Sex-Biased Expression and Response of microRNAs in Neurological Diseases and Neurotrauma
Abstract
1. Introduction
2. Overview of microRNAs
3. Sex-Biased miRNAs in Neurodegenerative Diseases and Neuropsychiatric Disorders
4. Sex-Biased miRNAs in Cerebral Vascular Diseases
5. Sex-Biased miRNAs in Traumatic Brain Injury (TBI)
6. Impacts of X Chromosome-Linked miRNAs
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirokova, O.; Zaborskaya, O.; Pchelin, P.; Kozliaeva, E.; Pershin, V.; Mukhina, I. Genetic and Epigenetic Sexual Dimorphism of Brain Cells during Aging. Brain Sci. 2023, 13, 195. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog. Brain Res. 2010, 186, 77–95. [Google Scholar]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: A Review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- May, T.; Adesina, I.; McGillivray, J.; Rinehart, N.J. Sex differences in neurodevelopmental disorders. Curr. Opin. Neurol. 2019, 32, 622–626. [Google Scholar] [CrossRef]
- Cahill, L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.-C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Sex differences in the developing brain as a source of inherent risk. Dialog. Clin. Neurosci. 2016, 18, 361–372. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.J. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology 2004, 145, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving Knowledge of Sex Differences in Brain Structure, Function, and Chemistry. Biol. Psychiatry 2007, 62, 847–855. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Fisher, D.W.; Bennett, D.A.; Dong, H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol. Aging 2018, 70, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, C.; Odén, A.; Lycke, J. High nationwide prevalence of multiple sclerosis in Sweden. Mult. Scler. 2011, 17, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Wooten, G.F.; Currie, L.J.; Bovbjerg, V.E.; Lee, J.K.; Patrie, J. Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 2004, 75, 637–639. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Epidemiology of amyotrophic lateral sclerosis. Adv. Neurol. 1982, 36, 281–302. [Google Scholar] [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Pawelec, P.; Ziabska, K.; Zalewska, T. Sex Differences in Brain Disorders. Int. J. Mol. Sci. 2023, 24, 14571. [Google Scholar] [CrossRef]
- Payami, H.; Montee, K.R.; Kaye, J.A.; Bird, T.D.; Yu, C.E.; Wijsman, E.M.; Schellenberg, G.D. Alzheimer’s disease, apolipoprotein E4, and gender. JAMA 1994, 271, 1316–1317. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; Van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Ryu, H.-S.; Shin, E.; Park, Y.; Jeon, S.R.; Kim, S.Y.; Kim, J.S.; Koh, S.-B.; Chung, S.J. Ethnicity- and sex-specific genome wide association study on Parkinson’s disease. npj Park. Dis. 2023, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; McCullough, L.D. Sex differences in stroke: The contribution of coagulation. Exp. Neurol. 2014, 259, 16–27. [Google Scholar] [CrossRef]
- Martínez-Sánchez, P.; Fuentes, B.; Fernández-Domínguez, J.; Ortega-Casarrubios, M.d.l.Á.; Aguilar-Amar, M.J.; Abenza-Abildúa, M.J.; Idrovo-Freire, L.; Díez-Tejedor, E. Young Women Have Poorer Outcomes than Men after Stroke. Cerebrovasc. Dis. 2011, 31, 455–463. [Google Scholar] [CrossRef]
- Mo, C.; Hannan, A.J.; Renoir, T. Environmental factors as modulators of neurodegeneration: Insights from gene-environment interactions in Huntington’s disease. Neurosci. Biobehav. Rev. 2015, 52, 178–192. [Google Scholar] [CrossRef]
- Meoni, S.; Macerollo, A.; Moro, E. Sex differences in movement disorders. Nat. Rev. Neurol. 2020, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, D.; Marinus, J.; Roos, R.A.; De Michele, G. The influence of gender on phenotype and disease progression in patients with Huntington’s disease. Parkinsonism Relat. Disord. 2013, 19, 192–197. [Google Scholar] [CrossRef]
- Zielonka, D.; Stawinska-Witoszynska, B. Gender Differences in Non-sex Linked Disorders: Insights From Huntington’s Disease. Front. Neurol. 2020, 11, 571. [Google Scholar] [CrossRef]
- Levin, H.S.; Temkin, N.R.; Barber, J.; Nelson, L.D.; Robertson, C.; Brennan, J.; Stein, M.B.; Yue, J.K.; Giacino, J.T.; McCrea, M.A.; et al. Association of Sex and Age With Mild Traumatic Brain Injury–Related Symptoms: A TRACK-TBI Study. JAMA Netw. Open 2021, 4, e213046. [Google Scholar] [CrossRef]
- Gupte, R.P.; Brooks, W.M.; Vukas, R.R.; Pierce, J.D.; Harris, J.L. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef] [PubMed]
- Kirkness, C.J.; Burr, R.L.; Mitchell, P.H.; Newell, D.W. Is there a sex difference in the course following traumatic brain injury? Biol. Res. Nurs. 2004, 5, 299–310. [Google Scholar] [CrossRef]
- Mikolic, A.; van Klaveren, D.; Groeniger, J.O.; Wiegers, E.; Lingsma, H.F.; Zeldovich, M.; von Steinbuechel, N.; Maas, A.; van Lennep, J.E.R.; Polinder, S. Differences between Men and Women in Treatment and Outcome after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 235–251. [Google Scholar] [CrossRef]
- Bretzin, A.C.; Esopenko, C.; D’Alonzo, B.A.; Wiebe, D.J. Clinical Recovery Timelines After Sport-Related Concussion in Men’s and Women’s Collegiate Sports. J. Athl. Train. 2022, 57, 678–687. [Google Scholar] [CrossRef]
- Musko, P.A.; Demetriades, A.K. Are Sex Differences in Collegiate and High School Sports-Related Concussion Reflected in the Guidelines? A Scoping Review. Brain Sci. 2023, 13, 1310. [Google Scholar] [CrossRef]
