Exploring the Genetic Landscape of Mild Behavioral Impairment as an Early Marker of Cognitive Decline: An Updated Review Focusing on Alzheimer’s Disease
Abstract
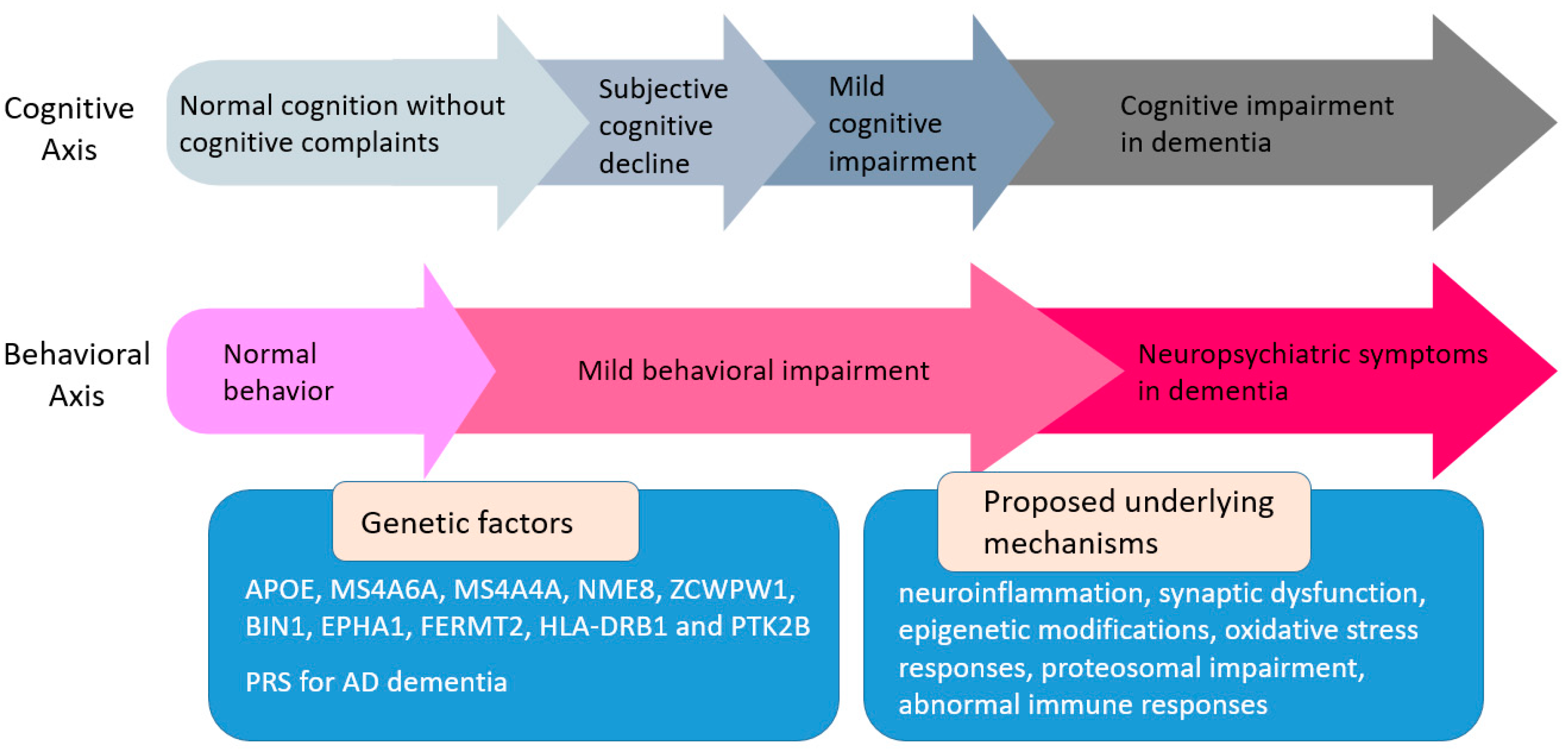
1. Introduction
2. The Relationship between Apolipoprotein E (APOE) Genotype and Mild Behavioral Impairment (MBI)
3. The Relationship between Other Alzheimer’s Disease (AD)-Related Genetic Factors and MBI
3.1. MS4A Genetic Variants and Mild Behavioral Impairment (MBI)
3.2. NME8 Genetic Variants and MBI
3.3. ZCWPW1 Genetic Variants and MBI
3.4. BIN1 Genetic Variants and MBI
3.5. EPHA1 Genetic Variants and MBI
3.6. FERMT2 Genetic Variants and MBI
3.7. HLA-DRB1 Genetic Variants and MBI
3.8. PTK2B Genetic Variants and MBI
4. The Relationship between Polygenic Risk Scores (PRSs) for AD and MBI
4.1. The Association between PRSs for AD and MBI
4.2. The Interaction between PRSs for AD and MBI on Cognition
5. Challenges and Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Creese, B.; Ismail, Z. Mild behavioral impairment: Measurement and clinical correlates of a novel marker of preclinical Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 2. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Shaikh, M.F.; Piperi, C. Flotillin: A Promising Biomarker for Alzheimer’s Disease. J. Pers. Med. 2020, 10, 20. [Google Scholar] [CrossRef]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: Challenges and advances in diagnosis and treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Seripa, D.; D’Onofrio, G.; Santamato, A.; Masullo, C.; Logroscino, G.; Solfrizzi, V.; Pilotto, A. Apolipoprotein E genotypes and neuropsychiatric symptoms and syndromes in late-onset Alzheimer’s disease. Ageing Res. Rev. 2012, 11, 87–103. [Google Scholar] [CrossRef]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef]
- Geda, Y.E.; Roberts, R.O.; Mielke, M.M.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Boeve, B.F.; Sochor, O.; Tangalos, E.G.; Petersen, R.C.; et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am. J. Psychiatry 2014, 171, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.N.; Cano, J.; Zhao, X.; Ismail, Z.; Chen, C.L.; Xu, X. Prevalence, Clinical Correlates, Cognitive Trajectories, and Dementia Risk Associated with Mild Behavioral Impairment in Asians. J. Clin. Psychiatry 2022, 83, 21m14105. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Xu, J.; Liao, Z.; Zhang, Y.; Wang, Y.; Sun, W.; Yu, E. A review of current evidence for mild behavioral impairment as an early potential novel marker of Alzheimer’s disease. Front. Psychiatry 2023, 14, 1099333. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.S.; Yu, D.S.; Chau, P.H.; Li, P.W.; Ismail, Z. Reliability and Validity of the Traditional Chinese Version of the Mild Behavioral Impairment—Checklist Among Persons with Mild Cognitive Impairment—A Validation Study. J. Geriatr. Psychiatry Neurol. 2023, 36, 26–38. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Clark, K.; Leung, Y.Y.; Lee, W.P.; Voight, B.; Wang, L.S. Polygenic Risk Scores in Alzheimer’s Disease Genetics: Methodology, Applications, Inclusion, and Diversity. J. Alzheimer’s Dis. 2022, 89, 1–12. [Google Scholar] [CrossRef]
- Ridge, P.G.; Hoyt, K.B.; Boehme, K.; Mukherjee, S.; Crane, P.K.; Haines, J.L.; Mayeux, R.; Farrer, L.A.; Pericak-Vance, M.A.; Schellenberg, G.D.; et al. Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol. Aging 2016, 41, 200.e13–200.e20. [Google Scholar] [CrossRef]
- Sweet, R.A.; Nimgaonkar, V.L.; Devlin, B.; Lopez, O.L.; DeKosky, S.T. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology 2002, 58, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Nowrangi, M.A.; Outen, J.D.; Kim, J.; Avramopoulos, D.; Lyketsos, C.G.; Rosenberg, P.B. Neuropsychiatric Symptoms of Alzheimer’s Disease: An Anatomic-Genetic Framework for Treatment Development. J. Alzheimer’s Dis. 2023, 95, 53–68. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, H.J.; Myung, W.; Suh, S.W.; Seong, S.J.; Hwang, J.Y.; Ryu, J.I.; Park, S.C. Clinical Approaches to Late-Onset Psychosis. J. Pers. Med. 2022, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Jeste, D.V.; Meeks, T.W.; Kim, D.S.; Zubenko, G.S. Research agenda for DSM-V: Diagnostic categories and criteria for neuropsychiatric syndromes in dementia. J. Geriatr. Psychiatry Neurol. 2006, 19, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Brandt, J.; Albert, M.; Devanand, D.P.; Marder, K.; Bell, K.; Ciappa, A.; Tycko, B.; Stern, Y. Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology 2002, 58, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Krell-Roesch, J.; Sambuchi, N.; Michel, B.F. Neuropsychiatric Symptoms and Neuroimaging Biomarkers in Alzheimer Disease: “Which is the Cart and Which is the Horse?”. Am. J. Geriatr. Psychiatry 2017, 25, 694–696. [Google Scholar] [CrossRef]
