Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research
Abstract
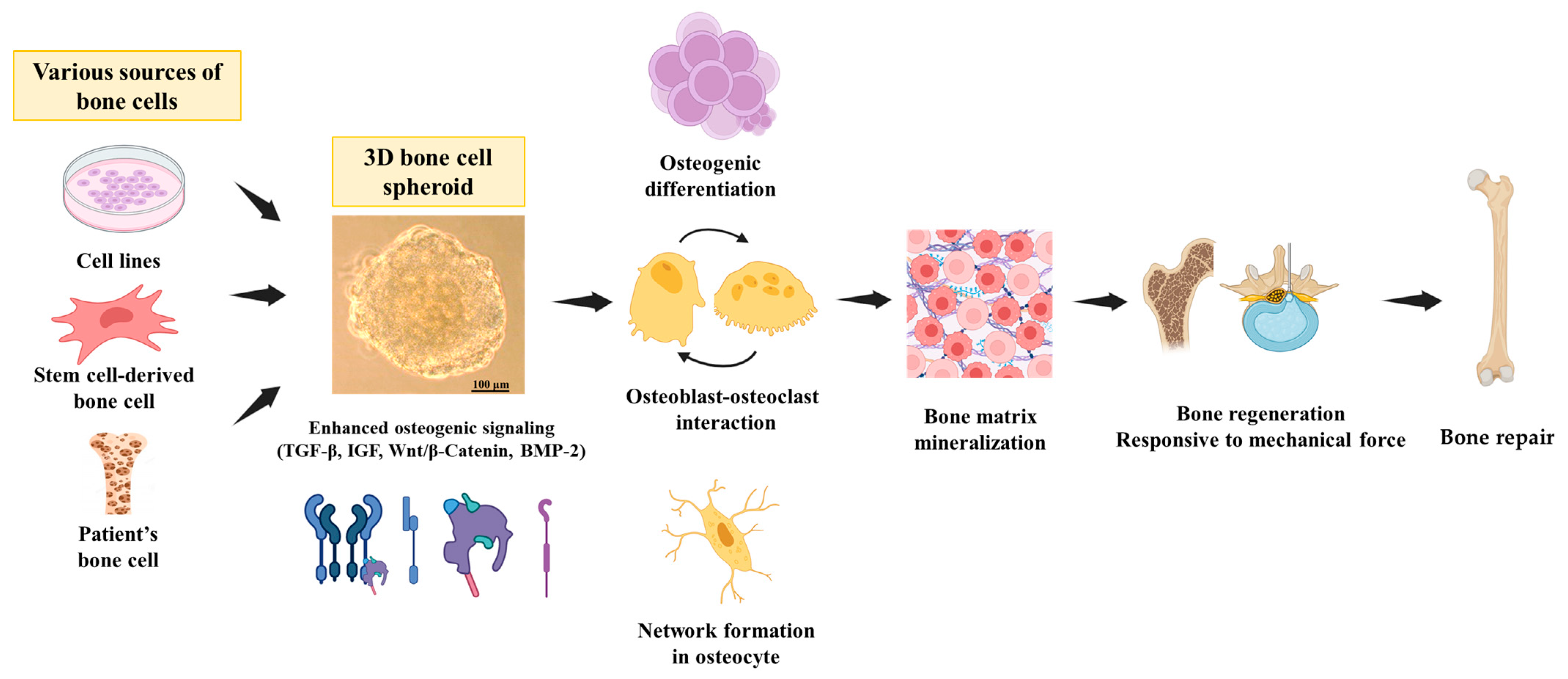
:1. Introduction
2. General Advantages of 3D Cell Culture System
3. The Purpose and Advantages of Using 3D Culture in Bone Research
3.1. General Physiology of the Bone
3.2. Importance and Advantages of 3D Culture Systems for Bone Study
3.3. Molecular Similarities between 3D Culture Systems and the Bone Environment
4. Application of Bone Differentiation in 3D Culture Systems
4.1. Studies Showcasing Successful Bone Differentiation Using 3D Culture Models
4.2. Highlighting Key Factors and Signaling Pathways Involved in Bone Homeostasis of 3D Spheroids
5. Application of 3D Culture Systems for Toxicological and Pharmacological Evaluation in Bone Research
5.1. Advantages of 3D Cultures of Bone Models in Toxicological and Pharmacological Studies
5.2. Benefits for Toxicological and Pharmacological Bone Research in 3D Culture Systems
6. Challenges and Future Prospects of 3D Culture Systems in Bone Research
6.1. Challenges Associated with Bone 3D Spheroid Systems in Toxicological and Pharmacological Development
6.2. Proposing Potential Advancements and Research Directions of Bone 3D Spheroids for Toxicological and Pharmacological Assessments
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef]
- Uhlenberg, P. Children in an Aging Society. J. Gerontol. Ser. B 2009, 64B, 489–496. [Google Scholar] [CrossRef]
- Bicer, M.; Cottrell, G.S. Widera, Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- María Verónica Cuevas-González, F.S.-O.; Cuevas-González, J.C.; Álvarez-Pérez, M.A. 3D spheroid cell cultures and their role in bone regeneration: A systematic review. Odovtos Int. J. Dent. Sci. 2022, 24, 44–57. [Google Scholar]
- Vanderburgh, J.P.; Guelcher, S.A.; Sterling, J.A. 3D bone models to study the complex physical and cellular interactions between tumor and the bone microenvironment. J. Cell. Biochem. 2018, 119, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Chae, S.; Hong, J.; Hwangbo, H.; Kim, G. The utility of biomedical scaffolds laden with spheroids in various tissue engineering applications. Theranostics 2021, 11, 6818–6832. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Thippabhotla, S.; Zhong, C.; He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Three-dimensional cell cultures: From molecular mechanisms to clinical applications. Am. J. Physiol. Cell Physiol. 1997, 273, C1109–C1123. [Google Scholar] [CrossRef]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Balawender, K. Structural and Metabolic Changes in Bone. Animals 2022, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Ono, N. The diverse origin of bone-forming osteoblasts. J. Bone Miner. Res. 2021, 36, 1432–1447. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Wang, X.; Chen, T.; Tao, F.; Liu, C.; Tu, Q.; Shen, G.; Chen, J.J. Irisin deficiency disturbs bone metabolism. J. Cell. Physiol. 2021, 236, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M. An overview of bone cells and their regulating factors of differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Ottewell, P.D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 2016, 5, 124–127. [Google Scholar] [CrossRef]
- Dallas, S.L.; Bonewald, L.F. Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 2010, 1192, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.B.; Kennedy, O.D. Osteocyte signaling in bone. Curr. Osteoporos. Rep. 2012, 10, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Castellani, C.M.; Popp, K.L.; Guerriere, K.I.; Matheny, R.W.; Nindl, B.C.; Bouxsein, M.L. The Central Role of Osteocytes in the Four Adaptive Pathways of Bone’s Mechanostat. Exerc. Sport Sci. Rev. 2020, 48, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, G.; Xiao, Y.; Luo, G.; Li, G.; Li, Z. Friend or Foe? Essential Roles of Osteoclast in Maintaining Skeletal Health. BioMed Res. Int. 2020, 2020, 4791786. [Google Scholar] [CrossRef]
- Carter, P.H.; Schipani, E. The roles of parathyroid hormone and calcitonin in bone remodeling: Prospects for novel therapeutics. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 59–76. [Google Scholar] [CrossRef]