- Bretzin, A.C.; Covassin, T.; Fox, M.E.; Petit, K.M.; Savage, J.L.; Walker, L.F.; Gould, D. Sex Differences in the Clinical Incidence of Concussions, Missed School Days, and Time Loss in High School Student-Athletes: Part 1. Am. J. Sports Med. 2018, 46, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Covassin, T.; Moran, R.; Elbin, R.J. Sex Differences in Reported Concussion Injury Rates and Time Loss From Participation: An Update of the National Collegiate Athletic Association Injury Surveillance Program From 2004–2005 Through 2008–2009. J. Athl. Train. 2016, 51, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.; Malmevik, J.; Fasching, L.; Åkerblom, M.; Jakobsson, J. miRNAs in brain development. Exp. Cell Res. 2014, 321, 84–89. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Lodish, H.F. MicroRNAs as regulators of mammalian hematopoiesis. Semin. Immunol. 2005, 17, 155–165. [Google Scholar] [CrossRef]
- Coolen, M.; Bally-Cuif, L. MicroRNAs in brain development and physiology. Curr. Opin. Neurobiol. 2009, 19, 461–470. [Google Scholar] [CrossRef]
- Schratt, G. Fine-tuning neural gene expression with microRNAs. Curr. Opin. Neurobiol. 2009, 19, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila MicroRNA Mir-14 Suppresses Cell Death and Is Required for Normal Fat Metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef]
- Giraldez, A.J.; Cinalli, R.M.; Glasner, M.E.; Enright, A.J.; Thomson, J.M.; Baskerville, S.; Hammond, S.M.; Bartel, D.P.; Schier, A.F. MicroRNAs regulate brain morphogenesis in zebrafish. Science 2005, 308, 833–838. [Google Scholar] [CrossRef]
- Rao, Y.S.; Pak, T.R. microRNAs and the adolescent brain: Filling the knowledge gap. Neurosci. Biobehav. Rev. 2016, 70, 313–322. [Google Scholar] [CrossRef]
- Rao, Y.S.; Mott, N.N.; Wang, Y.; Chung, W.C.; Pak, T.R. MicroRNAs in the Aging Female Brain: A Putative Mechanism for Age-Specific Estrogen Effects. Endocrinology 2013, 154, 2795–2806. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of Novel Genes Coding for Small Expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.-P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. MicroRNA precursors in motion: Exportin-5 mediates their nuclear export. Trends Cell Biol. 2004, 14, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Joshua-Tor, L. The Argonautes. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. Anatomy of RISC: How do small RNAs and chaperones activate Argonaute proteins? Wiley Interdiscip. Rev. RNA 2016, 7, 637–660. [Google Scholar] [CrossRef]
- Park, M.S.; Araya-Secchi, R.; Brackbill, J.A.; Phan, H.-D.; Kehling, A.C.; El-Wahab, E.W.A.; Dayeh, D.M.; Sotomayor, M.; Nakanishi, K. Multidomain Convergence of Argonaute during RISC Assembly Correlates with the Formation of Internal Water Clusters. Mol. Cell 2019, 75, 725–740.e6. [Google Scholar] [CrossRef]
- Alló, M.; Agirre, E.; Bessonov, S.; Bertucci, P.; Acuña, L.G.; Buggiano, V.; Bellora, N.; Singh, B.; Petrillo, E.; Blaustein, M.; et al. Argonaute-1 binds transcriptional enhancers and controls constitutive and alternative splicing in human cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15622–15629. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.-J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 Is the Catalytic Engine of Mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.-J.; Barad, D.; Gleicher, N.; Hammes, S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef]
- McFall, T.; McKnight, B.; Rosati, R.; Kim, S.; Huang, Y.; Viola-Villegas, N.; Ratnam, M. Progesterone receptor A promotes invasiveness and metastasis of luminal breast cancer by suppressing regulation of critical microRNAs by estrogen. J. Biol. Chem. 2018, 293, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Maillot, G.; Lacroix-Triki, M.; Pierredon, S.; Gratadou, L.; Schmidt, S.; Bénès, V.; Roché, H.; Dalenc, F.; Auboeuf, D.; Millevoi, S.; et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009, 69, 8332–8340. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- Dai, R.; Ahmed, S.A. Sexual dimorphism of miRNA expression: A new perspective in understanding the sex bias of autoimmune diseases. Ther. Clin. Risk Manag. 2014, 10, 151–163. [Google Scholar] [PubMed]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA regulation of gene expression: No smoke, just miRs. Biol. Sex Differ. 2012, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Pak, T.R.; Rao, Y.S.; Prins, S.A.; Mott, N.N. An emerging role for microRNAs in sexually dimorphic neurobiological systems. Pflüg. Arch. 2013, 465, 655–667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Modzelewski, A.J.; Holmes, R.J.; Hilz, S.; Grimson, A.; Cohen, P.E. AGO4 Regulates Entry into Meiosis and Influences Silencing of Sex Chromosomes in the Male Mouse Germline. Dev. Cell 2012, 23, 251–264. [Google Scholar] [CrossRef]
- Koturbash, I.; Zemp, F.; Kolb, B.; Kovalchuk, O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat. Res. 2011, 722, 114–118. [Google Scholar] [CrossRef]
- Ciaudo, C.; Servant, N.; Cognat, V.; Sarazin, A.; Kieffer, E.; Viville, S.; Colot, V.; Barillot, E.; Heard, E.; Voinnet, O. Highly Dynamic and Sex-Specific Expression of microRNAs During Early ES Cell Differentiation. PLoS Genet. 2009, 5, e1000620. [Google Scholar] [CrossRef]