- Geda, Y.E.; Schneider, L.S.; Gitlin, L.N.; Miller, D.S.; Smith, G.S.; Bell, J.; Evans, J.; Lee, M.; Porsteinsson, A.; Lanctot, K.L.; et al. Neuropsychiatric symptoms in Alzheimer’s disease: Past progress and anticipation of the future. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 602–608. [Google Scholar] [CrossRef]
- Andrews, S.J.; Ismail, Z.; Anstey, K.J.; Mortby, M. Association of Alzheimer’s genetic loci with mild behavioral impairment. Am. J. Med. Genetics. Part B Neuropsychiatr. Genet. 2018, 177, 727–735. [Google Scholar] [CrossRef]
- Feng, F.; Lu, S.S.; Hu, C.Y.; Gong, F.F.; Qian, Z.Z.; Yang, H.Y.; Wu, Y.L.; Zhao, Y.Y.; Bi, P.; Sun, Y.H. Association between apolipoprotein E gene polymorphism and depression. J. Clin. Neurosci. 2015, 22, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I.; Waern, M.; Duberstein, P.; Blennow, K.; Zetterberg, H.; Borjesson-Hanson, A.; Ostling, S.; Guo, X.; Kern, J.; Gustafson, D.; et al. A 9-year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biol. Psychiatry 2015, 78, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Metti, A.L.; Cauley, J.A.; Newman, A.B.; Ayonayon, H.N.; Barry, L.C.; Kuller, L.M.; Satterfield, S.; Simonsick, E.M.; Yaffe, K. Plasma beta amyloid level and depression in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 74–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, P.; Olesen, P.J.; Simoni, M.; Pantoni, L.; Ostling, S.; Kern, S.; Guo, X.; Skoog, I. White matter lesions and temporal lobe atrophy related to incidence of both dementia and major depression in 70-year-olds followed over 10 years. Eur. J. Neurol. 2015, 22, 781-e50. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Xiao, N.; Zeng, G.; Bi, D.; Dai, X.; Mi, X.; Ye, Q.; Chen, X.; Zhang, J. APOE4 genotype exacerbates the depression-like behavior of mice during aging through ATP decline. Transl. Psychiatry 2021, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Bonk, S.; Kirchner, K.; Ameling, S.; Garvert, L.; Volzke, H.; Nauck, M.; Volker, U.; Grabe, H.J.; Van der Auwera, S. APOE epsilon4 in Depression-Associated Memory Impairment-Evidence from Genetic and MicroRNA Analyses. Biomedicines 2022, 10, 1560. [Google Scholar] [CrossRef]
- van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briede, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Valero, S.; Marquie, M.; De Rojas, I.; Espinosa, A.; Moreno-Grau, S.; Orellana, A.; Montrreal, L.; Hernandez, I.; Mauleon, A.; Rosende-Roca, M.; et al. Interaction of neuropsychiatric symptoms with APOE epsilon4 and conversion to dementia in MCI patients in a Memory Clinic. Sci. Rep. 2020, 10, 20058. [Google Scholar] [CrossRef] [PubMed]
- Raber, J. Role of apolipoprotein E in anxiety. Neural Plast. 2007, 2007, 91236. [Google Scholar] [CrossRef] [PubMed]
- de Leon, M.J.; McRae, T.; Rusinek, H.; Convit, A.; De Santi, S.; Tarshish, C.; Golomb, J.; Volkow, N.; Daisley, K.; Orentreich, N.; et al. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer’s disease. J. Clin. Endocrinol. Metab. 1997, 82, 3251–3259. [Google Scholar] [CrossRef][Green Version]
- Nijhuis, E.W.; Oostervink, F.; Hinloopen, B.; Rozing, J.; Nagelkerken, L. Differences in dexamethasone-sensitivity between lymphocytes from patients with Alzheimer’s disease and patients with multi-infarct dementia. Brain Behav. Immun. 1996, 10, 115–125. [Google Scholar] [CrossRef]
- den Heijer, T.; Oudkerk, M.; Launer, L.J.; van Duijn, C.M.; Hofman, A.; Breteler, M.M. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 2002, 59, 746–748. [Google Scholar] [CrossRef]
- Mosca, A.; Sperduti, S.; Pop, V.; Ciavardelli, D.; Granzotto, A.; Punzi, M.; Stuppia, L.; Gatta, V.; Assogna, F.; Banaj, N.; et al. Influence of APOE and RNF219 on Behavioral and Cognitive Features of Female Patients Affected by Mild Cognitive Impairment or Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 92. [Google Scholar] [CrossRef]
- Woods, D.L.; Bushnell, B.; Kim, H.; Geschwind, D.; Cummings, J. Apolipoprotein epsilon4 status is associated with behavioral symptoms in nursing home residents with dementia. Int. Psychogeriatr. 2009, 21, 722–728. [Google Scholar] [CrossRef]
- Banning, L.C.P.; Ramakers, I.; Deckers, K.; Verhey, F.R.J.; Aalten, P. Apolipoprotein E and affective symptoms in mild cognitive impairment and Alzheimer’s disease dementia: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 302–315. [Google Scholar] [CrossRef]
- Creese, B.; Arathimos, R.; Brooker, H.; Aarsland, D.; Corbett, A.; Lewis, C.; Ballard, C.; Ismail, Z. Genetic risk for Alzheimer’s disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimer’s Dement. 2021, 13, e12164. [Google Scholar] [CrossRef] [PubMed]
- Creese, B.; Arathimos, R.; Aarsland, D.; Ballard, C.; Brooker, H.; Hampshire, A.; Corbett, A.; Ismail, Z. Late-life onset psychotic symptoms and incident cognitive impairment in people without dementia: Modification by genetic risk for Alzheimer’s disease. Alzheimer’s Dement. 2023, 9, e12386. [Google Scholar] [CrossRef]
