic brain injury elevates glycogen and induces tolerance to ischemia in rat brain. J. Neurotrauma 2004, 21, 707–718. [Google Scholar] [CrossRef]
- Regenold, W.T.; Hisley, K.C.; Phatak, P.; Marano, C.M.; Obuchowski, A.; Lefkowitz, D.M.; Sassan, A.; Ohri, S.; Phillips, T.L.; Dosanjh, N.; et al. Relationship of cerebrospinal fluid glucose metabolites to MRI deep white matter hyperintensities and treatment resistance in bipolar disorder patients. Bipolar Disord. 2008, 10, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, C.; van Zuylen, M.L.; Hulst, A.H.; Preckel, B.; van Beek, A.P.; Kema, I.P.; Hermanides, J.; Absalom, A.R. Elevated cerebrospinal fluid glucose levels and diabetes mellitus are associated with activation of the neurotoxic polyol pathway. Diabetologia 2022, 65, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Barnea-Goraly, N.; Raman, M.; Mazaika, P.; Marzelli, M.; Hershey, T.; Weinzimer, S.A.; Aye, T.; Buckingham, B.; Mauras, N.; White, N.H.; et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care 2014, 37, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.C.; Cheung, A.K.; Hung, V.K.; Yeung, C.M.; He, Q.Y.; Chiu, J.F.; Chung, S.S.; Chung, S.K. Deletion of aldose reductase leads to protection against cerebral ischemic injury. J. Cereb. Blood Flow Metab. 2007, 27, 1496–1509. [Google Scholar] [CrossRef]
- Zeman, R.J.; Wen, X.; Ouyang, N.; Brown, A.M.; Etlinger, J.D. Role of the Polyol Pathway in Locomotor Recovery and Wallerian Degeneration after Spinal Cord Contusion Injury. Neurotrauma Rep. 2021, 2, 411–423. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, I.; Carpenter, K.L.; Helmy, A.; Carpenter, T.A.; Menon, D.K.; Hutchinson, P.J. Glucose metabolism following human traumatic brain injury: Methods of assessment and pathophysiological findings. Metab. Brain Dis. 2015, 30, 615–632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribnau, A.; van Zuylen, M.L.; Coles, J.P.; Plummer, M.P.; Hermanns, H.; Hermanides, J. Cerebral Glucose Metabolism following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review. Int. J. Mol. Sci. 2024, 25, 2513. https://doi.org/10.3390/ijms25052513
Gribnau A, van Zuylen ML, Coles JP, Plummer MP, Hermanns H, Hermanides J. Cerebral Glucose Metabolism following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review. International Journal of Molecular Sciences. 2024; 25(5):2513. https://doi.org/10.3390/ijms25052513
Chicago/Turabian StyleGribnau, Annerixt, Mark L. van Zuylen, Jonathan P. Coles, Mark P. Plummer, Henning Hermanns, and Jeroen Hermanides. 2024. "Cerebral Glucose Metabolism following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review" International Journal of Molecular Sciences 25, no. 5: 2513. https://doi.org/10.3390/ijms25052513
APA StyleGribnau, A., van Zuylen, M. L., Coles, J. P., Plummer, M. P., Hermanns, H., & Hermanides, J. (2024). Cerebral Glucose Metabolism following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review. International Journal of Molecular Sciences, 25(5), 2513. https://doi.org/10.3390/ijms25052513