- Henriksen, K.; Sørensen, M.G.; Nielsen, R.H.; Gram, J.; Schaller, S.; Dziegiel, M.H.; Everts, V.; Bollerslev, J.; Karsdal, M.A. Degradation of the organic phase of bone by osteoclasts: A secondary role for lysosomal acidification. J. Bone Miner. Res. 2006, 21, 58–66. [Google Scholar] [CrossRef]
- Boyce, B.F.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, F.; Pujic, N.; Liu, Y.; Laroche, N.; Vico, L.; Cancedda, R. Osteoblast and osteoclast differentiation in an in vitro three-dimensional model of bone. Tissue Eng. Part A 2009, 15, 2373–2383. [Google Scholar] [CrossRef]
- Ayoubi, M.; van Tol, A.F.; Weinkamer, R.; Roschger, P.; Brugger, P.C.; Berzlanovich, A.; Bertinetti, L.; Roschger, A.; Fratzl, P. 3D Interrelationship between Osteocyte Network and Forming Mineral during Human Bone Remodeling. Adv. Healthc. Mater. 2021, 10, e2100113. [Google Scholar] [CrossRef]
- Chen, J.; Dosier, C.R.; Park, J.H.; De, S.; Guldberg, R.E.; Boyan, B.D.; Schwartz, Z. Mineralization of three-dimensional osteoblast cultures is enhanced by the interaction of 1α,25-dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J. Tissue Eng. Regen. Med. 2016, 10, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Guillaume, L.; Rigal, L.; Fehrenbach, J.; Severac, C.; Ducommun, B.; Lobjois, V. Characterization of the physical properties of tumor-derived spheroids reveals critical insights for pre-clinical studies. Sci. Rep. 2019, 9, 6597. [Google Scholar] [CrossRef] [PubMed]
- Gaitán-Salvatella, I.; López-Villegas, E.O.; González-Alva, P.; Susate-Olmos, F.; Álvarez-Pérez, M.A. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci. 2021, 8, 672518. [Google Scholar] [CrossRef] [PubMed]
- Kwakwa, K.A.; Vanderburgh, J.P.; Guelcher, S.A.; Sterling, J.A. Engineering 3D Models of Tumors and Bone to Understand Tumor-Induced Bone Disease and Improve Treatments. Curr. Osteoporos. Rep. 2017, 15, 247–254. [Google Scholar] [CrossRef]
- Mittler, F.; Obeïd, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-Content Monitoring of Drug Effects in a 3D Spheroid Model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef]
- Plantz, M.; Lyons, J.B.; Yamaguchi, J.T.; Greene, A.C.B.; Ellenbogen, D.J.B.; Hallman, M.J.B.; Shah, V.B.; Yun, C.; Jakus, A.E.; Procissi, D.; et al. Preclinical Safety of a 3D-Printed Hydroxyapatite-Demineralized Bone Matrix Scaffold for Spinal Fusion. Spine 2022, 47, 82–89. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsu, E.L.; Stupp, S.I. Supramolecular self-assembling peptides to deliver bone morphogenetic proteins for skeletal regeneration. Bone 2020, 141, 115565. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: A perspective on construction and application. Bioact. Mater. 2022, 18, 15–25. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Lu, A.; Liang, C. Organoids as Innovative Models for Bone and Joint Diseases. Cells 2023, 12, 1590. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Liao, S.; Chan, C.K.; Ramakrishna, S. Enhanced osteogenic differentiation with 3D electrospun nanofibrous scaffolds. Nanomedicine 2012, 7, 1561–1575. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Spheroid model for functional osteogenic evaluation of human adipose derived stem cells. J. Biomed. Mater. Res. A 2017, 105, 1230–1236. [Google Scholar] [CrossRef]
- Wongwitwichot, P.; Kaewsrichan, J. Osteogenic differentiation of mesenchymal stem cells is impaired by bone morphogenetic protein 7. Adv. Med. Sci. 2017, 62, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Naujoks, C.; Smeets, R.; Berr, K.; Depprich, R.; Kübler, N.; Handschel, J. Scaffold-free microtissues: Differences from monolayer cultures and their potential in bone tissue engineering. Clin. Oral Investig. 2013, 17, 9–17. [Google Scholar] [CrossRef]