- Liew, T.M. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer’s and non-Alzheimer’s dementia. Alzheimer’s Res. Ther. 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Fischer, C.E.; Schweizer, T.A.; Munoz, D.G. Association between Psychosis Phenotype and APOE Genotype on the Clinical Profiles of Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jonas, K.; Clouston, S.; Li, K.; Fochtmann, L.J.; Lencz, T.; Malhotra, A.K.; Cicero, D.; Perlman, G.; Bromet, E.J.; Kotov, R. Apolipoprotein E-epsilon4 allele predicts escalation of psychotic symptoms in late adulthood. Schizophr. Res. 2019, 206, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Vellone, D.; Ghahremani, M.; Goodarzi, Z.; Forkert, N.D.; Smith, E.E.; Ismail, Z. Apathy and APOE in mild behavioral impairment, and risk for incident dementia. Alzheimer’s Dement. 2022, 8, e12370. [Google Scholar] [CrossRef]
- Sheikh, F.; Ismail, Z.; Mortby, M.E.; Barber, P.; Cieslak, A.; Fischer, K.; Granger, R.; Hogan, D.B.; Mackie, A.; Maxwell, C.J.; et al. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int. Psychogeriatr. 2018, 30, 233–244. [Google Scholar] [CrossRef]
- Pink, A.; Stokin, G.B.; Bartley, M.M.; Roberts, R.O.; Sochor, O.; Machulda, M.M.; Krell-Roesch, J.; Knopman, D.S.; Acosta, J.I.; Christianson, T.J.; et al. Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: A population-based study. Neurology 2015, 84, 935–943. [Google Scholar] [CrossRef]
- Ebrahim, I.M.; Ghahremani, M.; Camicioli, R.; Smith, E.E.; Ismail, Z. Effects of race, baseline cognition, and APOE on the association of affective dysregulation with incident dementia: A longitudinal study of dementia-free older adults. J. Affect. Disord. 2023, 332, 9–18. [Google Scholar] [CrossRef]
- Tan, E.Y.L.; Kohler, S.; Hamel, R.E.G.; Munoz-Sanchez, J.L.; Verhey, F.R.J.; Ramakers, I. Depressive Symptoms in Mild Cognitive Impairment and the Risk of Dementia: A Systematic Review and Comparative Meta-Analysis of Clinical and Community-Based Studies. J. Alzheimer’s Dis. 2019, 67, 1319–1329. [Google Scholar] [CrossRef]
- Dean, K.; Oulhaj, A.; Zamboni, G.; deJager, C.A.; Wilcock, G.K. Role of depression in predicting time to conversion to mild cognitive impairment. Am. J. Geriatr. Psychiatry 2014, 22, 727–734. [Google Scholar] [CrossRef]
- Nathan, S.; Gill, S.; Ismail, Z. ApoE ε4 status in pre-dementia risk states, mild behavioural impairment and subjective cognitive decline, and the risk of incident cognitive decline. Alzheimer’s Dement. 2020, 16, e046615. [Google Scholar] [CrossRef]
- Efthymiou, A.G.; Goate, A.M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 2017, 12, 43. [Google Scholar] [CrossRef]
- Eon Kuek, L.; Leffler, M.; Mackay, G.A.; Hulett, M.D. The MS4A family: Counting past 1, 2 and 3. Immunol. Cell Biol. 2016, 94, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, G.; Schindler, S.E.; Christensen, J.; McKay, N.S.; Liu, J.; Wang, S.; Sun, Z.; Hassenstab, J.; Su, Y.; et al. Baseline Microglial Activation Correlates with Brain Amyloidosis and Longitudinal Cognitive Decline in Alzheimer Disease. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1152. [Google Scholar] [CrossRef]
- Schaffer Aguzzoli, C.; Ferreira, P.C.L.; Povala, G.; Ferrari-Souza, J.P.; Bellaver, B.; Soares Katz, C.; Zalzale, H.; Lussier, F.Z.; Rohden, F.; Abbas, S.; et al. Neuropsychiatric Symptoms and Microglial Activation in Patients with Alzheimer Disease. JAMA Netw. Open 2023, 6, e2345175. [Google Scholar] [CrossRef]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.W.; Sprague, D.; Barrera, J.; Chiba-Falek, O. Shared genetic etiology underlying Alzheimer’s disease and major depressive disorder. Transl. Psychiatry 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Das, R.; Emon, M.P.Z.; Shahriar, M.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, S.N.; Islam, M.R. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021, 295, 113568. [Google Scholar] [CrossRef]
- Duriez, B.; Duquesnoy, P.; Escudier, E.; Bridoux, A.M.; Escalier, D.; Rayet, I.; Marcos, E.; Vojtek, A.M.; Bercher, J.F.; Amselem, S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 2007, 104, 3336–3341. [Google Scholar] [CrossRef]
- Kim, S.H.; Fountoulakis, M.; Cairns, N.J.; Lubec, G. Human brain nucleoside diphosphate kinase activity is decreased in Alzheimer’s disease and Down syndrome. Biochem. Biophys. Res. Commun. 2002, 296, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J.T.; Wang, H.F.; Hao, X.K.; Yang, Y.F.; Jiang, T.; Zhu, X.C.; Cao, L.; Zhang, D.Q.; Tan, L. Association between NME8 locus polymorphism and cognitive decline, cerebrospinal fluid and neuroimaging biomarkers in Alzheimer’s disease. PLoS ONE 2014, 9, e114777. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, M.S.; Wang, H.F.; Zhang, W.; Wang, Z.X.; Jiang, T.; Yu, J.T.; Tan, L. ZCWPW1 is associated with late-onset Alzheimer’s disease in Han Chinese: A replication study and meta-analyses. Oncotarget 2016, 7, 20305–20311. [Google Scholar] [CrossRef]
