Inhibitory Effects of Eicosapentaenoic Acid on Vascular Endothelial Growth Factor-Induced Monocyte Chemoattractant Protein-1, Interleukin-6, and Interleukin-8 in Human Vascular Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. VEGF-Induced Gene Expression and Protein Secretion of MCP-1 in HUVECs
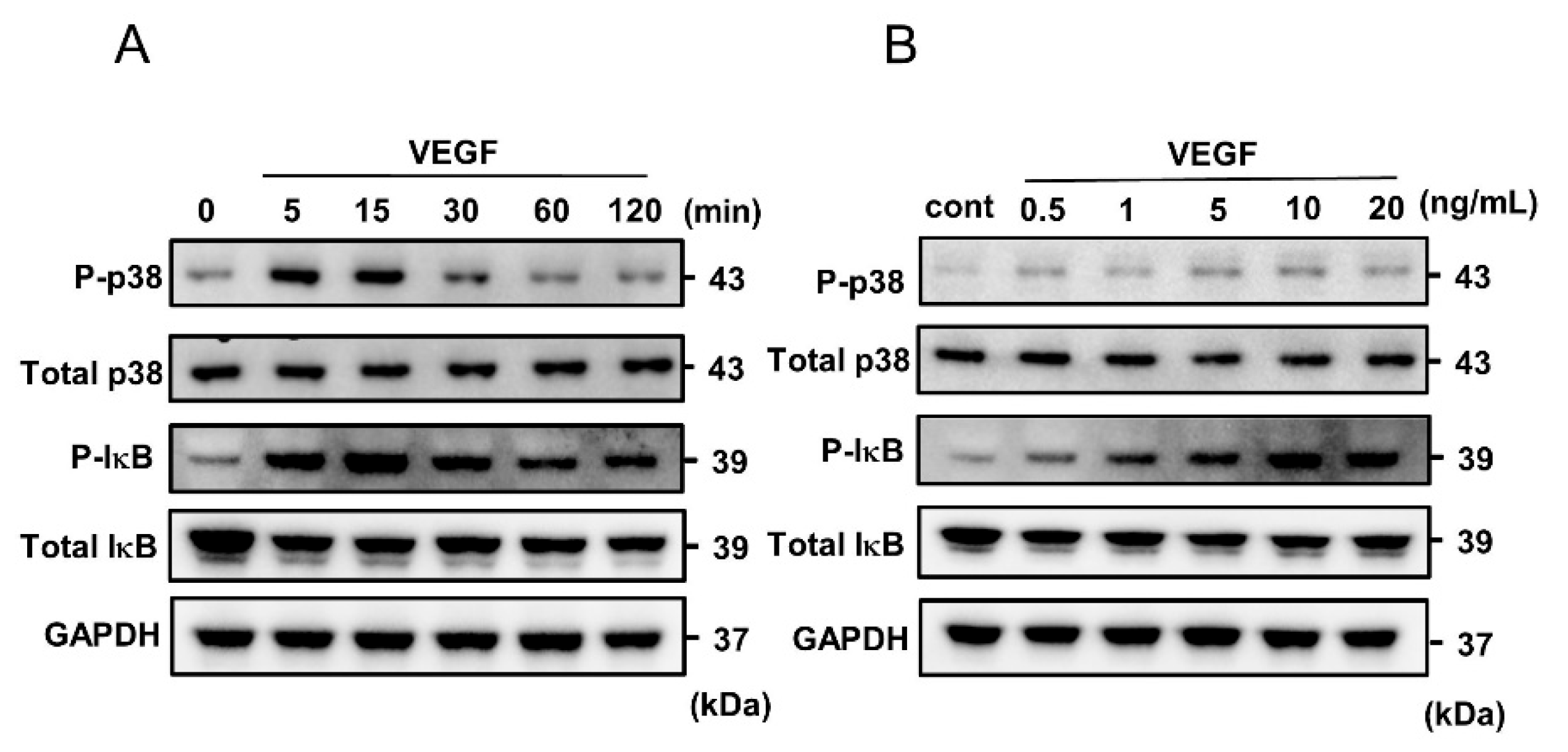
2.2. VEGF-Induced Phosphorylation of p38 MAPK and IκB in HUVECs
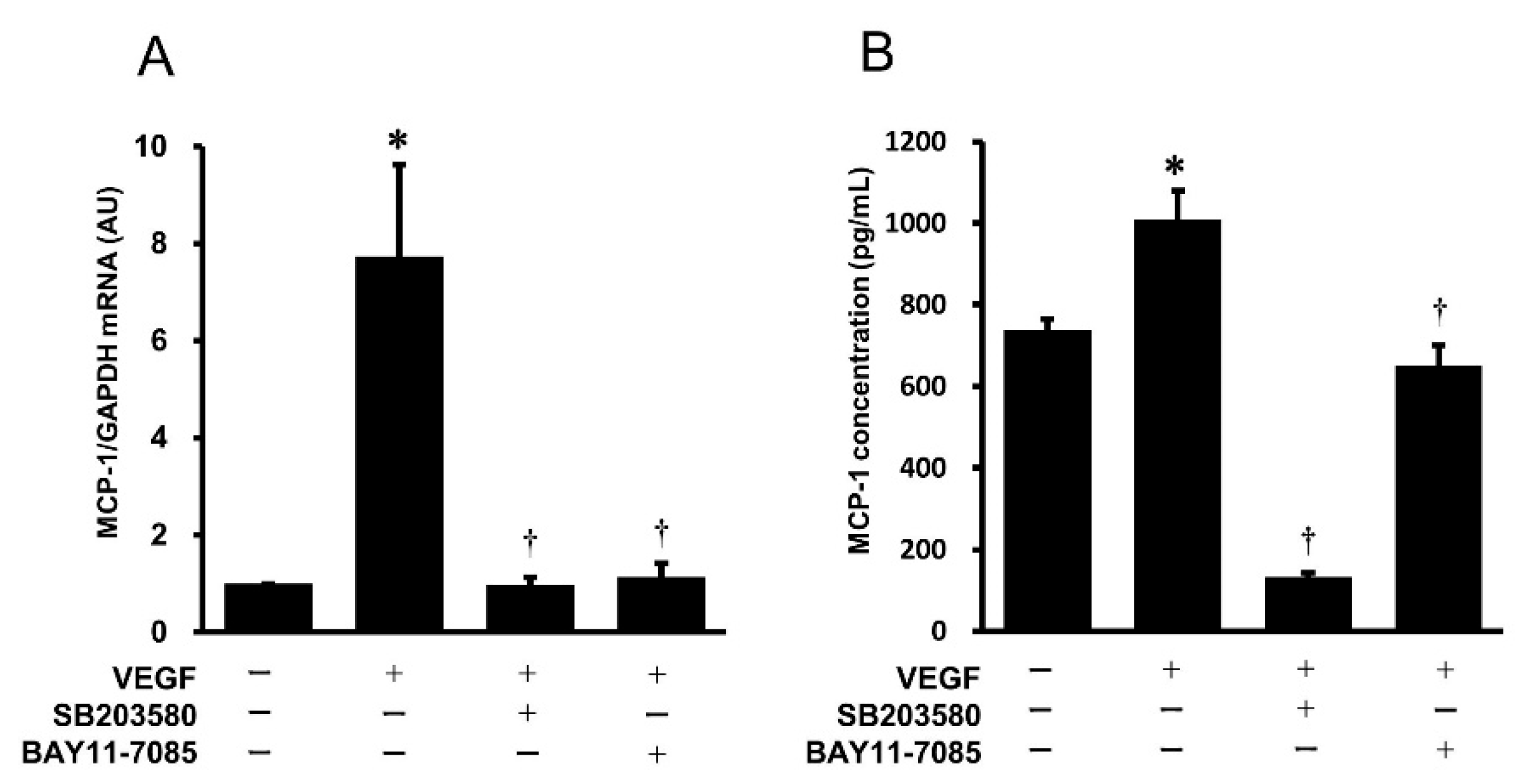
2.3. Effects of Pharmacological Inhibitors of the p38 MAPK and NF-κB Signaling Pathways on VEGF-Induced Gene Expression and Protein Secretion of MCP-1 in HUVECs
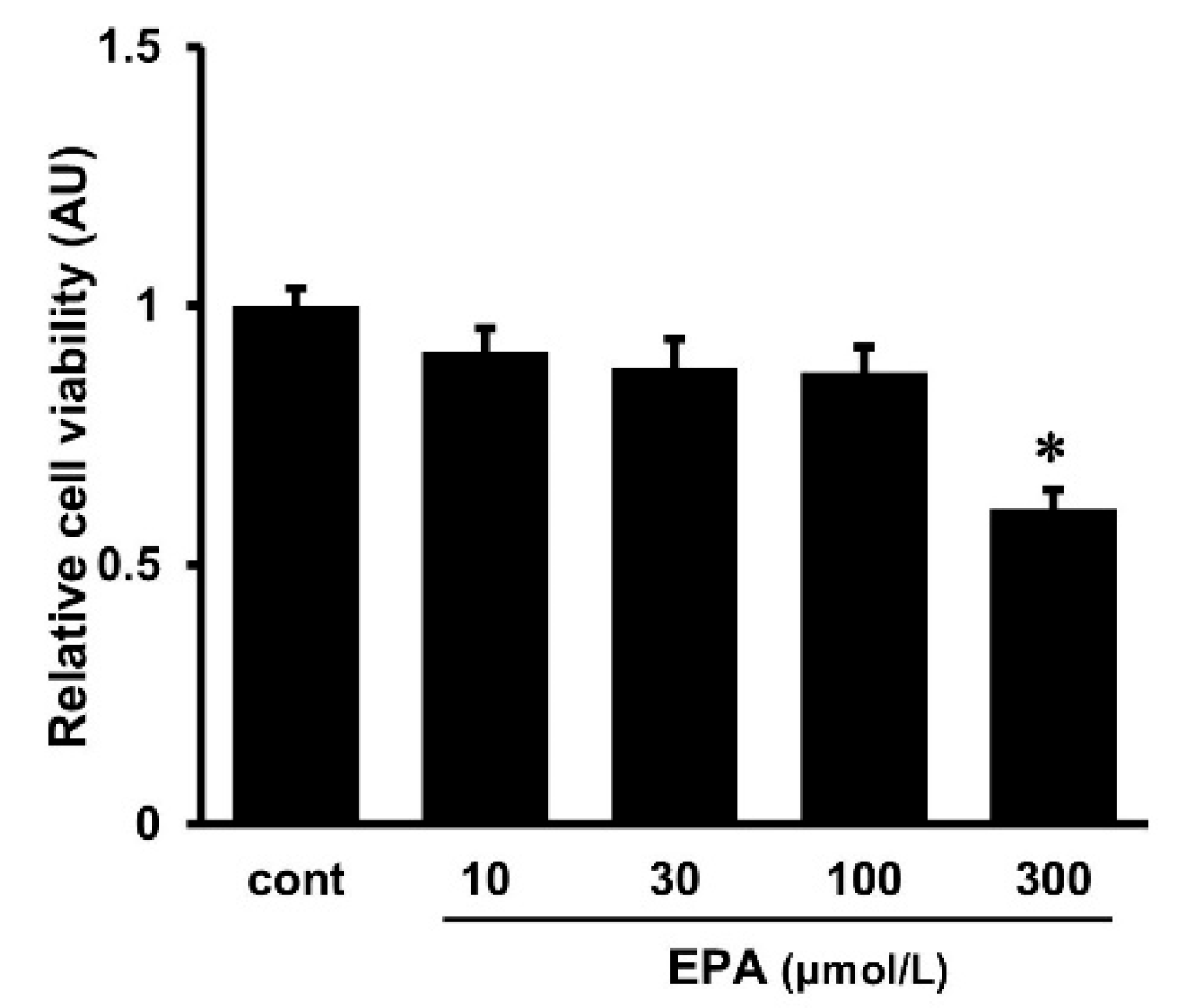
2.4. Effects of EPA on Cell Viability
2.5. Effects of EPA on the VEGF-Induced Gene Expression and Protein Secretion of MCP-1 in HUVECs
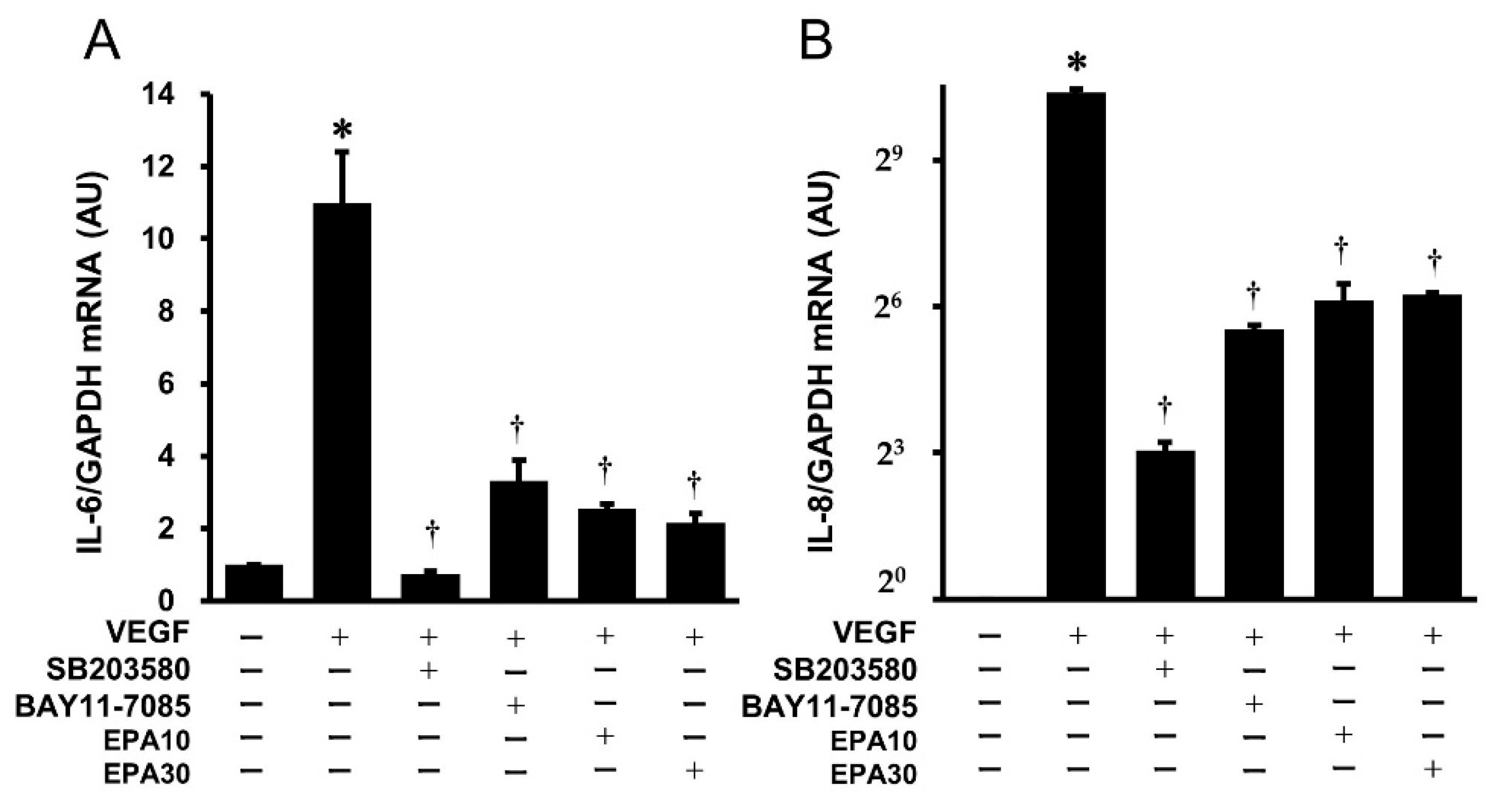
2.6. Effects of SB203580, BAY11-7085, and EPA on the VEGF-Induced Gene Expression of IL-6 and IL-8 in HUVECs
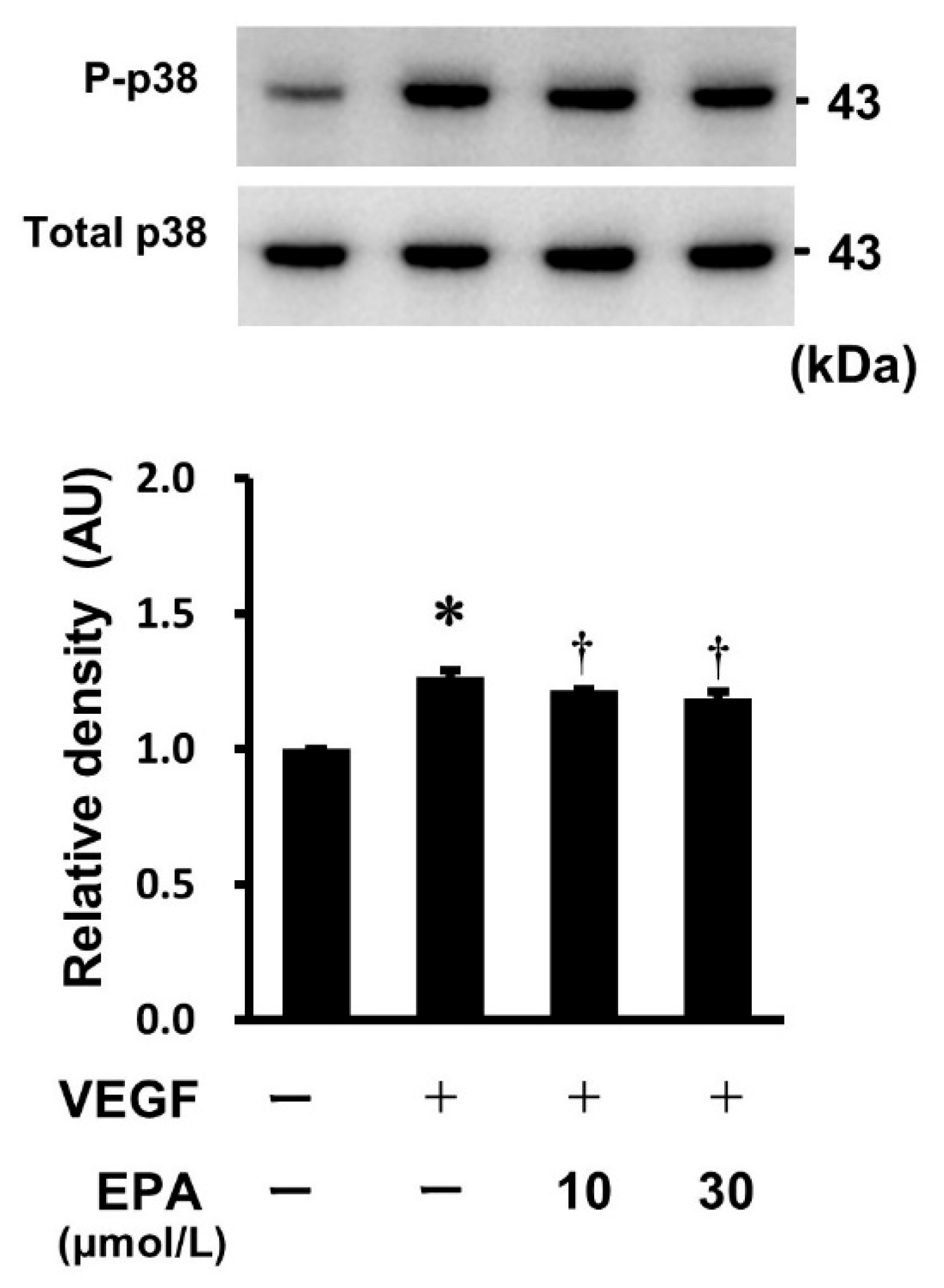
2.7. Effects of EPA on the VEGF-Stimulated Phosphorylation of p38 MAPK in HUVECs
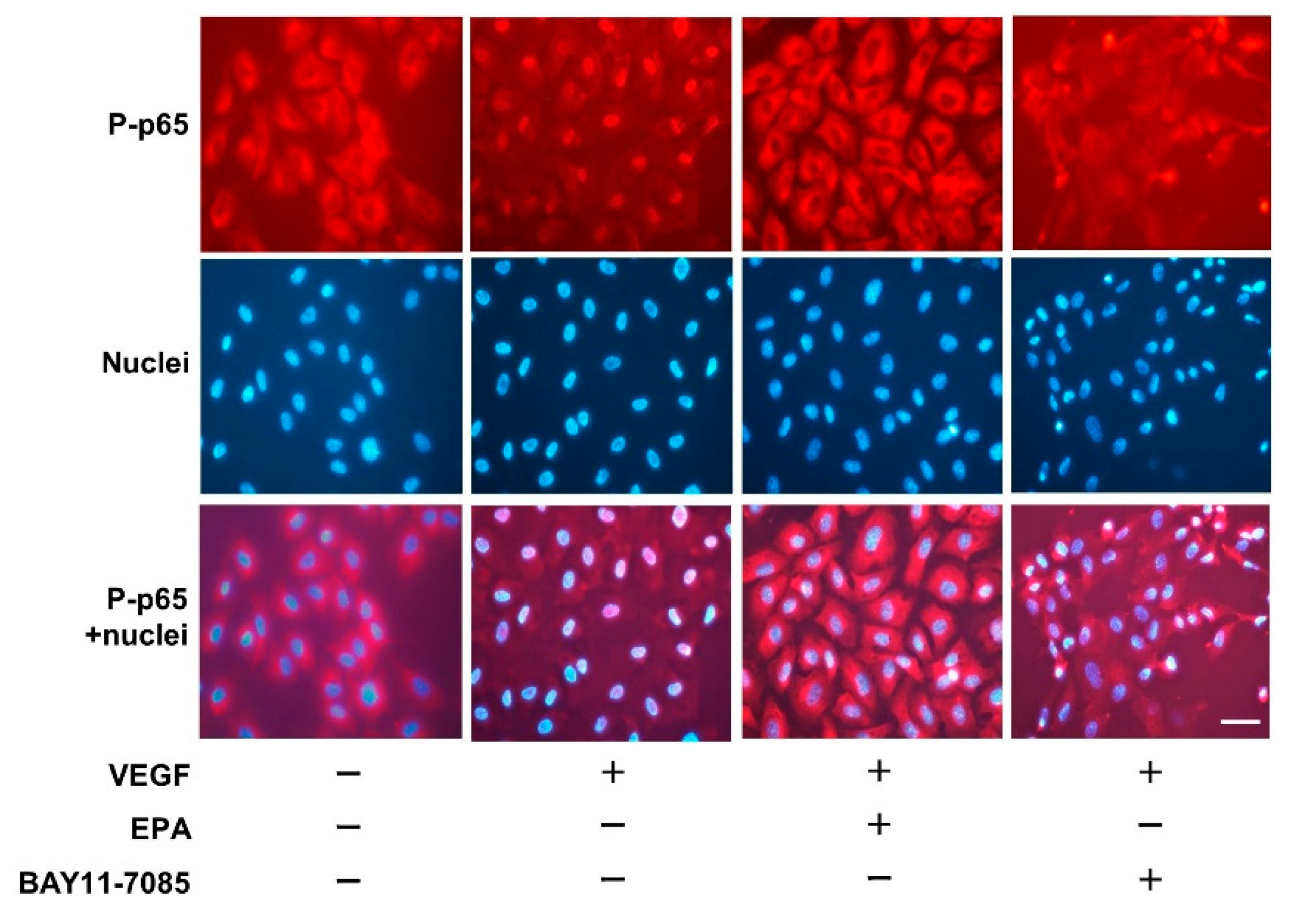
2.8. Immunofluorescence Staining