- Futrega, K.; Mosaad, E.; Chambers, K.; Lott, W.B.; Clements, J.; Doran, M.R. Bone marrow-derived stem/stromal cells (BMSC) 3D microtissues cultured in BMP-2 supplemented osteogenic induction medium are prone to adipogenesis. Cell Tissue Res. 2018, 374, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Lysdahl, H.; Baatrup, A.; Foldager, C.B.; Bünger, C. Preconditioning Human Mesenchymal Stem Cells with a Low Concentration of BMP2 Stimulates Proliferation and Osteogenic Differentiation In Vitro. Biores. Open Access 2014, 3, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kim, M.; Park, J.B. Bone morphogenetic protein 2-enhanced osteogenic differentiation of stem cell spheres by regulation of Runx2 expression. Exp. Ther. Med. 2020, 20, 79. [Google Scholar] [CrossRef]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.; Zhao, D.; Jin, H.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 226. [Google Scholar] [CrossRef]
- Wolff, A.; Frank, M.; Staehlke, S.; Springer, A.; Hahn, O.; Meyer, J.; Peters, K. 3D Spheroid Cultivation Alters the Extent and Progression of Osteogenic Differentiation of Mesenchymal Stem/Stromal Cells Compared to 2D Cultivation. Biomedicines 2023, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Bouet, G.; Cruel, M.; Laurent, C.; Vico, L.; Malaval, L.; Marchat, D. Validation of an in vitro 3D bone culture model with perfused and mechanically stressed ceramic scaffold. Eur. Cells Mater. 2015, 29, 250–266, discussion 266–267. [Google Scholar] [CrossRef]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Z.; Gao, W.; Gao, W.; He, R.; Li, Y.; Crawford, R.; Zhou, Y.; Xiao, L.; Xiao, Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels 2022, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Licata, J.P.; Schwab, K.H.; Har-El, Y.-E.; Gerstenhaber, J.A.; Lelkes, P.I. Bioreactor Technologies for Enhanced Organoid Culture. Int. J. Mol. Sci. 2023, 24, 11427. [Google Scholar] [CrossRef]
- Cho, S.; Choi, H.; Jeong, H.; Kwon, S.Y.; Roh, E.J.; Jeong, K.-H.; Baek, I.; Kim, B.J.; Lee, S.-H.; Han, I. Preclinical Study of Human Bone Marrow-Derived Mesenchymal Stem Cells Using a 3-Dimensional Manufacturing Setting for Enhancing Spinal Fusion. Stem Cells Transl. Med. 2022, 11, 1072–1088. [Google Scholar] [CrossRef]
- Yamada, Y.; Okano, T.; Orita, K.; Makino, T.; Shima, F.; Nakamura, H. 3D-cultured small size adipose-derived stem cell spheroids promote bone regeneration in the critical-sized bone defect rat model. Biochem. Biophys. Res. Commun. 2022, 603, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hinkelmann, S.; Springwald, A.H.; Starke, A.; Kalwa, H.; Wölk, C.; Hacker, M.C.; Schulz-Siegmund, M. Microtissues from mesenchymal stem cells and siRNA-loaded cross-linked gelatin microparticles for bone regeneration. Mater. Today Bio 2022, 13, 100190. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Kampleitner, C.; Mohamed-Ahmed, S.; Yassin, M.A.; Dongre, H.; Costea, D.E.; Tangl, S.; Hassan, M.N.; Stavropoulos, A.; Bolstad, A.I.; et al. Ectopic Bone Tissue Engineering in Mice Using Human Gingiva or Bone Marrow-Derived Stromal/Progenitor Cells in Scaffold-Hydrogel Constructs. Front. Bioeng. Biotechnol. 2021, 9, 783468. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ayan, B.; Dey, M.; Banerjee, D.; Wee, H.; Lewis, G.S.; Ozbolat, I.T. Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering. Biofabrication 2020, 13, 015013. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.; Kajiya, H.; Fujisaki, S.; Maeshiba, M.; Yanagi, T.; Kojima, H.; Ohno, J. Three-dimensional spheroids of mesenchymal stem/stromal cells promote osteogenesis by activating stemness and Wnt/β-catenin. Biochem. Biophys. Res. Commun. 2020, 523, 458–464. [Google Scholar] [CrossRef]
- Lawrence, L.M.; Cottrill, A.; Valluri, A.; Marenzi, G.; Denning, K.L.; Valluri, J.; Claudio, P.P.; Day, J.B. Minimally Manipulative Method for the Expansion of Human Bone Marrow Mesenchymal Stem Cells to Treat Osseous Defects. Int. J. Mol. Sci. 2019, 20, 612. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, C.S.; Rath, S.N. Enhanced osteodifferentiation of MSC spheroids on patterned electrospun fiber mats—An advanced 3D double strategy for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 703–712. [Google Scholar] [CrossRef]