- Ponnusamy, M.; Wang, S.; Yuksel, M.; Hansen, M.T.; Blazier, D.M.; McMillan, J.D.; Zhang, X.; Dammer, E.B.; Collier, L.; Thinakaran, G. Loss of forebrain BIN1 attenuates hippocampal pathology and neuroinflammation in a tauopathy model. Brain J. Neurol. 2023, 146, 1561–1579. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Ossenkoppele, R.; Brendel, M.; Rubinski, A.; Smith, R.; Kumar, A.; Mattsson-Carlgren, N.; Strandberg, O.; Duering, M.; Buerger, K.; et al. The BIN1 rs744373 Alzheimer’s disease risk SNP is associated with faster Aβ-associated tau accumulation and cognitive decline. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.S.; Kirkwood, C.M.; Gray, M.C.; Fish, K.N.; Ikonomovic, M.D.; Hamilton, R.L.; Kofler, J.K.; Klunk, W.E.; Lopez, O.L.; Sweet, R.A. Hyperphosphorylated tau is elevated in Alzheimer’s disease with psychosis. J. Alzheimer’s Dis. 2014, 39, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.F.; Lee, W.J.; Yeh, Y.C.; Liao, Y.C.; Wang, S.J.; Yang, Y.H.; Chen, C.S.; Fuh, J.L. Genetics of neuropsychiatric symptoms in patients with Alzheimer’s disease: A 1-year follow-up study. Psychiatry Clin. Neurosci. 2020, 74, 645–651. [Google Scholar] [CrossRef]
- Buhl, E.; Kim, Y.A.; Parsons, T.; Zhu, B.; Santa-Maria, I.; Lefort, R.; Hodge, J.J.L. Effects of Eph/ephrin signalling and human Alzheimer’s disease-associated EphA1 on Drosophila behaviour and neurophysiology. Neurobiol. Dis. 2022, 170, 105752. [Google Scholar] [CrossRef]
- Eysert, F.; Coulon, A.; Boscher, E.; Vreulx, A.C.; Flaig, A.; Mendes, T.; Hughes, S.; Grenier-Boley, B.; Hanoulle, X.; Demiautte, F.; et al. Alzheimer’s genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner. Mol. Psychiatry 2021, 26, 5592–5607. [Google Scholar] [CrossRef]
- Qin, W.; Liu, C.; Sodhi, M.; Lu, H. Meta-analysis of sex differences in gene expression in schizophrenia. BMC Syst. Biol. 2016, 10 (Suppl. 1), 9. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, J.; Cheng, S.; Pan, C.; Liu, L.; Meng, P.; Yang, X.; Wei, W.; Liu, H.; Jia, Y.; et al. Associations of classical HLA alleles with depression and anxiety. HLA 2023, 103, e15173. [Google Scholar] [CrossRef] [PubMed]
- Vica, M.L.; Delcea, C.; Dumitrel, G.A.; Vuscan, M.E.; Matei, H.V.; Teodoru, C.A.; Siserman, C.V. The Influence of HLA Alleles on the Affective Distress Profile. Int. J. Environ. Res. Public Health 2022, 19, 12608. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.; Donaldson, P.T.; Underhill, J.A.; Choudhuri, K.; Doherty, D.G.; Murray, R.M. Genetic association of the HLA DRB1 gene locus on chromosome 6p21.3 with schizophrenia. Am. J. Psychiatry 1996, 153, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Wang, H.F.; Tan, L.; Liu, J.; Wan, Y.; Sun, F.R.; Tan, M.S.; Tan, C.C.; Jiang, T.; Tan, L.; et al. Effects of HLA-DRB1/DQB1 Genetic Variants on Neuroimaging in Healthy, Mild Cognitive Impairment, and Alzheimer’s Disease Cohorts. Mol. Neurobiol. 2017, 54, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Choo, I.H.; Lee, D.Y.; Oh, J.S.; Lee, J.S.; Lee, D.S.; Song, I.C.; Youn, J.C.; Kim, S.G.; Kim, K.W.; Jhoo, J.H.; et al. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2010, 31, 772–779. [Google Scholar] [CrossRef]
- Lee, P.L.; Chou, K.H.; Chung, C.P.; Lai, T.H.; Zhou, J.H.; Wang, P.N.; Lin, C.P. Posterior Cingulate Cortex Network Predicts Alzheimer’s Disease Progression. Front. Aging Neurosci. 2020, 12, 608667. [Google Scholar] [CrossRef]
- Jones, D.T.; Knopman, D.S.; Gunter, J.L.; Graff-Radford, J.; Vemuri, P.; Boeve, B.F.; Petersen, R.C.; Weiner, M.W.; Jack, C.R., Jr. Cascading network failure across the Alzheimer’s disease spectrum. Brain J. Neurol. 2016, 139, 547–562. [Google Scholar] [CrossRef]
- Chen, Y.; Dang, M.; Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Mol. Neurodegener. 2021, 16, 38. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Bozi, M.; Simitsi, A.M.; Koros, C.; Antonelou, R.; Papagiannakis, N.; Maniati, M.; Poula, D.; Stamelou, M.; Vassilatis, D.K.; et al. Clinical differences between early-onset and mid-and-late-onset Parkinson’s disease: Data analysis of the Hellenic Biobank of Parkinson’s disease. J. Neurol. Sci. 2022, 442, 120405. [Google Scholar] [CrossRef]
- Brody, A.H.; Nies, S.H.; Guan, F.; Smith, L.M.; Mukherjee, B.; Salazar, S.A.; Lee, S.; Lam, T.K.T.; Strittmatter, S.M. Alzheimer risk gene product Pyk2 suppresses tau phosphorylation and phenotypic effects of tauopathy. Mol. Neurodegener. 2022, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Montalban, E.; Al-Massadi, O.; Sancho-Balsells, A.; Brito, V.; de Pins, B.; Alberch, J.; Gines, S.; Girault, J.A.; Giralt, A. Pyk2 in the amygdala modulates chronic stress sequelae via PSD-95-related micro-structural changes. Transl. Psychiatry 2019, 9, 3. [Google Scholar] [CrossRef]