3. Discussion
4. Materials and Methods
4.1. Regents
4.2. Cell Culture of HUVECs
4.3. Total RNA Extraction and Real-Time RT-PCR
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Immunoblot Analysis
4.6. Cell Viability
4.7. Immunofluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | activator protein-1 |
| BLAST | basic local alignment search tool |
| CCL2 | CC-motif ligand 2 |
| ELISA | enzyme-linked immunosorbent assay |
| EPA | eicosapentaenoic acid |
| ERK | extracellular signal-regulated kinase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HUVECs | human vascular endothelial cells |
| IκB | inhibitor of nuclear factor-kappa B |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| NF-κB | nuclear factor-kappa B |
| PI3K | phosphoinositide-3-kinase |
| RT-PCR | reverse transcription polymerase chain reaction |
| TNF | tissue necrosis factor |
| VEGF | vascular endothelial growth factor |
References
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Shibuya, M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 2001, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Khotina, V.A.; Omelchenko, A.V.; Kalmykov, V.A.; Orekhov, A.N. The Role of the VEGF Family in Atherosclerosis Development and Its Potential as Treatment Targets. Int. J. Mol. Sci. 2022, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Marumo, T.; Schini-Kerth, V.B.; Busse, R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes 1999, 48, 1131–1137. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Yamada, M.; Kim, S.; Egashira, K.; Takeya, M.; Ikeda, T.; Mimura, O.; Iwao, H. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1996–2001. [Google Scholar] [CrossRef]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; Bellik, L.; Brogelli, L.; Filippi, S.; Ledda, F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1978–H1984. [Google Scholar] [CrossRef]
- Mukaida, N.; Harada, A.; Matsushima, K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998, 9, 9–23. [Google Scholar] [CrossRef]
- Boisvert, W.A. Modulation of atherogenesis by chemokines. Trends Cardiovasc. Med. 2004, 14, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Budoff, M. Triglycerides and Triglyceride-Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am. J. Cardiol. 2016, 118, 138–145. [Google Scholar] [CrossRef]