- Langevin, S.M.; Stone, R.A.; Bunker, C.H.; Grandis, J.R.; Sobol, R.W.; Taioli, E. MicroRNA-137 promoter methylation in oral rinses from patients with squamous cell carcinoma of the head and neck is associated with gender and body mass index. Carcinog. 2010, 31, 864–870. [Google Scholar] [CrossRef]
- Siegel, C.; Li, J.; Liu, F.; Benashski, S.E.; McCullough, L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 2011, 108, 11662–11667. [Google Scholar] [CrossRef]
- Gumerov, V.; Hegyi, H. MicroRNA-derived network analysis of differentially methylated genes in schizophrenia, implicating GABA receptor B1 [GABBR1] and protein kinase B [AKT1]. Biol. Direct. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, J.; Sun, G.; Yan, J.; Noltner, K.; Li, W.; Buzin, C.H.; Longmate, J.; Heston, L.L.; Rossi, J.; Sommer, S.S. Evidence for X-Chromosomal Schizophrenia Associated with microRNA Alterations. PLoS ONE 2009, 4, e6121. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, S.; Logvinenko, T.; Volpe, M.V.; Nielsen, H.C. miRNA regulated pathways in late stage murine lung development. BMC Dev. Biol. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA-mRNA networks: Examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus. Biol. Sex Differ. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.P.; Bale, T.L. Early Prenatal Stress Epigenetically Programs Dysmasculinization in Second-Generation Offspring via the Paternal Lineage. J. Neurosci. 2011, 31, 11748–11755. [Google Scholar] [CrossRef] [PubMed]
- Szakats, S.; McAtamney, A.; Cross, H.; Wilson, M.J. Sex-biased gene and microRNA expression in the developing mouse brain is associated with neurodevelopmental functions and neurological phenotypes. Biol. Sex Differ. 2023, 14, 57. [Google Scholar] [CrossRef]
- Ameling, S.; Kacprowski, T.; Chilukoti, R.K.; Malsch, C.; Liebscher, V.; Suhre, K.; Pietzner, M.; Friedrich, N.; Homuth, G.; Hammer, E.; et al. Associations of circulating plasma microRNAs with age, body mass index and sex in a population-based study. BMC Med. Genom. 2015, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, W.; Shi, J.; Zhou, Y.; Yang, J.; Cui, Q.; Zhou, Y. Identification and Analysis of Human Sex-biased MicroRNAs. Genom. Proteom. Bioinform. 2018, 16, 200–211. [Google Scholar] [CrossRef]
- Zhong, B.; Cui, C.; Cui, Q. Identification and Analysis of Sex-Biased MicroRNAs in Human Diseases. Genes 2023, 14, 1688. [Google Scholar] [CrossRef]
- Murphy, S.J.; Lusardi, T.A.; Phillips, J.I.; Saugstad, J.A. Sex differences in microRNA expression during development in rat cortex. Neurochem. Int. 2014, 77, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chum, P.P.; Hakim, A.; Behringer, E.J. Cerebrovascular microRNA Expression Profile During Early Development of Alzheimer’s Disease in a Mouse Model. J. Alzheimer’s Dis. 2022, 85, 91–113. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Jicha, G.A.; Nelson, P.T.; Chan, C. Blood serum miRNA: Non-invasive biomarkers for Alzheimer’s disease. Exp. Neurol. 2012, 235, 491–496. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Chan, C. MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid β, novel targets in sporadic Alzheimer’s disease. J. Neurosci. 2011, 31, 14820–14830. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Moldawer, L.L.; Efron, P.A.; Foster, T.C. Influence of age and sex on microRNA response and recovery in the hippocampus following sepsis. Aging 2022, 14, 728–746. [Google Scholar] [CrossRef]
- Lusardi, T.A.; Murphy, S.J.; Phillips, J.I.; Chen, Y.; Davis, C.M.; Young, J.M.; Thompson, S.J.; Saugstad, J.A. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front. Mol. Neurosci. 2014, 7, 11. [Google Scholar] [CrossRef]
- Kodama, L.; Guzman, E.; Etchegaray, J.I.; Li, Y.; Sayed, F.A.; Zhou, L.; Zhou, Y.; Zhan, L.; Le, D.; Udeochu, J.C.; et al. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat. Neurosci. 2020, 23, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, V.; Merico, A.; Angelini, C. Micro-RNAs in ALS muscle: Differences in gender, age at onset and disease duration. J. Neurol. Sci. 2017, 380, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vallelunga, A.; Iannitti, T.; Somma, G.; Russillo, M.C.; Picillo, M.; De Micco, R.; Vacca, L.; Cilia, R.; Cicero, C.E.; Zangaglia, R.; et al. Gender differences in microRNA expression in levodopa-naive PD patients. J. Neurol. 2023, 270, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, Y.; Yu, M.; Wu, L.; Liu, F.; Ni, J.; Wang, Z.; Wang, J.; Fei, J.; Wang, W.; et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson’s disease. Sci. Rep. 2017, 7, 5411. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Piscopo, P.; Talarico, G.; Ricci, L.; Crestini, A.; Tosto, G.; Gasparini, M.; Bruno, G.; Denti, M.A.; Confaloni, A. Plasma microRNA profiling distinguishes patients with frontotemporal dementia from healthy subjects. Neurobiol. Aging 2019, 84, 240.e1–240.e12. [Google Scholar] [CrossRef]
- Mellios, N.; Galdzicka, M.; Ginns, E.; Baker, S.P.; Rogaev, E.; Xu, J.; Akbarian, S. Gender-Specific Reduction of Estrogen-Sensitive Small RNA, miR-30b, in Subjects With Schizophrenia. Schizophr. Bull. 2012, 38, 433–443. [Google Scholar] [CrossRef]
- Matarrese, P.; Tieri, P.; Anticoli, S.; Ascione, B.; Conte, M.; Franceschi, C.; Malorni, W.; Salvioli, S.; Ruggieri, A. X-chromosome-linked miR548am-5p is a key regulator of sex disparity in the susceptibility to mitochondria-mediated apoptosis. Cell Death Dis. 2019, 10, 673. [Google Scholar] [CrossRef]