- Gaitán-Salvatella, I.; González-Alva, P.; Montesinos, J.J.; Alvarez-Perez, M.A. In Vitro Bone Differentiation of 3D Microsphere from Dental Pulp-Mesenchymal Stem Cells. Bioengineering 2023, 10, 571. [Google Scholar] [CrossRef]
- Shanbhag, S.; Suliman, S.; Mohamed-Ahmed, S.; Kampleitner, C.; Hassan, M.N.; Heimel, P.; Dobsak, T.; Tangl, S.; Bolstad, A.I.; Mustafa, K. Bone regeneration in rat calvarial defects using dissociated or spheroid mesenchymal stromal cells in scaffold-hydrogel constructs. Stem Cell Res. Ther. 2021, 12, 575. [Google Scholar] [CrossRef]
- Sato, T.; Anada, T.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Sakai, S.; Baba, K.; Sasaki, K.; Suzuki, O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomater. 2019, 88, 477–490. [Google Scholar] [CrossRef]
- Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Eap, S.; Brasse, D.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Nanoengineered implant as a new platform for regenerative nanomedicine using 3D well-organized human cell spheroids. Int. J. Nanomed. 2017, 12, 447–457. [Google Scholar] [CrossRef]
- Guerrero, J.; Oliveira, H.; Catros, S.; Siadous, R.; Derkaoui, S.-M.; Bareille, R.; Letourneur, D.; Amédée, J. The use of total human bone marrow fraction in a direct three-dimensional expansion approach for bone tissue engineering applications: Focus on angiogenesis and osteogenesis. Tissue Eng. Part A 2015, 21, 861–874. [Google Scholar] [CrossRef]
- Gorkun, A.A.; Revokatova, D.P.; Zurina, I.M.; Nikishin, D.A.; Bikmulina, P.Y.; Timashev, P.S.; Shpichka, A.I.; Kosheleva, N.V.; Kolokoltsova, T.D.; Saburina, I.N. The Duo of Osteogenic and Angiogenic Differentiation in ADSC-Derived Spheroids. Front. Cell Dev. Biol. 2021, 9, 572727. [Google Scholar] [CrossRef]
- Inglis, S.; Kanczler, J.M.; Oreffo, R.O.C. 3D human bone marrow stromal and endothelial cell spheres promote bone healing in an osteogenic niche. FASEB J. 2019, 33, 3279–3290. [Google Scholar] [CrossRef]
- Ahmad, T.; Lee, J.; Shin, Y.M.; Shin, H.J.; Perikamana, S.K.M.; Park, S.H.; Kim, S.W.; Shin, H. Hybrid-spheroids incorporating ECM like engineered fragmented fibers potentiate stem cell function by improved cell/cell and cell/ECM interactions. Acta Biomater. 2017, 64, 161–175. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, R.-X.; Mao, J.-P.; Xiao, B.; Chen, D.-F.; Tian, W. Three-dimensional spheroid culture promotes the stemness maintenance of cranial stem cells by activating PI3K/AKT and suppressing NF-κB pathways. Biochem. Biophys. Res. Commun. 2017, 488, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Walser, R.; Metzger, W.; Görg, A.; Pohlemann, T.; Menger, M.D.; Laschke, M.W. Generation of co-culture spheroids as vascularisation units for bone tissue engineering. Eur. Cells Mater. 2013, 26, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.S.; Rasmussen, P.L.; Larsen, K.H.; Schrøder, H.D.; Kassem, M. Parameters in three-dimensional osteospheroids of telomerized human mesenchymal (stromal) stem cells grown on osteoconductive scaffolds that predict in vivo bone-forming potential. Tissue Eng. Part A 2010, 16, 2331–2342. [Google Scholar] [CrossRef]
- Amaral, I.F.; Sampaio, P.; Barbosa, M.A. Three-dimensional culture of human osteoblastic cells in chitosan sponges: The effect of the degree of acetylation. J. Biomed. Mater. Res. A 2006, 76, 335–346. [Google Scholar] [CrossRef]
- Duque, G.; Huang, D.C.; Dion, N.; Macoritto, M.; Rivas, D.; Li, W.; Yang, X.F.; Li, J.; Lian, J.; Marino, F.T.; et al. Interferon-γ plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J. Bone Miner. Res. 2011, 26, 1472–1483. [Google Scholar] [CrossRef]
- Vermeulen, S.; Birgani, Z.T.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef]
- Yanagi, T.; Kajiya, H.; Fujisaki, S.; Maeshiba, M.; Yanagi-S, A.; Yamamoto-M, N.; Kakura, K.; Kido, H.; Ohno, J. Three-dimensional spheroids of dedifferentiated fat cells enhance bone regeneration. Regen. Ther. 2021, 18, 472–479. [Google Scholar] [CrossRef]