- Creese, B.; Arathimos, R.; Brooker, H.; Corbett, A.; Aarsland, D.; Lewis, C.; Ballard, X.; Ismail, Z. Self and informant-rated mild behavioral impairment and genetic risk for AD: The respondent matters. Alzheimer’s Dement. 2021, 17 (Suppl. 6), e055314. [Google Scholar] [CrossRef]
- Azocar, I.; Rapaport, P.; Burton, A.; Meisel, G.; Orgeta, V. Risk factors for apathy in Alzheimer’s disease: A systematic review of longitudinal evidence. Ageing Res. Rev. 2022, 79, 101672. [Google Scholar] [CrossRef]
- Flirski, M.; Sieruta, M.; Golanska, E.; Kloszewska, I.; Liberski, P.P.; Sobow, T. PRND 3′UTR polymorphism may be associated with behavioral disturbances in Alzheimer disease. Prion 2012, 6, 73–80. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.; Scheltens, P. Heterogeneity of Alzheimer’s disease: Consequence for drug trials? Alzheimer’s Res. Ther. 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Escott-Price, V.; Sims, R.; Bannister, C.; Harold, D.; Vronskaya, M.; Majounie, E.; Badarinarayan, N.; Gerad/Perades; Consortia, I.; Morgan, K.; et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain J. Neurol. 2015, 138, 3673–3684. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.J.; Hill, W.D.; Marioni, R.E.; Davies, G.; Hagenaars, S.P.; Harris, S.E.; Cox, S.R.; Taylor, A.M.; Corley, J.; Pattie, A.; et al. Polygenic predictors of age-related decline in cognitive ability. Mol. Psychiatry 2020, 25, 2584–2598. [Google Scholar] [CrossRef]
- Harris, S.E.; Davies, G.; Luciano, M.; Payton, A.; Fox, H.C.; Haggarty, P.; Ollier, W.; Horan, M.; Porteous, D.J.; Starr, J.M.; et al. Polygenic risk for Alzheimer’s disease is not associated with cognitive ability or cognitive aging in non-demented older people. J. Alzheimer’s Dis. 2014, 39, 565–574. [Google Scholar] [CrossRef]
- Darst, B.F.; Koscik, R.L.; Racine, A.M.; Oh, J.M.; Krause, R.A.; Carlsson, C.M.; Zetterberg, H.; Blennow, K.; Christian, B.T.; Bendlin, B.B.; et al. Pathway-Specific Polygenic Risk Scores as Predictors of Amyloid-β Deposition and Cognitive Function in a Sample at Increased Risk for Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 473–484. [Google Scholar] [CrossRef]
- Kassam, F.; Chen, H.; Nosheny, R.L.; McGirr, A.; Williams, T.; Ng, N.; Camacho, M.; Mackin, R.S.; Weiner, M.W.; Ismail, Z. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int. Psychogeriatr. 2023, 35, 643–652. [Google Scholar] [CrossRef]
- Creese, B.; Griffiths, A.; Brooker, H.; Corbett, A.; Aarsland, D.; Ballard, C.; Ismail, Z. Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in cognitively normal older adults. Int. Psychogeriatr. 2020, 32, 705–717. [Google Scholar] [CrossRef]
- Papastefanopoulou, V.; Stanitsa, E.; Koros, C.; Simoudis, A.; Florou-Hatziyiannidou, C.; Beratis, I.; Antonelou, R.; Andronas, N.; Voskou, P.; Angelopoulou, E.; et al. APOE Allele Frequency in Southern Greece: Exploring the Role of Geographical Gradient in the Greek Population. Geriatrics 2022, 8, 1. [Google Scholar] [CrossRef]
- Ringman, J.M.; Liang, L.J.; Zhou, Y.; Vangala, S.; Teng, E.; Kremen, S.; Wharton, D.; Goate, A.; Marcus, D.S.; Farlow, M.; et al. Early behavioural changes in familial Alzheimer’s disease in the Dominantly Inherited Alzheimer Network. Brain J. Neurol. 2015, 138, 1036–1045. [Google Scholar] [CrossRef][Green Version]
- Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Chan, Y.H.; Jiang, L.; Therriault, J.; Benedet, A.L.; Shin, M.; Kandiah, N.; Greenwood, C.M.T.; et al. Neuropsychiatric symptoms are early indicators of an upcoming metabolic decline in Alzheimer’s disease. Transl. Neurodegener. 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Deste, G.; Smieskova, R.; Barlati, S.; Yung, A.R.; Howes, O.; Stieglitz, R.D.; Vita, A.; McGuire, P.; Borgwardt, S. Cognitive functioning in prodromal psychosis: A meta-analysis. Arch. Gen. Psychiatry 2012, 69, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Kremen, W.S.; Seidman, L.J.; Pepple, J.R.; Lyons, M.J.; Tsuang, M.T.; Faraone, S.V. Neuropsychological risk indicators for schizophrenia: A review of family studies. Schizophr. Bull. 1994, 20, 103–119. [Google Scholar] [CrossRef]
- Sitskoorn, M.M.; Aleman, A.; Ebisch, S.J.; Appels, M.C.; Kahn, R.S. Cognitive deficits in relatives of patients with schizophrenia: A meta-analysis. Schizophr. Res. 2004, 71, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Seidman, L.J.; Giuliano, A.J.; Smith, C.W.; Stone, W.S.; Glatt, S.J.; Meyer, E.; Faraone, S.V.; Tsuang, M.T.; Cornblatt, B. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: Results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr. Bull. 2006, 32, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.P.; Berman, K.F. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacol. 2010, 35, 258–277. [Google Scholar] [CrossRef]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef]
- Mattay, V.S.; Goldberg, T.E.; Fera, F.; Hariri, A.R.; Tessitore, A.; Egan, M.F.; Kolachana, B.; Callicott, J.H.; Weinberger, D.R. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. USA 2003, 100, 6186–6191. [Google Scholar] [CrossRef]
