Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives
Abstract
1. Introduction
1.1. Epidemiology
1.2. Risk Factors
1.3. Pathogenesis/Oncogenesis
1.4. Diagnostic Techniques
1.5. Vaccination
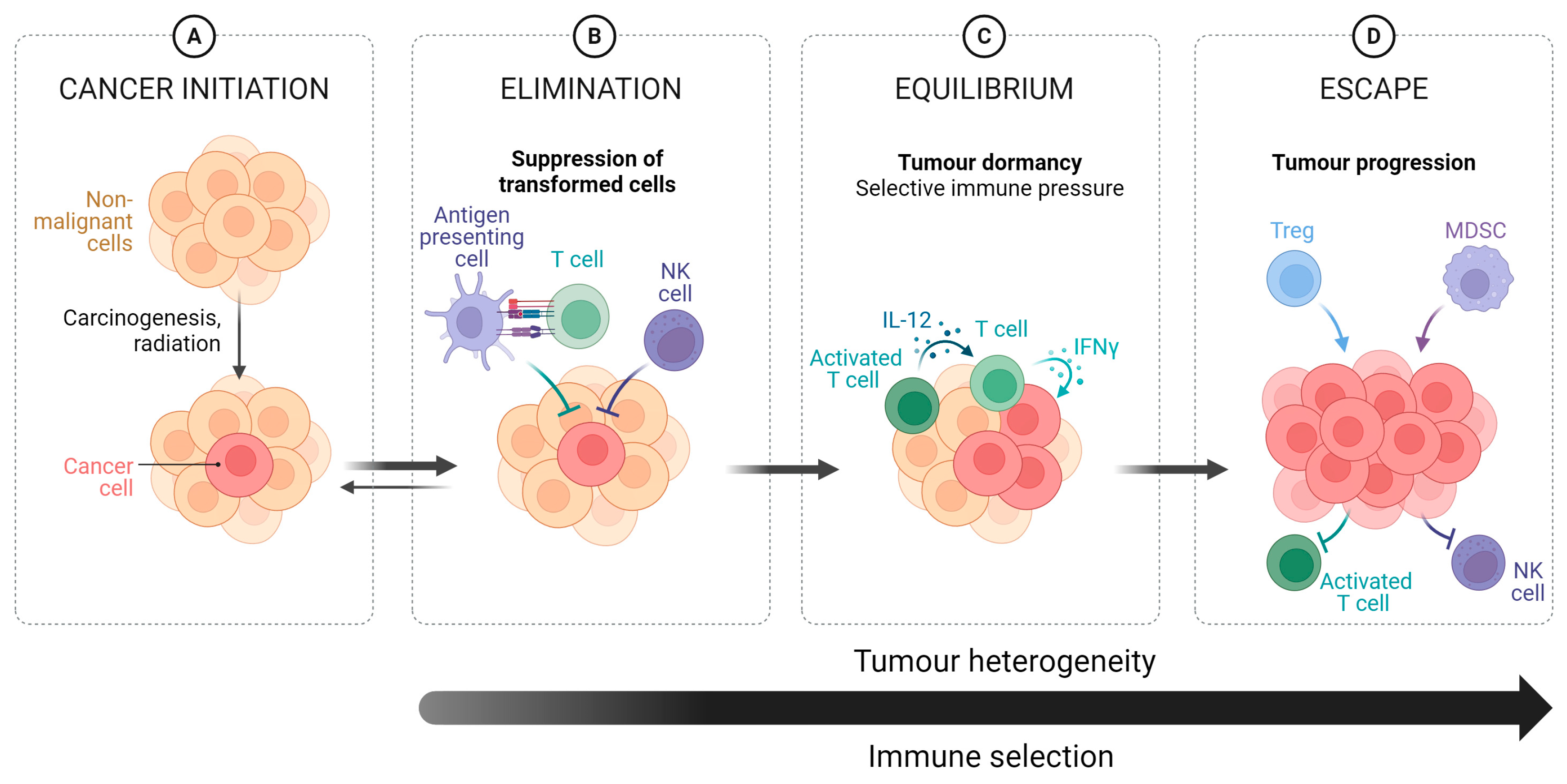
2. Role of the Immune System
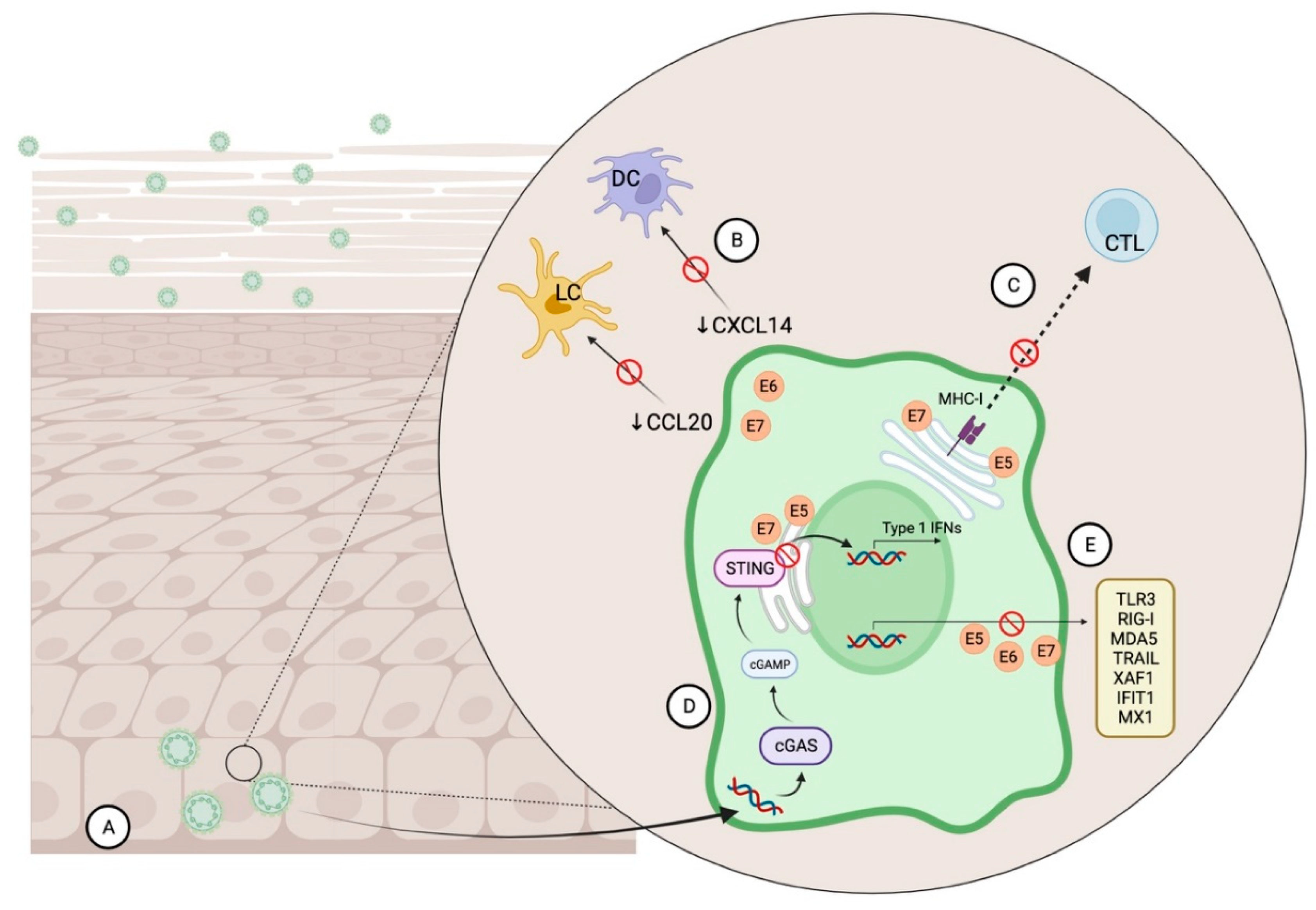
2.1. HPV & Immune System Evasion
2.2. HPV+OPSCC and the Tumour Microenvironment
2.3. Immune Checkpoint Inhibitors
3. Emerging Therapeutic Strategies
3.1. Small Molecule Immunotherapy
3.1.1. RTK
3.1.2. STAT
3.1.3. STING
3.1.4. PPAR
3.1.5. AHR
3.1.6. NET Based Therapies
Polyanions
3.1.7. NET Modulators or Preventors
3.1.8. NET Degraders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV+OPSCC | Human Papillomavirus Positive Oropharyngeal Squamous Cell Carcinoma |
| HPV−OPSCC | Human Papillomavirus Negative Oropharyngeal Squamous Cell Carcinoma |
References
- Syrjänen, K.J.; Pyrhönen, S.; Syrjänen, S.M.; Lamberg, M.A. Immunohistochemical demonstration of Human papilloma virus (HPV) antigens in oral squamous cell lesions. Br. J. Oral Surg. 1983, 21, 147–153. [Google Scholar] [CrossRef]
- Ferreira, C.C. The relation between human papillomavirus (HPV) and oropharyngeal cancer: A review. PeerJ 2023, 11, e15568. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Guthrie, V.B.; Masica, D.L.; Tokheim, C.; Kang, H.; Richmon, J.; Agrawal, N.; Fakhry, C.; Quon, H.; Subramaniam, R.M.; et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann. Oncol. 2015, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Santegoets, S.J.; van der Burg, S.H. The Tumor Microenvironment and Immunotherapy of Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 545385. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Mellin, H.; Friesland, S.; Lewensohn, R.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer 2000, 89, 300–304. [Google Scholar] [CrossRef]