- Zhang, J.; Griesbach, J.; Ganeyev, M.; Zehnder, A.-K.; Zeng, P.; Schädli, G.N.; de Leeuw, A.; Lai, Y.; Rubert, M. Long-term mechanical loading is required for the formation of 3D bioprinted functional osteocyte bone organoids. Biofabrication 2022, 14, 035018. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Hallman, M.; Driscoll, J.A.; Lubbe, R.; Jeong, S.; Chang, K.; Haleem, M.; Jakus, A.; Pahapill, R.; Yun, C.; Shah, R.N.; et al. Influence of Geometry and Architecture on the In Vivo Success of 3D-Printed Scaffolds for Spinal Fusion. Tissue Eng. Part A 2021, 27, 26–36. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Arrigoni, C.; Talò, G.; Zamai, M.; Zagra, L.; Caiolfa, V.; Moretti, M. Human in vitro 3D co-culture model to engineer vascularized bone-mimicking tissues combining computational tools and statistical experimental approach. Biomaterials 2016, 76, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Shie, M.-Y.; Lai, Y.-H.; Foo, N.-P.; Lee, M.-J.; Yao, C.-H. Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration. Polymers 2021, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Grafe, I.; Bae, Y.; Chen, S.; Chen, Y.; Bertin, T.K.; Jiang, M.-M.; Ambrose, C.G.; Lee, B. E-selectin ligand 1 regulates bone remodeling by limiting bioactive TGF-β in the bone microenvironment. Proc. Natl. Acad. Sci. USA 2013, 110, 7336–7341. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Arnsdorf, E.J.; Tummala, P.; Jacobs, C.R. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS ONE 2009, 4, e5388. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.; Kajiya, H.; Fujisaki, S.; Maeshiba, M.; Yanagi, T.; Kojima, H.; Ohno, J. Corrigendum to “Three-dimensional spheroids of mesenchymal stem/stromal cells promote osteogenesis by activating stemness and Wnt/β-catenin” [Biochem Biophys Res Commun. 2020 523(2) 458–464]. Biochem. Biophys. Res. Commun. 2020, 525, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Herencia, C.; Martínez-Moreno, J.M.; Herrera, C.; Corrales, F.; Santiago-Mora, R.; Espejo, I.; Barco, M.; Almadén, Y.; de la Mata, M.; Rodríguez-Ariza, A.; et al. Nuclear translocation of β-catenin during mesenchymal stem cells differentiation into hepatocytes is associated with a tumoral phenotype. PLoS ONE 2012, 7, e34656. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.C.; Adams, A.M.; Goodson, J.M.; McDonald, C.E.; Potter, J.C.; Berndt, J.D.; Biechele, T.L.; Taylor, R.J.; Moon, R.T. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA 2012, 109, 4485–4490. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [PubMed]
- Bord, S.; Ireland, D.; Beavan, S.; Compston, J. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef]
- Trouvin, A.P.; Goëb, V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: Maintaining the balance to prevent bone loss. Clin. Interv. Aging 2010, 5, 345–354. [Google Scholar]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, Y.-M.; Baek, S.; Park, Y.-H.; Park, J.-B. Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina 2021, 57, 1271. [Google Scholar] [CrossRef]
- Schröder, M.; Riksen, E.A.; He, J.; Skallerud, B.H.; Møller, M.E.; Lian, A.; Syversen, U.; Reseland, J.E. Vitamin K2 Modulates Vitamin D-Induced Mechanical Properties of Human 3D Bone Spheroids In Vitro. JBMR Plus 2020, 4, e10394. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Advanced cell culture techniques for cancer drug discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Weinhart, M.; Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D organ models-Revolution in pharmacological research? Pharmacol. Res. 2019, 139, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)evolution: Unveiling Tumor–Stroma Interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, L.; Zheng, Y.; Li, W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci. Rep. 2018, 38, BSR20180448. [Google Scholar] [CrossRef]
- Cox, C.R.; Lynch, S.; Goldring, C.; Sharma, P. Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury. Front. Med. Technol. 2020, 2, 611913. [Google Scholar] [CrossRef]
- Fong, E.L.S.; Toh, T.B.; Yu, H.; Chow, E.K.-H. 3D Culture as a Clinically Relevant Model for Personalized Medicine. SLAS Technol. 2017, 22, 245–253. [Google Scholar] [CrossRef]