- Prajapati, P.; Wang, W.-X.; Pesina, S.A.; Geleta, U.; Springer, J.E. Sex-Specific Alterations in Inflammatory MicroRNAs in Mouse Brain and Bone Marrow CD11b+ Cells Following Traumatic Brain Injury. Cell. Mol. Neurobiol. 2021, 43, 423–429. [Google Scholar] [CrossRef]
- Delaloy, C.; Liu, L.; Lee, J.-A.; Su, H.; Shen, F.; Yang, G.-Y.; Young, W.L.; Ivey, K.N.; Gao, F.-B. MicroRNA-9 Coordinates Proliferation and Migration of Human Embryonic Stem Cell-Derived Neural Progenitors. Cell Stem Cell 2010, 6, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; King, K.S.; Donahue, C.P.; Khrapko, K.; Kosik, K.S. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 2003, 9, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Ziats, M.N.; Rennert, O.M. Identification of differentially expressed microRNAs across the developing human brain. Mol. Psychiatry 2014, 19, 848–852. [Google Scholar] [CrossRef]
- Markham, J.A.; Koenig, J.I. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology 2011, 214, 89–106. [Google Scholar] [CrossRef]
- Kinney, D.K.; Miller, A.M.; Crowley, D.J.; Huang, E.; Gerber, E. Autism Prevalence Following Prenatal Exposure to Hurricanes and Tropical Storms in Louisiana. J. Autism Dev. Disord. 2008, 38, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Khashan, A.S.; Abel, K.M.; McNamee, R.; Pedersen, M.G.; Webb, R.T.; Baker, P.N.; Kenny, L.C.; Mortensen, P.B. Higher Risk of Offspring Schizophrenia Following Antenatal Maternal Exposure to Severe Adverse Life Events. Arch. Gen. Psychiatry 2008, 65, 146–152. [Google Scholar] [CrossRef] [PubMed]
- van Os, J.; Selten, J.P. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br. J. Psychiatry 1998, 172, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. Gender Differences in the Effects of Prenatal Stress on Brain Development and Behaviour. Neurochem. Res. 2007, 32, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Grundwald, N.J.; Brunton, P.J. Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology 2015, 62, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: An update. Stress 2011, 14, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic Transgenerational Actions of Vinclozolin on Promoter Regions of the Sperm Epigenome. PLoS ONE 2010, 5, e13100. [Google Scholar] [CrossRef]
- Maher, B. Personal genomes: The case of the missing heritability. Nature 2008, 456, 18–21. [Google Scholar] [CrossRef]
- Slatkin, M. Epigenetic Inheritance and the Missing Heritability Problem. Genetics 2009, 182, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.I.; Mori, H.; Kawata, M. Epigenetic mechanisms are involved in sexual differentiation of the brain. Rev. Endocr. Metab. Disord. 2012, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.R.; Bale, T.L. Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Wang, W.X.; Rajeev, B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008, 18, 130–138. [Google Scholar] [CrossRef]
- Liu, N.-K.; Xu, X.-M. MicroRNA in central nervous system trauma and degenerative disorders. Physiol. Genom. 2011, 43, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Barbato, C.; Giorgi, C.; Catalanotto, C.; Cogoni, C. Thinking about RNA? MicroRNAs in the brain. Mamm. Genome 2008, 19, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Kuss, A.W.; Chen, W. MicroRNAs in brain function and disease. Curr. Neurol. Neurosci. Rep. 2008, 8, 190–197. [Google Scholar] [CrossRef]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J. Alzheimers Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef]
- Piscopo, P.; Grasso, M.; Puopolo, M.; D’acunto, E.; Talarico, G.; Crestini, A.; Gasparini, M.; Campopiano, R.; Gambardella, S.; Castellano, A.E.; et al. Circulating miR-127-3p as a Potential Biomarker for Differential Diagnosis in Frontotemporal Dementia. J. Alzheimer’s Dis. 2018, 65, 455–464. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef]
- Chung, H.Y.; Wickel, J.; Brunkhorst, F.M.; Geis, C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J. Clin. Med. 2020, 9, 703. [Google Scholar] [CrossRef]
- Gracner, T.; Agarwal, M.; Murali, K.P.; Stone, P.W.; Larson, E.L.; Furuya, E.Y.; Harrison, J.M.; Dick, A.W. Association of Infection-Related Hospitalization With Cognitive Impairment Among Nursing Home Residents. JAMA Netw. Open 2021, 4, e217528. [Google Scholar] [CrossRef]
- Kaidonis, G.; Rao, A.N.; Ouyang, Y.-B.; Stary, C.M. Elucidating sex differences in response to cerebral ischemia: Immunoregulatory mechanisms and the role of microRNAs. Prog. Neurobiol. 2019, 176, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Golomb, M.R.; Fullerton, H.J.; Nowak-Gottl, U.; Deveber, G. Male predominance in childhood ischemic stroke: Findings from the international pediatric stroke study. Stroke 2009, 40, 52–57. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Reed, J.C. IAP family proteins—Suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Florijn, B.W.; Bijkerk, R.; Kruyt, N.D.; van Zonneveld, A.J.; Wermer, M.J.H. Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 11888. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 3–13. [Google Scholar] [PubMed]
- Felderhoff-Mueser, U.; Ikonomidou, C. Mechanisms of neurodegeneration after paediatric brain injury. Curr. Opin. Neurol. 2000, 13, 141–145. [Google Scholar] [CrossRef]
- Ray, S.K.; Dixon, C.E.; Banik, N.L. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol. Histopathol. 2002, 17, 1137–1152. [Google Scholar]