- Bertolino, A.; Rubino, V.; Sambataro, F.; Blasi, G.; Latorre, V.; Fazio, L.; Caforio, G.; Petruzzella, V.; Kolachana, B.; Hariri, A.; et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 2006, 60, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Chowdari, K.V.; Mirnics, K.; Semwal, P.; Wood, J.; Lawrence, E.; Bhatia, T.; Deshpande, S.N.; Thelma, B.K.; Ferrell, R.E.; Middleton, F.A.; et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum. Mol. Genet. 2002, 11, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.M.; Chowdari, K.V.; Nimgaonkar, V.L.; Talkowski, M.E.; Lewis, D.A.; Keshavan, M.S. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol. Psychiatry 2005, 10, 213–219. [Google Scholar] [CrossRef]
- Marenco, S.; Steele, S.U.; Egan, M.F.; Goldberg, T.E.; Straub, R.E.; Sharrief, A.Z.; Weinberger, D.R. Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate measures in the dorsolateral prefrontal cortex. Am. J. Psychiatry 2006, 163, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Straub, R.E.; Goldberg, T.E.; Yakub, I.; Callicott, J.H.; Hariri, A.R.; Mattay, V.S.; Bertolino, A.; Hyde, T.M.; Shannon-Weickert, C.; et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2004, 101, 12604–12609. [Google Scholar] [CrossRef]
- Delano-Wood, L.; Houston, W.S.; Emond, J.A.; Marchant, N.L.; Salmon, D.P.; Jeste, D.V.; Thal, L.J.; Bondi, M.W. APOE genotype predicts depression in women with Alzheimer’s disease: A retrospective study. Int. J. Geriatr. Psychiatry 2008, 23, 632–636. [Google Scholar] [CrossRef]
- Xing, Y.; Tang, Y.; Jia, J. Sex Differences in Neuropsychiatric Symptoms of Alzheimer’s Disease: The Modifying Effect of Apolipoprotein E epsilon4 Status. Behav. Neurol. 2015, 2015, 275256. [Google Scholar] [CrossRef]
- Wood, M.E.; Xiong, L.Y.; Wong, Y.Y.; Buckley, R.F.; Swardfager, W.; Masellis, M.; Lim, A.S.P.; Nichols, E.; Joie, R.; Casaletto, K.B.; et al. Sex differences in associations between APOE epsilon2 and longitudinal cognitive decline. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.S.; Tan, Y.B.; Bowie, C.R.; Butters, M.A.; Flint, A.J.; Gallagher, D.; Golas, A.C.; Herrmann, N.; Ismail, Z.; Kennedy, J.L.; et al. Sex Modifies the Associations of APOEvarepsilon4 with Neuropsychiatric Symptom Burden in Both at-Risk and Clinical Cohorts of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Zhou, X.; Wang, Z.; Zhang, Y.; Wang, Z.; Zhou, G.; Zhao, Y.; Liu, M.; Lu, H.; Zhao, H. Sex-specific genetic association between psychiatric disorders and cognition, behavior and brain imaging in children and adults. Transl. Psychiatry 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Perry, R.; Barber, R.; Gholkar, A.; Thomas, A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann. N. Y. Acad. Sci. 2000, 903, 482–489. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, F.E.; Richard, F.; de Groot, J.C.; van Duijn, C.M.; Hofman, A.; Van Gijn, J.; Breteler, M.M. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 2004, 35, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Laukka, E.J.; Dekhtyar, S.; Papenberg, G.; Speh, A.; Fratiglioni, L.; Kalpouzos, G.; Qiu, C. Association between Behavioral, Biological, and Genetic Markers of Cardiovascular Health and MRI Markers of Brain Aging: A Cohort Study. Neurology 2023, 100, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Rutten-Jacobs, L.C.A.; Tozer, D.J.; Duering, M.; Malik, R.; Dichgans, M.; Markus, H.S.; Traylor, M. Genetic Study of White Matter Integrity in UK Biobank (N = 8448) and the Overlap with Stroke, Depression, and Dementia. Stroke 2018, 49, 1340–1347. [Google Scholar] [CrossRef]
- Schork, N.J.; Elman, J.A. Pathway-Specific Polygenic Risk Scores Correlate with Clinical Status and Alzheimer’s Disease-Related Biomarkers. J. Alzheimer’s Dis. 2023, 95, 915–929. [Google Scholar] [CrossRef]
- Lussier, F.Z.; Pascoal, T.A.; Chamoun, M.; Therriault, J.; Tissot, C.; Savard, M.; Kang, M.S.; Mathotaarachchi, S.; Benedet, A.L.; Parsons, M.; et al. Mild behavioral impairment is associated with β-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2020, 16, 192–199. [Google Scholar] [CrossRef]
- Ramezani, M.; Ruskey, J.A.; Martens, K.; Kibreab, M.; Javer, Z.; Kathol, I.; Hammer, T.; Cheetham, J.; Leveille, E.; Martino, D.; et al. Association between BDNF Val66Met Polymorphism and Mild Behavioral Impairment in Patients with Parkinson’s Disease. Front. Neurol. 2020, 11, 587992. [Google Scholar] [CrossRef]
- Nagata, T.; Kobayashi, N.; Shinagawa, S.; Yamada, H.; Kondo, K.; Nakayama, K. Plasma BDNF levels are correlated with aggressiveness in patients with amnestic mild cognitive impairment or Alzheimer disease. J. Neural Transm. 2014, 121, 433–441. [Google Scholar] [CrossRef]
- Nacmias, B.; Tedde, A.; Forleo, P.; Piacentini, S.; Guarnieri, B.M.; Bartoli, A.; Ortenzi, L.; Petruzzi, C.; Serio, A.; Marcon, G.; et al. Association between 5-HT(2A) receptor polymorphism and psychotic symptoms in Alzheimer’s disease. Biol. Psychiatry 2001, 50, 472–475. [Google Scholar] [CrossRef]