- Hilleman, D.; Smer, A. Prescription Omega-3 Fatty Acid Products and Dietary Supplements Are Not Interchangeable. Manag. Care 2016, 25, 46–52. [Google Scholar]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Koto, T.; Nagai, N.; Mochimaru, H.; Kurihara, T.; Izumi-Nagai, K.; Satofuka, S.; Shinoda, H.; Noda, K.; Ozawa, Y.; Inoue, M.; et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4328–4334. [Google Scholar] [CrossRef]
- Calviello, G.; Di Nicuolo, F.; Gragnoli, S.; Piccioni, E.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Palozza, P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 2004, 25, 2303–2310. [Google Scholar] [CrossRef]
- Cawood, A.L.; Ding, R.; Napper, F.L.; Young, R.H.; Williams, J.A.; Ward, M.J.; Gudmundsen, O.; Vige, R.; Payne, S.P.; Ye, S.; et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 252–259. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Patel, A.A.; Budoff, M.J. Effects of eicosapentaenoic acid and docosahexaenoic acid on lipoproteins in hypertriglyceridemia. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 145–149. [Google Scholar] [CrossRef]
- Tagawa, H.; Shimokawa, H.; Tagawa, T.; Kuroiwa-Matsumoto, M.; Hirooka, Y.; Takeshita, A. Long-term treatment with eicosapentaenoic acid augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilatation in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 1999, 33, 633–640. [Google Scholar] [CrossRef]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Wardhana; Surachmanto, E.S.; Datau, E.A. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med. Indones. 2011, 43, 138–143. [Google Scholar] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Abekura, Y.; Ono, I.; Kawashima, A.; Takizawa, K.; Koseki, H.; Miyata, H.; Shimizu, K.; Oka, M.; Kushamae, M.; Miyamoto, S.; et al. Eicosapentaenoic acid prevents the progression of intracranial aneurysms in rats. J. Neuroinflamm. 2020, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Diaz Encarnacion, M.M.; Warner, G.M.; Cheng, J.; Gray, C.E.; Nath, K.A.; Grande, J.P. n-3 Fatty acids block TNF-α-stimulated MCP-1 expression in rat mesangial cells. Am. J. Physiol. Renal Physiol. 2011, 300, F1142–F1151. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Morita, I.; Murota, S.I. Eicosapentaenoic acid attenuates vascular endothelial growth factor-induced proliferation via inhibiting Flk-1 receptor expression in bovine carotid artery endothelial cells. J. Cell. Physiol. 1998, 176, 342–349. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, G.; Li, L.; Lin, G.; Deng, Z. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. J. Cardiovasc. Transl. Res. 2013, 6, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Tevar, R.; Jho, D.H.; Babcock, T.; Helton, W.S.; Espat, N.J. Omega-3 fatty acid supplementation reduces tumor growth and vascular endothelial growth factor expression in a model of progressive non-metastasizing malignancy. JPEN J. Parenter. Enteral Nutr. 2002, 26, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Cook, M.D.; Lane-Cordova, A.D. Making cell culture more physiological: A call for a more comprehensive assessment of racial disparities in endothelial cell culture studies. Am. J. Physiol. Cell Physiol. 2020, 318, C238–C241. [Google Scholar] [CrossRef] [PubMed]
- Umebashi, K.; Tokito, A.; Yamamoto, M.; Jougasaki, M. Interleukin-33 induces interleukin-8 expression via JNK/c-Jun/AP-1 pathway in human umbilical vein endothelial cells. PLoS ONE 2018, 13, e0191659. [Google Scholar] [CrossRef]
- Yamamoto, M.; Umebashi, K.; Tokito, A.; Imamura, J.; Jougasaki, M. Interleukin-33 induces growth-regulated oncogene-alpha expression and secretion in human umbilical vein endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R272–R279. [Google Scholar] [CrossRef]
- Umebashi, K.; Yamamoto, M.; Tokito, A.; Sudou, K.; Takenoshita, Y.; Jougasaki, M. Inhibitory Effects of Simvastatin on IL-33-Induced MCP-1 via the Suppression of the JNK Pathway in Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 13015. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequences (Forward/Reverse) | Accession Number |
|---|---|---|
| MCP-1 | F: 5′-CATAGCAGCCACCTTCATTCC-3′ R: 5′-TCTCCTTGGCCACAATGGTC-3′ | NM_002982.3 |
| IL-6 | F: 5′-ACTCACCTCTTCAGAACGAATTG-3′ R: 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ | NM_000600.3 |
| IL-8 | F: 5′-AAGAAACCACCGGAAGGAAC-3′ R: 5′-ACTCCTTGGCAAAACTGCAC-3′ | NM_000584.3 |