- Patt, S.; Brodhun, M. Neuropathological sequelae of traumatic injury in the brain. An overview. Exp. Toxicol. Pathol. 1999, 51, 119–123. [Google Scholar] [CrossRef]
- Intosh, M.; Saatman, K.; Raghupathi, R.; Graham, D.; Smith, D.H.; Lee, V.M.; Trojanowski, J.Q. gThe Dorothy Russell Memorial Lecture* The molecular and cellular sequelae of experimental traumatic brain injury: Pathogenetic mechanisms. Neuropathol. Appl. Neurobiol. 1998, 24, 251–267. [Google Scholar]
- Rosenfeld, J.V.; Maas, A.I.; Bragge, P.; Morganti-Kossmann, M.C.; Manley, G.T.; Gruen, R.L. Early management of severe traumatic brain injury. Lancet 2012, 380, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.; Havlik, R.; Steffens, D.; Helms, M.; Newman, T.; Drosdick, D.; Phillips, C.; Gau, B.; Welsh–Bohmer, K.; Burke, J.; et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef]
- Wang, H.-K.; Lin, S.-H.; Sung, P.-S.; Wu, M.-H.; Hung, K.-W.; Wang, L.-C.; Huang, C.-Y.; Lu, K.; Chen, H.-J.; Tsai, K.-J. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1080–1085. [Google Scholar] [CrossRef]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Kaup, A.; Barnes, D.E.; Yaffe, K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014, 71, 1490–1497. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Hou, S.-W.; Lee, C.-C.; Hsu, C.-Y.; Huang, Y.-S.; Su, Y.-C. Increased Risk of Dementia in Patients with Mild Traumatic Brain Injury: A Nationwide Cohort Study. PLoS ONE 2013, 8, e62422. [Google Scholar] [CrossRef]
- Nordström, P.; Michaëlsson, K.; Gustafson, Y.; Nordström, A. Traumatic brain injury and young onset dementia: A nationwide cohort study. Ann. Neurol. 2014, 75, 374–381. [Google Scholar] [CrossRef]
- Gardner, R.C.; Langa, K.M.; Yaffe, K. Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study. PLoS Med. 2017, 14, e1002246. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Leech, R.; Sharp, D.J. Alzheimer’s Disease Neuroimaging Initiative. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Arena, J.D.; Smith, D.H. Traumatic Brain Injury as a Trigger of Neurodegeneration. Adv. Neurobiol. 2017, 15, 383–400. [Google Scholar]
- Zaloshnja, E.; Miller, T.; Langlois, J.A.; Selassie, A.W. Prevalence of Long-Term Disability From Traumatic Brain Injury in the Civilian Population of the United States, 2005. J. Head Trauma Rehabil. 2008, 23, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Farace, E.; Alves, W.M. Do women fare worse: A metaanalysis of gender differences in traumatic brain injury outcome. J. Neurosurg. 2000, 93, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Master, C.L.; Katz, B.P.; Arbogast, K.B.; McCrea, M.A.; McAllister, T.W.; Pasquina, P.F.; Lapradd, M.; Zhou, W.; Broglio, S.P. Differences in sport-related concussion for female and male athletes in comparable collegiate sports: A study from the NCAA-DoD Concussion Assessment, Research and Education (CARE) Consortium. Br. J. Sports Med. 2021, 55, 1387–1394. [Google Scholar] [CrossRef]
- Berry, C.; Ley, E.J.; Tillou, A.; Gil Cryer, G.; Margulies, D.R.; Salim, A. The Effect of Gender on Patients With Moderate to Severe Head Injuries. J. Trauma Inj. Infect. Crit. Care 2009, 67, 950–953. [Google Scholar] [CrossRef]
- Beijer, E.; van Wonderen, S.F.; Zuidema, W.P.; Visser, M.C.; Edwards, M.J.R.; Verhofstad, M.H.J.; Tromp, T.N.; Brom, C.E.v.D.; van Lieshout, E.M.M.; Bloemers, F.W.; et al. Sex Differences in Outcome of Trauma Patients Presented with Severe Traumatic Brain Injury: A Multicenter Cohort Study. J. Clin. Med. 2023, 12, 6892. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Roof, R.L.; Hall, E.D. Estrogen-Related Gender Difference in Survival Rate and Cortical Blood Flow after Impact-Acceleration Head Injury in Rats. J. Neurotrauma 2000, 17, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, H.M.; Dietrich, W.D. Neuropathological Protection after Traumatic Brain Injury in Intact Female Rats Versus Males or Ovariectomized Females. J. Neurotrauma 2001, 18, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; Burke, J.F.; Fu, A.H.; McCabe, J.T. Neuropsychiatric Symptom Modeling in Male and Female C57BL/6J Mice after Experimental Traumatic Brain Injury. J. Neurotrauma 2017, 34, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, A.C.; Kim, H.; Salcedo, E.; Yonchek, J.C.; Rodgers, K.M.; Orfila, J.E.; Dietz, R.M.; Quillinan, N.; Traystman, R.J.; Herson, P.S. Endogenous Sex Steroids Dampen Neuroinflammation and Improve Outcome of Traumatic Brain Injury in Mice. J. Mol. Neurosci. 2018, 64, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Liu, Y.; Dash, P.K. Traumatic brain injury alters expression of hippocampal microRNAs: Potential regulators of multiple pathophysiological processes. J. Neurosci. Res. 2009, 87, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, T.; Liu, Z.; Chen, X.; Zhao, L.; Qu, G.; Li, Q. Traumatic Brain Injury Dysregulates MicroRNAs to Modulate Cell Signaling in Rat Hippocampus. PLoS ONE 2014, 9, e103948. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Visavadiya, N.P.; Pandya, J.D.; Nelson, P.T.; Sullivan, P.G.; Springer, J.E. Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp. Neurol. 2015, 265, 84–93. [Google Scholar] [CrossRef]
- Wang, W.-X.; Springer, J.; Prajapati, P.; Vekaria, H.J.; Spry, M.; Cloud, A.L.; Sullivan, P.G. Temporal changes in inflammatory mitochondria-enriched microRNAs following traumatic brain injury and effects of miR-146a nanoparticle delivery. Neural Regen. Res. 2021, 16, 514–522. [Google Scholar] [CrossRef]