- Posner, M.R.; Lorch, J.H.; Goloubeva, O.; Tan, M.; Schumaker, L.M.; Sarlis, N.J.; Haddad, R.I.; Cullen, K.J. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann. Oncol. 2011, 22, 1071–1077. [Google Scholar] [CrossRef]
- McIlwain, W.R.; Sood, A.J.; Nguyen, S.A.; Day, T.A. Initial Symptoms in Patients with HPV-Positive and HPV-Negative Oropharyngeal Cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 441–447. [Google Scholar] [CrossRef]
- Gillison, M.L. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 2007, 29, 779–792. [Google Scholar] [CrossRef]
- Gillison, M.L.; Zhang, Q.; Jordan, R.; Xiao, W.; Westra, W.H.; Trotti, A.; Spencer, S.; Harris, J.; Chung, C.H.; Ang, K.K. Tobacco Smoking and Increased Risk of Death and Progression for Patients with p16-Positive and p16-Negative Oropharyngeal Cancer. J. Clin. Oncol. 2012, 30, 2102–2111. [Google Scholar] [CrossRef]
- Tramacere, I.; Negri, E.; Bagnardi, V.; Garavello, W.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; Boffetta, P.; La Vecchia, C. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: Overall results and dose-risk relation. Oral Oncol. 2010, 46, 497–503. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Bos, P.; van den Brekel, M.W.M.; Gouw, Z.A.R.; Al-Mamgani, A.; Waktola, S.; Aerts, H.; Beets-Tan, R.G.H.; Castelijns, J.A.; Jasperse, B. Clinical variables and magnetic resonance imaging-based radiomics predict human papillomavirus status of oropharyngeal cancer. Head Neck 2021, 43, 485–495. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- O’Sullivan, B. Head and neck tumours. UICC TNM Classif. Malig. Tumours 2017, 8, 17–54. [Google Scholar]
- Morris, L.G.; Sikora, A.G.; Patel, S.G.; Hayes, R.B.; Ganly, I. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol. 2011, 29, 739–746. [Google Scholar] [CrossRef]