- Abraham, D.M.; Herman, C.; Witek, L.; Cronstein, B.N.; Flores, R.L.; Coelho, P.G. Self-assembling human skeletal organoids for disease modeling and drug testing. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Fevre, R.; Mary, G.; Vertti-Quintero, N.; Durand, A.; Tomasi, R.F.-X.; Del Nery, E.; Baroud, C.N. Combinatorial drug screening on 3D Ewing sarcoma spheroids using droplet-based microfluidics. iScience 2023, 26, 106651. [Google Scholar] [CrossRef]
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S.-Y. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, e1702787. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, A.; Hughes, E.A.B.; Joseph, S.; Hill, E.J.; Grover, L.M.; Metcalfe, A.D. Trabecular bone organoids: A micron-scale ‘humanised’ prototype designed to study the effects of microgravity and degeneration. NPJ Microgravity 2021, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures-Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Tang, J.; Shi, J.; Liu, J. Editorial: Advances in 3D cell culture for drug screening and toxicology evaluation. Front. Bioeng. Biotechnol. 2023, 11, 1266506. [Google Scholar] [CrossRef]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.B.; Gellert, P.R.; Stolnik, S.; Grabowska, A.M.; Garnett, M.C. Penetration and Uptake of Nanoparticles in 3D Tumor Spheroids. Bioconjug. Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Huh, K.M.; Kang, S.W. Applications of Biomaterials in 3D Cell Culture and Contributions of 3D Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J. Orthop. Res. 2012, 30, 1507–1515. [Google Scholar] [CrossRef]
- García, J.R.; García, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Transl. Res. 2016, 6, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Wedzinska, A.; Kot, M.; Sarnowska, A. Effect of Long-Term 3D Spheroid Culture on WJ-MSC. Cells 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Castanheira, E.M.S.; Lanceros-Méndez, S.; Cardoso, V.F. Recent Advances on Cell Culture Platforms for In Vitro Drug Screening and Cell Therapies: From Conventional to Microfluidic Strategies. Adv. Healthc. Mater. 2023, 12, e2202936. [Google Scholar] [CrossRef]
- Chunduri, V.; Maddi, S. Role of in vitro two-dimensional (2D) and three-dimensional (3D) cell culture systems for ADME-Tox screening in drug discovery and development: A comprehensive review. ADMET DMPK 2023, 11, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Baruffaldi, D.; Palmara, G.; Pirri, C.; Frascella, F. 3D Cell Culture: Recent Development in Materials with Tunable Stiffness. ACS Appl. Bio Mater. 2021, 4, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.A.; Lubbe, R.; Jakus, A.E.; Chang, K.; Haleem, M.; Yun, C.; Singh, G.; Schneider, A.D.; Katchko, K.M.; Soriano, C.; et al. 3D-Printed Ceramic-Demineralized Bone Matrix Hyperelastic Bone Composite Scaffolds for Spinal Fusion. Tissue Eng. Part A 2020, 26, 157–166. [Google Scholar] [CrossRef]
- Tse, H.M.; Gardner, G.; Dominguez-Bendala, J.; Fraker, C.A. The Importance of Proper Oxygenation in 3D Culture. Front. Bioeng. Biotechnol. 2021, 9, 634403. [Google Scholar] [CrossRef]
- Radetzki, F.; Mendel, T.; Noser, H.; Stoevesandt, D.; Röllinghoff, M.; Gutteck, N.; Delank, K.S.; Wohlrab, D. Potentialities and limitations of a database constructing three-dimensional virtual bone models. Surg. Radiol. Anat. 2013, 35, 963–968. [Google Scholar] [CrossRef]
- Anthon, S.G.; Valente, K.P. Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. Int. J. Mol. Sci. 2022, 23, 14582. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Gordon, R.; Zhang, L.; Bergan, R.; Keene, D.R.; Jones, J.M.; Xie, H.; Chen, Z.; Tao, J.; et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat. Commun. 2019, 10, 3520. [Google Scholar] [CrossRef]
- Loewa, A.; Feng, J.J.; Hedtrich, S. Human disease models in drug development. Nat. Rev. Bioeng. 2023, 1, 545–559. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; White, D.L.; Genant, H.K. Micro CT and Micro MR imaging of 3D architecture of animal skeleton. J. Musculoskelet. Neuronal Interact. 2000, 1, 45–51. [Google Scholar] [PubMed]