- Devoto, C.; Lai, C.; Qu, B.-X.; Guedes, V.A.; Leete, J.; Wilde, E.A.; Walker, W.C.; Diaz-Arrastia, R.; Kenney, K.; Gill, J. Exosomal MicroRNAs in Military Personnel with Mild Traumatic Brain Injury: Preliminary Results from the Chronic Effects of Neurotrauma Consortium Biomarker Discovery Project. J. Neurotrauma 2020, 37, 2482–2492. [Google Scholar] [CrossRef]
- Ghai, V.; Fallen, S.; Baxter, D.; Scherler, K.; Kim, T.-K.; Zhou, Y.; Meabon, J.S.; Logsdon, A.F.; Banks, W.A.; Schindler, A.G.; et al. Alterations in Plasma microRNA and Protein Levels in War Veterans with Chronic Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1418–1430. [Google Scholar] [CrossRef]
- Atif, H.; Hicks, S.D. A Review of MicroRNA Biomarkers in Traumatic Brain Injury. J. Exp. Neurosci. 2019, 13, 117906951983228. [Google Scholar] [CrossRef]
- Di Pietro, V.; Porto, E.; Ragusa, M.; Barbagallo, C.; Davies, D.; Forcione, M.; Logan, A.; Di Pietro, C.; Purrello, M.; Grey, M.; et al. Salivary MicroRNAs: Diagnostic Markers of Mild Traumatic Brain Injury in Contact-Sport. Front. Mol. Neurosci. 2018, 11, 290. [Google Scholar] [CrossRef]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Slobounov, S.M.; Breiter, H.C.; Walter, A.; Bream, T.; Seidenberg, P.; Bailes, J.E.; Bravo, S.; Johnson, B.; Kaufman, D.; et al. Elevations in MicroRNA Biomarkers in Serum Are Associated with Measures of Concussion, Neurocognitive Function, and Subconcussive Trauma over a Single National Collegiate Athletic Association Division I Season in Collegiate Football Players. J. Neurotrauma 2019, 36, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Johnson, J.; Carney, M.C.; Bramley, H.; Olympia, R.P.; Loeffert, A.C.; Thomas, N.J. Overlapping MicroRNA Expression in Saliva and Cerebrospinal Fluid Accurately Identifies Pediatric Traumatic Brain Injury. J. Neurotrauma 2018, 35, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef] [PubMed]
- Späni, C.B.; Braun, D.J.; Van Eldik, L.J. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocr. 2018, 50, 52–66. [Google Scholar] [CrossRef]
- Biegon, A. Considering Biological Sex in Traumatic Brain Injury. Front. Neurol. 2021, 12, 576366. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, R.; Giurato, G.; Bruno, G.; Ravo, M.; Rizzo, F.; Salvati, A.; Ricciardi, L.; Marchese, G.; Cordella, A.; Rocco, T.; et al. The nuclear receptor ERβ engages AGO2 in regulation of gene transcription, RNA splicing and RISC loading. Genome Biol. 2017, 18, 189. [Google Scholar] [CrossRef] [PubMed]
- Linscott, M.L.; Yildiz, Y.; Flury, S.; Newby, M.L.; Pak, T.R. Age and 17β-Estradiol (E2) Facilitate Nuclear Export and Argonaute Loading of microRNAs in the Female Brain. Non-Coding RNA 2023, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.S.; Shults, C.L.; Pinceti, E.; Pak, T.R. Prolonged ovarian hormone deprivation alters the effects of 17β-estradiol on microRNA expression in the aged female rat hypothalamus. Oncotarget 2015, 6, 36965–36983. [Google Scholar] [CrossRef]
- Guo, X.; Su, B.; Zhou, Z.; Sha, J. Rapid evolution of mammalian X-linked testis microRNAs. BMC Genom. 2009, 10, 97. [Google Scholar] [CrossRef]
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 2011, 33, 791–802. [Google Scholar] [CrossRef]
- Carè, A.; Bellenghi, M.; Matarrese, P.; Gabriele, L.; Salvioli, S.; Malorni, W. Sex disparity in cancer: Roles of microRNAs and related functional players. Cell Death Differ. 2018, 25, 477–485. [Google Scholar] [CrossRef]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.H.; Potenza, N. What microRNAs could tell us about the human X chromosome. Cell. Mol. Life Sci. 2020, 77, 4069–4080, Erratum in Cell. Mol. Life Sci. 2021, 78, 4067. [Google Scholar] [CrossRef]
- Lyon, M.F. Gene Action in the X-chromosome of the Mouse (Mus musculus L.). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Lyon, M.F. X-chromosome inactivation: A repeat hypothesis. Cytogenet. Cell Genet. 1998, 80, 133–137. [Google Scholar] [CrossRef]
- Payer, B.; Lee, J.T. X Chromosome Dosage Compensation: How Mammals Keep the Balance. Annu. Rev. Genet. 2008, 42, 733–772. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Lyon, M.F. X-chromosome inactivation and human genetic disease. Acta Paediatr. Suppl. 2002, 91, 107–112. [Google Scholar] [CrossRef]
- Navarro-Cobos, M.J.; Balaton, B.P.; Brown, C.J. Genes that escape from X-chromosome inactivation: Potential contributors to Klinefelter syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 226–238. [Google Scholar] [CrossRef]
- Balaton, B.P.; Brown, C.J. Escape Artists of the X Chromosome. Trends Genet. 2016, 32, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef]
- Chen, K.; Ma, Y.; Wu, S.; Zhuang, Y.; Liu, X.; Lv, L.; Zhang, G. Construction and analysis of a lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveals functional lncRNAs in diabetic cardiomyopathy. Mol. Med. Rep. 2019, 20, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Syrett, C.M.; Kramer, M.C.; Basu, A.; Atchison, M.L.; Anguera, M.C. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl. Acad. Sci. USA 2016, 113, E2029–E2038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sinke, L.; Jonkman, T.H.; Slieker, R.C.; van Zwet, E.W.; Daxinger, L.; Heijmans, B.T. BIOS Consortium The inactive X chromosome accumulates widespread epigenetic variability with age. Clin. Epigenet. 2023, 15, 135. [Google Scholar] [CrossRef]