| GAPDH | F: 5′-GCACCGTCAAGGCTGAGAAC-3′ R: 5′-TGGTGAAGACGCCAGTGGA-3′ | NM_002046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenoshita, Y.; Tokito, A.; Jougasaki, M. Inhibitory Effects of Eicosapentaenoic Acid on Vascular Endothelial Growth Factor-Induced Monocyte Chemoattractant Protein-1, Interleukin-6, and Interleukin-8 in Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 2749. https://doi.org/10.3390/ijms25052749
Takenoshita Y, Tokito A, Jougasaki M. Inhibitory Effects of Eicosapentaenoic Acid on Vascular Endothelial Growth Factor-Induced Monocyte Chemoattractant Protein-1, Interleukin-6, and Interleukin-8 in Human Vascular Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(5):2749. https://doi.org/10.3390/ijms25052749
Chicago/Turabian StyleTakenoshita, Yoko, Akinori Tokito, and Michihisa Jougasaki. 2024. "Inhibitory Effects of Eicosapentaenoic Acid on Vascular Endothelial Growth Factor-Induced Monocyte Chemoattractant Protein-1, Interleukin-6, and Interleukin-8 in Human Vascular Endothelial Cells" International Journal of Molecular Sciences 25, no. 5: 2749. https://doi.org/10.3390/ijms25052749






