Effects of Alterations in Acid–Base Effects on Insulin Signaling
Abstract
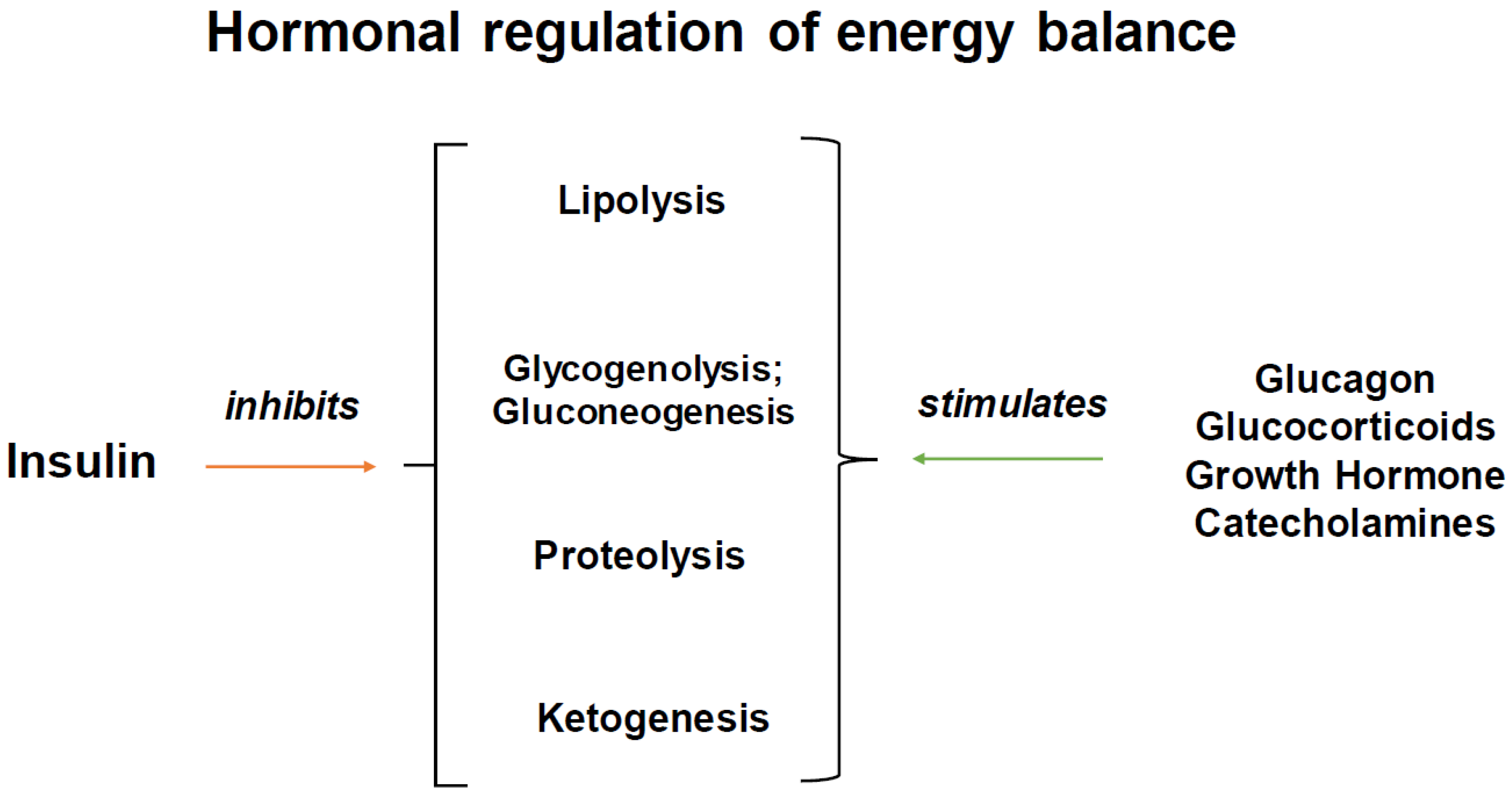
:1. Introduction
Regulation of Acid–Base Balance
2. Chronic Acid Stress and Metabolic Acidosis Develop with Increasing Age and Renal Functional Decline
3. Insulin Receptors and Insulin Signaling: Its Very Broad Effects in Tissues Besides Glucose Uptake
3.1. Liver
3.2. Adipose Tissue
3.3. Muscle
3.4. Central Nervous System
3.5. Growth
4. Evidence of Acid Stress and Metabolic Acidosis’ Effects on Insulin Action
4.1. Kidney
4.2. Muscle
4.3. Adipose
4.4. Liver
4.5. Central Nervous System
5. Evidence That Bicarbonate Treatment Improves Insulin Signaling
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rose, B.D. Acid-base physiology. In Clinical Physiology of Acid-Base and Electrolyte Disorders, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1994. [Google Scholar]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef]
- Wesson, D.E. The Continuum of Acid Stress. Clin. J. Am. Soc. Nephrol. 2021, 16, 1292–1299. [Google Scholar] [CrossRef]
- Rose, B.D. Hypokalemia. In Clinical Physiology of Acid-Base and Electrolyte Disorders, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1994. [Google Scholar]
- Zampieri, F.G.; Kellum, J.A.; Park, M.; Ranzani, O.T.; Barbeiro, H.V.; de Souza, H.P.; da Cruz Neto, L.M.; da Silva, F.P. Relationship between acid-base status and inflammation in the critically ill. Crit. Care 2014, 18, R154. [Google Scholar] [CrossRef]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef]
- Frassetto, L.; Sebastian, A. Age and systemic acid-base equilibrium: Analysis of published data. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, B91–B99. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Morris, R.C., Jr.; Sebastian, A. Effect of age on blood acid-base composition in adult humans: Role of age-related renal functional decline. Am. J. Physiol. 1996, 271 Pt 2, F1114–F1122. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Buysse, J.M.; Bushinsky, D.A. Mechanisms of Metabolic Acidosis-Induced Kidney Injury in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Attie, A.D.; Tang, Q.Q.; Bornfeldt, K.E. The insulin centennial-100 years of milestones in biochemistry. J. Lipid Res. 2021, 62, 100132. [Google Scholar] [CrossRef] [PubMed]
- White, M.F.; Kahn, C.R. Insulin action at a molecular level—100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Sonksen, P.; Sonksen, J. Insulin: Understanding its action in health and disease. Br. J. Anaesth. 2000, 85, 69–79. [Google Scholar] [CrossRef]
- Brüning, J.C.; Michael, M.D.; Winnay, J.N.; Hayashi, T.; Hörsch, D.; Accili, D.; Goodyear, L.J.; Kahn, C.R. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 1998, 2, 559–569. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef]