- Radojevic, B.; Dragasevic-Miskovic, N.T.; Marjanovic, A.; Brankovic, M.; Dobricic, V.; Milovanovic, A.; Tomic, A.; Svetel, M.; Petrovic, I.; Jancic, I.; et al. Clinical and Genetic Analysis of Psychosis in Parkinson’s Disease. J. Park. Dis. 2021, 11, 1973–1980. [Google Scholar] [CrossRef]
- Huey, E.D.; Fremont, R.; Manoochehri, M.; Gazes, Y.; Lee, S.; Cosentino, S.; Tierney, M.; Wassermann, E.M.; Momeni, P.; Grafman, J. Effect of Functional BDNF and COMT Polymorphisms on Symptoms and Regional Brain Volume in Frontotemporal Dementia and Corticobasal Syndrome. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 362–369. [Google Scholar] [CrossRef]
- Yokoyama, J.S.; Bonham, L.W.; Sturm, V.E.; Adhimoolam, B.; Karydas, A.; Coppola, G.; Miller, B.L.; Rankin, K.P. The 5-HTTLPR variant in the serotonin transporter gene modifies degeneration of brain regions important for emotion in behavioral variant frontotemporal dementia. NeuroImage Clin. 2015, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Papageorgiou, S.G.; Piperi, C. APOE Genotype and Alzheimer’s Disease: The Influence of Lifestyle and Environmental Factors. ACS Chem. Neurosci. 2021, 12, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hong, Y.; Li, W.; Wang, A.; Jiang, S.; Jiang, T.; Wang, Y.; Wang, L.; Yang, S.; Ren, Q.; et al. Chain Mediation Analysis of the Effects of Nutrition and Cognition on the Association of Apolipoprotein E varepsilon4 with Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 96, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, J.; Yang, Y.; Zhang, Z.; Zhong, H.; Zeng, G.; Zhou, D.; Nowakowski, R.S.; Long, J.; Wu, C.; et al. Identification of candidate DNA methylation biomarkers related to Alzheimer’s disease risk by integrating genome and blood methylome data. Transl. Psychiatry 2023, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, S.; Gingerich, D.; Lutz, M.W.; Chiba-Falek, O. Differential Gene Expression and DNA Methylation in the Risk of Depression in LOAD Patients. Biomolecules 2022, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Su, M.H.; Liao, S.C.; Chen, H.C.; Lu, M.L.; Chen, W.Y.; Hsiao, P.C.; Chen, C.H.; Huang, M.C.; Kuo, P.H. The association of personality polygenic risk score, psychosocial protective factors and suicide attempt in mood disorder. J. Psychiatr. Res. 2022, 156, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, S.; Liu, H.; Luo, S.; Lutz, M.W.; Chiba-Falek, O. Polygenic Risk Score Effectively Predicts Depression Onset in Alzheimer’s Disease Based on Major Depressive Disorder Risk Variants. Front. Neurosci. 2022, 16, 827447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Robinson, D.; Yu, J.; Gallego, J.; Fleischhacker, W.W.; Kahn, R.S.; Crespo-Facorro, B.; Vazquez-Bourgon, J.; Kane, J.M.; Malhotra, A.K.; et al. Schizophrenia Polygenic Risk Score as a Predictor of Antipsychotic Efficacy in First-Episode Psychosis. Am. J. Psychiatry 2019, 176, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.S.; Gottesman, R.T.; Manoochehri, M.; Chapman, S.; Appleby, B.S.; Brushaber, D.; Devick, K.L.; Dickerson, B.C.; Domoto-Reilly, K.; Fields, J.A.; et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain J. Neurol. 2022, 145, 1079–1097. [Google Scholar] [CrossRef] [PubMed]
- Taragano, F.E.; Allegri, R.F.; Krupitzki, H.; Sarasola, D.R.; Serrano, C.M.; Lon, L.; Lyketsos, C.G. Mild behavioral impairment and risk of dementia: A prospective cohort study of 358 patients. J. Clin. Psychiatry 2009, 70, 584–592. [Google Scholar] [CrossRef]
| Study | Country | Study Type | Aims | Study Participants | MBI Assessment | Main Results |
|---|---|---|---|---|---|---|
| Creese et al., 2021 [40] (data derived from a PROTECT study) | United Kingdom | Cross-sectional | To investigate whether the stratification of cognitively older individuals by mild behavioral impairment (MBI) affects the association between polygenic risk scores (PRSs) for Alzheimer’s disease (AD) and cognitive function | Non-demented individuals, ≥50 years of age, with access to a computer and internet, with available genotype, cognitive function and MBI-Checlist (MBI-C) data, without mild cognitive impairment (MCI), Parkinson’s disease (PD), or stroke (n = 4458) | MBI-C (cut-off points: zero and six in a post-hoc analysis) |
|
| Creese et al., 2021 [40] (data derived from a PROTECT study) | United Kingdom | Cross-sectional | To examine the relationship between PRSs for AD and MBI domains using both proxy informant and self-rated MBI-C responses | Non-demented individuals, ≥65 years of age, with access to a computer and internet, with available genotype, and MBI-C data (n = 2529) | MBI-C (measured by proxy informants and self-ratings) |
|