- Peeters, S.B.; Posynick, B.J.; Brown, C.J. Out of the Silence: Insights into How Genes Escape X-Chromosome Inactivation. Epigenomes 2023, 7, 29. [Google Scholar] [CrossRef]
- Grigoryan, A.; Pospiech, J.; Krämer, S.; Lipka, D.; Liehr, T.; Geiger, H.; Kimura, H.; Mulaw, M.A.; Florian, M.C. Attrition of X Chromosome Inactivation in Aged Hematopoietic Stem Cells. Stem Cell Rep. 2021, 16, 708–716. [Google Scholar] [CrossRef]
- Mishima, T.; Takizawa, T.; Luo, S.-S.; Ishibashi, O.; Kawahigashi, Y.; Mizuguchi, Y.; Ishikawa, T.; Mori, M.; Kanda, T.; Goto, T.; et al. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction 2008, 136, 811–822. [Google Scholar] [CrossRef]
- Ro, S.; Park, C.; Sanders, K.M.; McCarrey, J.R.; Yan, W. Cloning and expression profiling of testis-expressed microRNAs. Dev. Biol. 2007, 311, 592–602. [Google Scholar] [CrossRef]
- Royo, H.; Seitz, H.; ElInati, E.; Peters, A.H.F.M.; Stadler, M.B.; Turner, J.M.A. Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation. PLoS Genet. 2015, 11, e1005461. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ro, S.; Michaels, J.D.; Park, C.; McCarrey, J.R.; Yan, W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat. Genet. 2009, 41, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.; Flores, L.; Yan, W.; McCarrey, J.R. Escape of X-linked miRNA genes from meiotic sex chromosome inactivation. Development 2015, 142, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Lemoine, F.; Soumillon, M.; Liechti, A.; Weier, M.; Guschanski, K.; Hu, H.; Khaitovich, P.; Kaessmann, H. Birth and expression evolution of mammalian microRNA genes. Genome Res. 2013, 23, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Buchold, G.M.; Coarfa, C.; Kim, J.; Milosavljevic, A.; Gunaratne, P.H.; Matzuk, M.M. Analysis of MicroRNA Expression in the Prepubertal Testis. PLoS ONE 2010, 5, e15317. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.F.; Piergiorge, R.M.; dos Santos, J.M.; Gusmão, J.; Pimentel, M.M.G.; Santos-Rebouças, C.B. Network Profiling of Brain-Expressed X-Chromosomal MicroRNA Genes Implicates Shared Key MicroRNAs in Intellectual Disability. J. Mol. Neurosci. 2019, 67, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Calvente, C.J.; Del Pilar, H.; Tameda, M.; Johnson, C.D.; Feldstein, A.E. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol. Ther. 2020, 28, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Rieger, A.; Schildberg, F.A.; Knolle, P.A.; Schmid-Burgk, J.L.; Hornung, V. NLRP3 Inflammasome Activity Is Negatively Controlled by miR-223. J. Immunol. 2012, 189, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.J.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Berg, N.; Lee, J.W.; Le, T.-T.; Neudecker, V.; Jing, N.; Eltzschig, H. MicroRNA miR-223 as regulator of innate immunity. J. Leukoc. Biol. 2018, 104, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, J.R.; Wang, W.X.; Hébert, S.S.; Wilfred, B.R.; Mao, G.; Nelson, P.T. The miR-15/107 group of microRNA genes: Evolutionary biology, cellular functions, and roles in human diseases. J. Mol. Biol. 2010, 402, 491–509. [Google Scholar] [CrossRef]
- Wang, W.X.; Danaher, R.J.; Miller, C.S.; Berger, J.R.; Nubia, V.G.; Wilfred, B.S.; Neltner, J.H.; Norris, C.M.; Nelson, P.T. Expression of miR-15/107 family microRNAs in human tissues and cultured rat brain cells. Genom. Proteom. Bioinform. 2014, 12, 19–30. [Google Scholar] [CrossRef]
- Wang, W.-X.; Wilfred, B.R.; Madathil, S.K.; Tang, G.; Hu, Y.; Dimayuga, J.; Stromberg, A.J.; Huang, Q.; Saatman, K.E.; Nelson, P.T. miR-107 Regulates Granulin/Progranulin with Implications for Traumatic Brain Injury and Neurodegenerative Disease. Am. J. Pathol. 2010, 177, 334–345. [Google Scholar] [CrossRef]
- Kataoka, Y.; Takeichi, M.; Uemura, T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells 2001, 6, 313–325. [Google Scholar] [CrossRef]
- Bernstein, E.; Kim, S.Y.; Carmell, M.A.; Murchison, E.P.; Alcorn, H.; Li, M.Z.; Mills, A.A.; Elledge, S.J.; Anderson, K.V.; Hannon, G.J. Dicer is essential for mouse development. Nat. Genet. 2003, 35, 215–217. [Google Scholar] [CrossRef]
- Alisch, R.S.; Jin, P.; Epstein, M.; Caspary, T.; Warren, S.T. Argonaute2 Is Essential for Mammalian Gastrulation and Proper Mesoderm Formation. PLoS Genet. 2007, 3, e227. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Megraw, M.S.; Giannakakis, A.; Liang, S.; Naylor, T.L.; Barchetti, A.; Ward, M.R.; et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 9136–9141. [Google Scholar] [CrossRef]
- Kwanhian, W.; Lenze, D.; Alles, J.; Motsch, N.; Barth, S.; Döll, C.; Imig, J.; Hummel, M.; Tinguely, M.; Trivedi, P.; et al. MicroRNA-142 is mutated in about 20% of diffuse large B-cell lymphoma. Cancer Med. 2012, 1, 141–155. [Google Scholar] [CrossRef]
- Sethupathy, P.; Borel, C.; Gagnebin, M.; Grant, G.R.; Deutsch, S.; Elton, T.S.; Hatzigeorgiou, A.G.; Antonarakis, S.E. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: A mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am. J. Hum. Genet. 2007, 81, 405–413. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; SanGiovanni, P.M.; Sapieha, P.; De Guire, V. miRNAs, single nucleotide polymorphisms (SNPs) and age-related macular degeneration (AMD). Clin. Chem. Lab. Med. 2017, 55, 763–775. [Google Scholar] [CrossRef]