- Kannan, A.; Minardi, S.; Ellenbogen, D.J.; Hallman, M.J.; Greene, A.C.; Yamaguchi, J.T.; Plantz, M.A.; Jeong, S.; Sana, K.C.; Shah, V.; et al. The effect of local steroid application on bony fusion in a rat posterolateral spinal arthrodesis model. JOR Spine 2021, 4, e1177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuang, B.; Rothrauff, B.B.; Tuan, R.S.; Lin, H. Robust bone regeneration through endochondral ossification of human mesenchymal stem cells within their own extracellular matrix. Biomaterials 2019, 218, 119336. [Google Scholar] [CrossRef]
- McClendon, M.T.; Ji, W.; Greene, A.C.; Sai, H.; Sangji, M.H.; Sather, N.A.; Chen, C.H.; Lee, S.S.; Katchko, K.; Jeong, S.S.; et al. A supramolecular polymer-collagen microparticle slurry for bone regeneration with minimal growth factor. Biomaterials 2023, 302, 122357. [Google Scholar] [CrossRef]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef] [PubMed]
| Research Category | Highlighted Result |
|---|---|
| MSC-based bone spheroid research | Mass-produced, quality-controlled MSCs were well integrated into the decorated bones of the lumbar spine [70]. Scaffold-free 3D cultured compact adipose-derived stem cell (ADSC) spheroids survived in vivo and in vitro conditions and promoted bone regeneration [71]. In combination with autologous hMSCs, microtissues can be an innovative alternative to autologous transplantation [72]. Spheroid culture facilitated ectopic mineralization in the composition of the BMSC [73]. Osteogenic differentiation was induced in hMSC/HUVEC spheroids for 10 days to produce bone tissue [74]. The 3D spheroid MSC culture was more stem-like than the 2D monolayer MSC culture, and accelerated osteoblast production and osteogenesis [75]. The 3D spheroid culture allowed the production of hBM mesenchymal cells that retained osteoblast differentiation [76]. Geometric clues and synergistic effects of 3D culture appeared when differentiating them into osteogenic systems [77]. |
| Scaffold 3D-based bone research | Dental pulp–mesenchymal stem cells’ microspheres exhibited osteogenesis, such as human fetal osteoblast microspheres [78]. The 3D-printed poly(L-lactide-co-trimethylene carbonate) scaffolds and modified human platelet lysate hydrogels construct represents a promising scaffold for bone tissue engineering applications [79]. Magnetic levitation culture enabled 3D stable osteoblast spheroids [44]. Oxychip promoted bone formation differentiation of MSCs. In vitro bone formation ability was very similar to that observed in vivo [80]. BMSC resulted in tissue compression rather than growth. Not all mineralized bone-like substrates were included in the bulk microtissue mass [59]. An advanced double 3D bone implant was developed combining a nanostructured bioactive biomaterial and predifferentiated osteogenic microtissues [81]. A bioactive matrix can be utilized for bone regeneration and vascularization, as it can promote spheroid formation and contribute to the formation of vascularized tissue from human whole bone marrow cells [82]. Osteogenic differentiation of 3D microtissues was enhanced by mimicking in vivo conditions more than 2D [58]. |
| Matrigel and methodological application-based bone research | By arranging ADSCs as spheroids, markers of both osteoblastic and angiogenesis can be obtained quickly and spontaneously compared with 2D incubation [83]. Hydroxyapatite and cancellous bone scaffolds exhibited improved cell integration and survival compared with other materials [76]. Spheroids containing HUVECs and human bone marrow stromal cells enhanced bone formation at defect sites in vivo [84]. The presence of fragmented fibers improved the stemness retention of human turbinate mesenchymal stem cells [85]. Polymer matrix-based 3D spheroids of cranial stem cells enhanced multipotency and proliferation while promoting the maintenance of stemness [86]. Co-cultured spheroids composed of primary human osteoblasts and human dermal microvascular endothelial cells represent valuable tools for vascularization in bone tissue engineering [87]. The three-dimensional cultures of hMSC-TERT combined with hydroxyapatite–tricalcium phosphate in osteogenic induction medium replicated many features of in vivo bone formation [88]. The degree of acetylation of chitosan played a crucial role in determining the affinity of human osteoblastic MG-63 cells towards the 3D substrates within the three-dimensional chitosan matrices [89]. |