- Blüher, M.; Michael, M.D.; Peroni, O.D.; Ueki, K.; Carter, N.; Kahn, B.B.; Kahn, C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 2002, 3, 25–38. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- O-Sullivan, I.; Zhang, W.; Wasserman, D.H.; Liew, C.W.; Liu, J.; Paik, J.; DePinho, R.A.; Stolz, D.B.; Kahn, C.R.; Schwartz, M.W.; et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015, 6, 7079. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Mottagui-Tabar, S.; Rydén, M.; Löfgren, P.; Faulds, G.; Hoffstedt, J.; Brookes, A.J.; Andersson, I.; Arner, P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia 2003, 46, 789–797. [Google Scholar] [CrossRef]
- Bennett, R.G.; Hamel, F.G.; Duckworth, W.C. Insulin inhibits the ubiquitin-dependent degrading activity of the 26S proteasome. Endocrinology 2000, 141, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Lotter, E.C.; McKay, L.D.; Porte, D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979, 282, 503–505. [Google Scholar] [CrossRef]
- Nicolucci, A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010, 47, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef]
- Singh, P.; Alex, J.M.; Bast, F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: Novel treatment strategies for cancer. Med. Oncol. 2014, 31, 805. [Google Scholar] [CrossRef]
- Waelbroeck, M. The pH dependence of insulin binding. A quantitative study. J. Biol. Chem. 1982, 257, 8284–8291. [Google Scholar] [CrossRef]
- Mak, R.H. Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int. 1998, 54, 603–607. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Beckles, A.D. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol. 1979, 236, E328–E334. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Vilier, A.; Bonnet, F.; Lajous, M.; Balkau, B.; Boutron-Rualt, M.C.; Clavel-Chapelon, F. Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia 2014, 57, 313–320. [Google Scholar] [CrossRef]
- Sutton, J.R.; Jones, N.L.; Toews, C.J. Effect of PH on muscle glycolysis during exercise. Clin. Sci. 1981, 61, 331–338. [Google Scholar] [CrossRef]
- Szendroedi, J.; Schmid, A.I.; Meyerspeer, M.; Cervin, C.; Kacerovsky, M.; Smekal, G.; Gräser-Lang, S.; Groop, L.; Roden, M. Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care 2009, 32, 677–679. [Google Scholar] [CrossRef]
- Relman, A.S. Metabolic consequences of acid-base disorders. Kidney Int. 1972, 1, 347–359. [Google Scholar] [CrossRef]
- Whittaker, J.; Cuthbert, C.; Hammond, V.A.; Alberti, K.G. The effects of metabolic acidosis in vivo on insulin binding to isolated rat adipocytes. Metabolism 1982, 31, 553–557. [Google Scholar] [CrossRef]
- Hems, R.; Ross, B.D.; Berry, M.N.; Krebs, H.A. Gluconeogenesis in the perfused rat liver. Biochem. J. 1966, 101, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Fine, A. Preliminary report: The effects of acute acidosis on alanine and glucose metabolism across the liver, gut, kidney, and muscle in the dog. Metabolism 1983, 32, 317–319. [Google Scholar] [CrossRef]