- Meola, N.; Gennarino, V.A.; Banfi, S. microRNAs and genetic diseases. PathoGenetics 2009, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, M.H.; Vallès, A.; Kirkels, L.A.M.H.; Mastebroek, M.; Loohuis, N.O.; Kos, A.; Wissink-Lindhout, W.M.; de Brouwer, A.P.M.; Nillesen, W.M.; Pfundt, R.; et al. Chromosome 1p21.3 microdeletions comprising DPYD and MIR137 are associated with intellectual disability. J. Med. Genet. 2011, 48, 810–818. [Google Scholar] [CrossRef]
- Galea, I.; Bechmann, I.; Perry, V.H. What is immune privilege (not)? Trends Immunol. 2007, 28, 12–18. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Gate, D.; Town, T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J. Neuroimmune Pharmacol. 2009, 4, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflamm. 2014, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Extracellular Vesicles miRNA Cargo for Microglia Polarization in Traumatic Brain Injury. Biomolecules 2020, 10, 901. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Taha, H.B.; Bitan, G. Biomarkers for parkinsonian disorders in CNS-originating EVs: Promise and challenges. Acta Neuropathol. 2023, 145, 515–540. [Google Scholar] [CrossRef] [PubMed]
| miRNAs | Species/Tissue | Disease/Condition | X-Linked Yes/No | Observation | Reference | |
|---|---|---|---|---|---|---|
| Pre-clinical studies | miR-200 family (miR-141-3p, miR200a-3P, miR200b-3P, and miR-429), miR-875 | Rat/cortex | P0/development | No | Upregulated in females | [85] |
| miR-200 family (miR-141-3p, miR200a-3P, miR200b-3P, and miR-429), miR-875 | Rat/cortex | P7, adult/development | No | Upregulated in males | [85] | |
| miR-935 | Rat/cortex | P0, P7, adult | Upregulated in males | [85] | ||
| miR-322, miR-574, and miR-873 | Mouse/brain | Prenatal stress | No | Downregulated in males | [80] | |
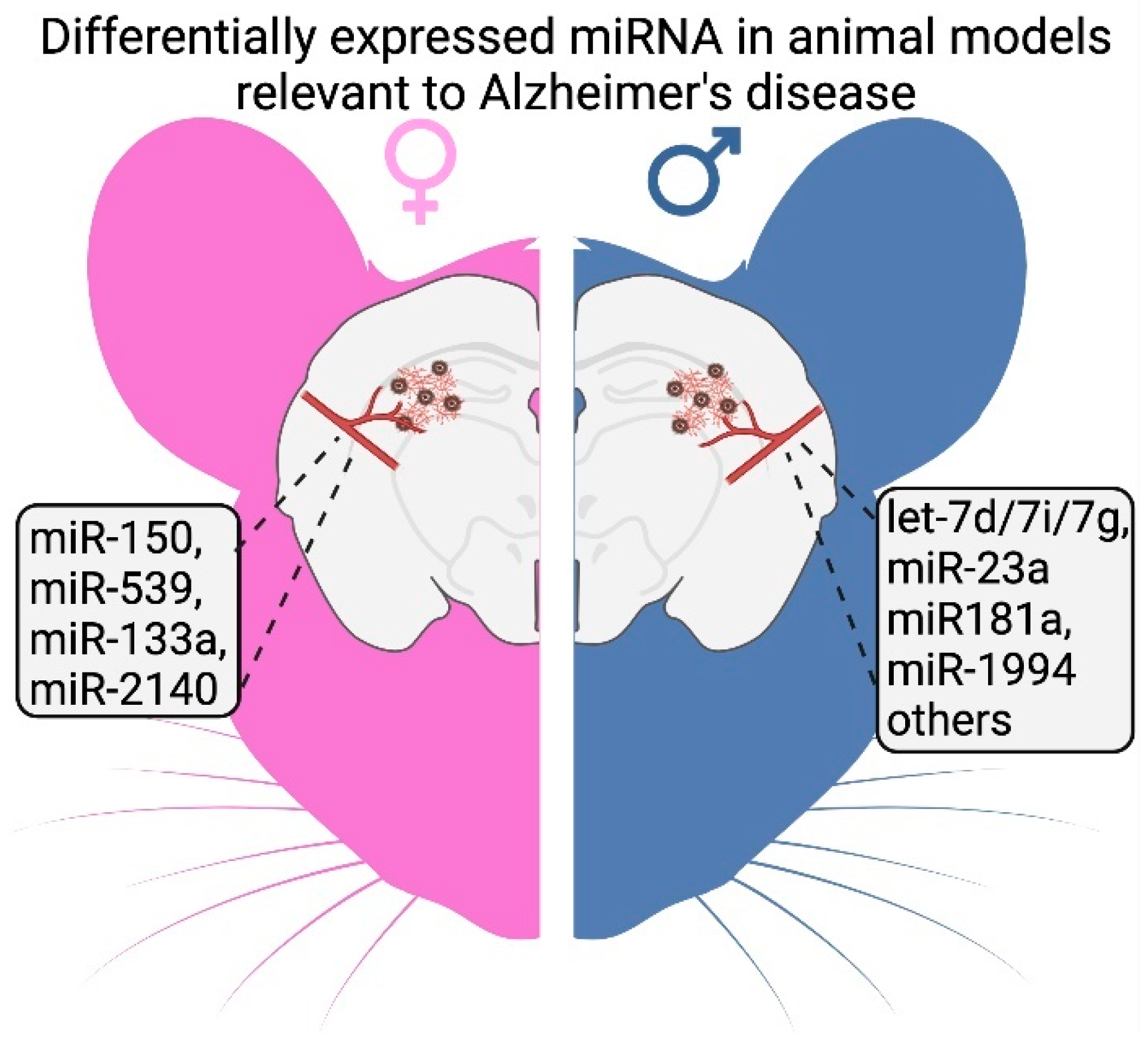
| Let-7g, miR-1944 | Mouse/cerebral vessels | 3xTg-AD, young to CI | No | Downregulated in males | [86] | |
| miR-133a, miR-2140 | Mouse/cerebral vessels | 3xTg-AD, young to CI | No | Downregulated in females | [86] | |
| miR-99a | Mouse/cerebral vessels | 3xTg-AD, CI to Aβ | No | Downregulated in males | [86] | |
| let-7d, let-7i, miR-23a, miR-34b-3p, miR-99a, miR-126-3p, miR-132, miR-150, miR-151-5p, miR-181a | Mouse/cerebral vessels | 3xTg-AD, pre-AD to AD | No | Changed in males | [86] | |
| miR-150, miR-539 | Mouse/cerebral vessels | 3xTg-AD, pre-AD to AD | No | Changed in females | [86] | |
| miR-137, miR-181c, miR-29a, miR29b-1 | Mouse/brain | C57/BL | No | Downregulated in females | [88] | |
| miR-137, miR-181c, miR29b-1 | Mouse/serum | High-fat diet/C57/BL | No | Downregulated in females | [87] | |
| miR-320 | Mouse/hippocampus | Sepsis/old mice | No | Changed in females not males | [89] | |
| miR-223-3p, miR-98-3p, and miR-662-5p | Mouse/hippocampus | Sepsis/old mice | Yes | Upregulated in males | [89] | |
| miR-23a | Mouse/brain | Cerebral ischemia | No | Upregulated in females | [75] | |
| miR-509-3p | Mouse/cortices | Cerebral ischemia | No | Upregulated in males downregulated in females | [90] | |
| miR-883b-3p | Mouse/cortices | Cerebral ischemia | No | Upregulated in females downregulated in males | [90] | |
| miR-142a-5p and 25 others | Mouse/microglia | B6C3F1/J/naive | Yes to some | Enriched in females | [91] | |
| miR-1298-5p and 60 others | Mouse/microglia | B6C3F1/J/naive | Yes to some | Enriched in males | [91] | |
| miR-150-5p, miR-155-5p | Mouse/CD11b+ | C57BL/6J/naïve | No | Upregulated in males | [98] | |
| miR-150-5p, miR-155-5p, miR-146a-5p, miR-223-3p | Mouse/CD11b+ | C57BL/6J/TBI | No, except miR-223-3p | Sex-specific response at different time points following TBI | [98] | |
| miR-29a, miR-29c | Mouse/brain | Radiation-induced brain injury | No | Upregulated in female | [72] | |
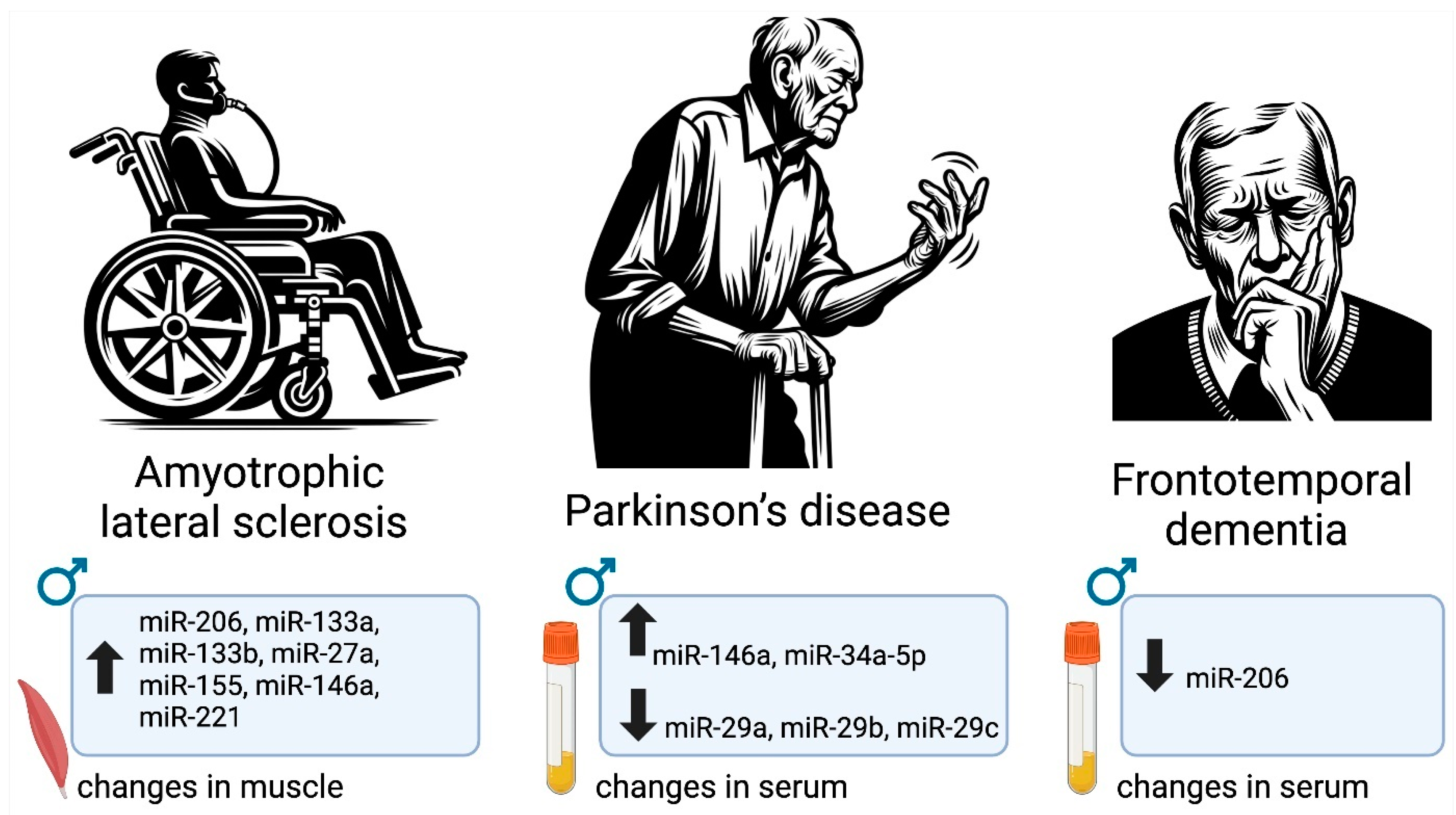
| Clinical studies | miR-206, miR-133a, miR-133b, miR-27a, miR-155, miR-146a, miR-221 | Human/muscle | ALS | No, except miR-222 | Upregulated in males | [92] |
| miR-146a, miR-34a-5p | Human/serum | PD | No | Upregulated in men | [93] | |
| miR-29a, miR-29b, miR-29c | Human/serum | PD | No | Downregulated in men | [94] | |
| miR-206 | Human/plasma | FTD | No | Downregulated in men | [95] | |
| miR-30b | Human/cortices | Schizophrenia | No | Downregulated in females | [96] | |
| miR-548am-5p | Human/primary dermal fibroblasts | XCI escape | Yes | Upregulated in females | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geleta, U.; Prajapati, P.; Bachstetter, A.; Nelson, P.T.; Wang, W.-X. Sex-Biased Expression and Response of microRNAs in Neurological Diseases and Neurotrauma. Int. J. Mol. Sci. 2024, 25, 2648. https://doi.org/10.3390/ijms25052648
Geleta U, Prajapati P, Bachstetter A, Nelson PT, Wang W-X. Sex-Biased Expression and Response of microRNAs in Neurological Diseases and Neurotrauma. International Journal of Molecular Sciences. 2024; 25(5):2648. https://doi.org/10.3390/ijms25052648
Chicago/Turabian StyleGeleta, Urim, Paresh Prajapati, Adam Bachstetter, Peter T. Nelson, and Wang-Xia Wang. 2024. "Sex-Biased Expression and Response of microRNAs in Neurological Diseases and Neurotrauma" International Journal of Molecular Sciences 25, no. 5: 2648. https://doi.org/10.3390/ijms25052648
APA StyleGeleta, U., Prajapati, P., Bachstetter, A., Nelson, P. T., & Wang, W.-X. (2024). Sex-Biased Expression and Response of microRNAs in Neurological Diseases and Neurotrauma. International Journal of Molecular Sciences, 25(5), 2648. https://doi.org/10.3390/ijms25052648