| Pros | Cons | |
|---|---|---|
| 3D | Physiological relevance: Better mimic the in vivo microenvironment of bone tissue, providing a physiologically relevant platform for studying drug toxicity and response [4,7]. | Technical challenges: Establishing and maintaining can be technically challenging and resource-intensive. May require specialized equipment and expertise [8]. |
| Complexity: Allow the recreation of the three-dimensional structure of bone tissue, including its cellular and matrix components. Complexity can yield insights into interactions [127]. | Variability: Variability can be higher due to the complexity of the system, making it harder to control experimental conditions [12]. | |
| Drug penetration: Often exhibit slower drug penetration compared with 2D systems, which can be advantageous for simulating drug distribution and metabolism more accurately [128,129]. | High-throughput: High-throughput screening for bone cells is still difficult, making it less suitable for rapid screening of large numbers of compounds [130]. | |
| Tissue engineering: Available tissue engineering applications, where researchers aim to regenerate or repair bone tissue [131]. | Lack of vascularization: Lack of vascularization within the engineered bone grafts inhibits osteogenesis and host integration; inhibits the healing of large bone defects [132,133]. | |
| Long-term studies: In many toxicological and pharmacological studies, 3D spheroid bone models can be maintained for extended periods, enabling the assessment of chronic exposure to drugs or toxins [134]. | ||
| 2D | Simplicity: Easier to set up and maintain the bone cell. Often cost-effective and amenable to high-throughput screening [7]. | Limited physiological relevance: Do not accurately represent the three-dimensional architecture and microenvironment of bone tissue. Can lead to discrepancies in drug responses and toxicity compared with in vivo conditions [7,68]. |
| Control: Easy control over experimental conditions. Easier to standardize experiments and achieve reproducible results [135]. | Limited interaction: Lack the spatial organization and intercellular interactions found in 3D systems, potentially leading to incomplete or misleading results [136]. | |
| Drug screening: Well suited for initial drug screening and toxicology studies for bone cells, allowing quick identification of potential candidates for further testing in more complex models [137]. | Drug penetration: Drugs may penetrate cells more rapidly than they would in vivo, leading to different pharmacokinetics [138]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.; Kim, S.H.; Kim, K.M.; Yang, M.H.; Byun, M.R.; Kim, J.-H.; Kwon, D.; Pham, H.T.M.; Kim, H.-S.; Kim, J.-H.; et al. Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. Int. J. Mol. Sci. 2024, 25, 2512. https://doi.org/10.3390/ijms25052512
Yun C, Kim SH, Kim KM, Yang MH, Byun MR, Kim J-H, Kwon D, Pham HTM, Kim H-S, Kim J-H, et al. Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. International Journal of Molecular Sciences. 2024; 25(5):2512. https://doi.org/10.3390/ijms25052512
Chicago/Turabian StyleYun, Chawon, Sou Hyun Kim, Kyung Mok Kim, Min Hye Yang, Mi Ran Byun, Joung-Hee Kim, Doyoung Kwon, Huyen T. M. Pham, Hyo-Sop Kim, Jae-Ho Kim, and et al. 2024. "Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research" International Journal of Molecular Sciences 25, no. 5: 2512. https://doi.org/10.3390/ijms25052512
APA StyleYun, C., Kim, S. H., Kim, K. M., Yang, M. H., Byun, M. R., Kim, J.-H., Kwon, D., Pham, H. T. M., Kim, H.-S., Kim, J.-H., & Jung, Y.-S. (2024). Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. International Journal of Molecular Sciences, 25(5), 2512. https://doi.org/10.3390/ijms25052512