- Chai, S.; Li, M.; Branigan, D.; Xiong, Z.G.; Simon, R.P. Activation of acid-sensing ion channel 1a (ASIC1a) by surface trafficking. J. Biol. Chem. 2010, 285, 13002–13011. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Wesson, D.E. Novel dietary and pharmacologic approaches for acid-base modulation to preserve kidney function and manage uremia. Curr. Opin. Nephrol. Hypertens. 2020, 29, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Surma, S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis. 2021, 7, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B.; UBI Study Investigators. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 158. [Google Scholar] [CrossRef]
- Reaich, D.; Graham, K.A.; Channon, S.M.; Hetherington, C.; Scrimgeour, C.M.; Wilkinson, R.; Goodship, T.H. Insulin-mediated changes in PD and glucose uptake after correction of acidosis in humans with CRF. Am. J. Physiol. 1995, 268 Pt 1, E121–E126. [Google Scholar] [CrossRef]
- Lennon, E.J.; Lemann, J., Jr.; Litzow, J.R. The effects of diet and stool composition on the net external acid balance of normal subjects. J. Clin. Investig. 1966, 45, 1601–1607. [Google Scholar] [CrossRef]
- Wu, T.; Seaver, P.; Lemus, H.; Hollenbach, K.; Wang, E.; Pierce, J.P. Associations between Dietary Acid Load and Biomarkers of Inflammation and Hyperglycemia in Breast Cancer Survivors. Nutrients 2019, 11, 1913. [Google Scholar] [CrossRef] [PubMed]
- Caferoglu, Z.; Erdal, B.; Hatipoglu, N.; Kurtoglu, S. The effects of diet quality and dietary acid load on insulin resistance in overweight children and adolescents. Endocrinol. Diabetes Nutr. Engl. Ed. 2022, 69, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.D. Metabolic Alkalosis. In Clinical Physiology of Acid-Base and Electrolyte Disorders, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1994. [Google Scholar]
- Raphael, K.L. Metabolic Acidosis and Subclinical Metabolic Acidosis in CKD. J. Am. Soc. Nephrol. 2018, 29, 376–382. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frassetto, L.A.; Masharani, U. Effects of Alterations in Acid–Base Effects on Insulin Signaling. Int. J. Mol. Sci. 2024, 25, 2739. https://doi.org/10.3390/ijms25052739
Frassetto LA, Masharani U. Effects of Alterations in Acid–Base Effects on Insulin Signaling. International Journal of Molecular Sciences. 2024; 25(5):2739. https://doi.org/10.3390/ijms25052739
Chicago/Turabian StyleFrassetto, Lynda A., and Umesh Masharani. 2024. "Effects of Alterations in Acid–Base Effects on Insulin Signaling" International Journal of Molecular Sciences 25, no. 5: 2739. https://doi.org/10.3390/ijms25052739
APA StyleFrassetto, L. A., & Masharani, U. (2024). Effects of Alterations in Acid–Base Effects on Insulin Signaling. International Journal of Molecular Sciences, 25(5), 2739. https://doi.org/10.3390/ijms25052739