| Creese et al., 2023 [41] (data derived from a PROTECT study) | United Kingdom | Longitudinal | To investigate the relationship between MBI psychosis and incident cognitive impairment [annually assessed by Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)] and whether this relationship was modified by gender and genetic risk for AD (APOE e4) | Non-demented individuals, ≥50 years of age, with access to a computer and internet, with available genotype, and MBI-C data, without MCI, and IQCODE < 3.6 at baseline, without PD, epilepsy, multiple sclerosis, or stroke (n = 2750) | MBI-C (the only domain of psychosis used in this study, with the cut-off of >0 in at least one of the five items of the MBI-C that are related to psychosis) |
|
| Nathan et al., 2020 [51] (data derived from the National Alzheimer’s Coordinating Center (NACC)) | USA | Longitudinal | To cross-sectionally determine the frequency of APOE e4 homozygosity among individuals with subjective cognitive decline (SCD), stratified by MBI status | Non-demented older individuals with normal cognition but with SCD (n = 5005) | Neuropsychiatric Inventory Questionnaire (NPI-Q) according to the published algorithm by Sheikh and colleagues, 2018 [46] |
|
| Andrews et al., 2018 [22] (data derived from the Personality and Total Health Through Life project (PATH)) | Australia | Cross-sectional | To investigate if PRSs for AD and specific genetic variants that are associated with a higher risk for AD have shared genetic factors related to MBI | Non-demented older individuals of European ancestry, ≥60 years of age, without APOE e2/e4 genotype and with normal cognition, or MCI (n = 1226) | NPI-Q (according to the published algorithm by Sheikh and colleagues, 2018 [46]) |
|
| Vellone et al., 2022 [45] (data derived from the National Alzheimer’s Coordinating Center (NACC)) | USA | Longitudinal | To investigate whether MBI apathy is associated with progression to dementia, and if this relationship is modified by sex, race, cognitive diagnosis, and APOE genotype | Non-demented individuals with normal cognition or MCI, without past psychiatric, developmental, or neurological conditions, including post-traumatic stress disorder, bipolar disorder, schizophrenia, obsessive-compulsive disorder, anxiety, depression, Down syndrome, Huntington’s disease, or PD, and with available data for APOE genotype, cognitive status, age, race, and years of education (n = 3932) | NPI-Q according to the published algorithm by Sheikh and colleagues in 2018 [46] at two consecutive annual visits (only the domain of apathy was investigated in this study; the MBI apathy group included participants with NPI-Q subscore for apathy > 0 in both visits, and no prior psychiatric diagnosis; the NPS apathy group included participants with NPI-Q subscores for apathy > 0 in the first visit without considering the psychiatric history) |
|
| Ebrahim et al., 2023 [48] (data derived from the National Alzheimer’s Coordinating Center (NACC)) | USA | Longitudinal | To investigate the longitudinal relationship between MBI affective dysregulation and incident dementia | Non-demented individuals with normal cognition or MCI, without past psychiatric or neurodevelopmental disorders, and with available data for APOE genotype, cognitive status, age, race, and years of education (n = 4984) | NPI-Q according to the published algorithm by Sheikh and colleagues in 2018 [46] at two consecutive annual visits (MBI affective dysregulation domain was defined as the NPI-Q subscore for depression, anxiety, or elation > 0 in both consecutive visits) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, E.; Koros, C.; Hatzimanolis, A.; Stefanis, L.; Scarmeas, N.; Papageorgiou, S.G. Exploring the Genetic Landscape of Mild Behavioral Impairment as an Early Marker of Cognitive Decline: An Updated Review Focusing on Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 2645. https://doi.org/10.3390/ijms25052645
Angelopoulou E, Koros C, Hatzimanolis A, Stefanis L, Scarmeas N, Papageorgiou SG. Exploring the Genetic Landscape of Mild Behavioral Impairment as an Early Marker of Cognitive Decline: An Updated Review Focusing on Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(5):2645. https://doi.org/10.3390/ijms25052645
Chicago/Turabian StyleAngelopoulou, Efthalia, Christos Koros, Alexandros Hatzimanolis, Leonidas Stefanis, Nikolaos Scarmeas, and Sokratis G. Papageorgiou. 2024. "Exploring the Genetic Landscape of Mild Behavioral Impairment as an Early Marker of Cognitive Decline: An Updated Review Focusing on Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 5: 2645. https://doi.org/10.3390/ijms25052645
APA StyleAngelopoulou, E., Koros, C., Hatzimanolis, A., Stefanis, L., Scarmeas, N., & Papageorgiou, S. G. (2024). Exploring the Genetic Landscape of Mild Behavioral Impairment as an Early Marker of Cognitive Decline: An Updated Review Focusing on Alzheimer’s Disease. International Journal of Molecular Sciences, 25(5), 2645. https://doi.org/10.3390/ijms25052645