- Chen, A.M. De-Escalation Treatment for Human Papillomavirus–Related Oropharyngeal Cancer: Questions for Practical Consideration. Oncology 2023, 37, 281–287. [Google Scholar]
- Silver, J.A.; Turkdogan, S.; Roy, C.F.; Subramaniam, T.; Henry, M.; Sadeghi, N. De-Escalation Strategies for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma—Where Are We Now? Curr. Oncol. 2022, 29, 3668–3697. [Google Scholar] [CrossRef]
- Elhalawani, H.; Mohamed, A.S.R.; Elgohari, B.; Lin, T.A.; Sikora, A.G.; Lai, S.Y.; Abusaif, A.; Phan, J.; Morrison, W.H.; Gunn, G.B.; et al. Tobacco exposure as a major modifier of oncologic outcomes in human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma. BMC Cancer 2020, 20, 912. [Google Scholar] [CrossRef]
- Lai, Y.H.; Su, C.C.; Wu, S.Y.; Hsueh, W.T.; Wu, Y.H.; Chen, H.H.W.; Hsiao, J.R.; Liu, C.H.; Tsai, Y.S. Impact of Alcohol and Smoking on Outcomes of HPV-Related Oropharyngeal Cancer. J. Clin. Med. 2022, 11, 6510. [Google Scholar] [CrossRef]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mariz, B.; Kowalski, L.P.; William, W.N., Jr.; de Castro, G., Jr.; Chaves, A.L.F.; Santos, M.; de Oliveira, T.B.; Araújo, A.L.D.; Normando, A.G.C.; Ribeiro, A.C.P.; et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 156, 103116. [Google Scholar] [CrossRef]
- Lechner, M.; Jones, O.S.; Breeze, C.E.; Gilson, R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 2019, 19, 131–132. [Google Scholar] [CrossRef]
- Faraji, F.; Rettig, E.M.; Tsai, H.L.; El Asmar, M.; Fung, N.; Eisele, D.W.; Fakhry, C. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 2019, 125, 761–769. [Google Scholar] [CrossRef]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Grønhøj, C.; Buchwald, C.V. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, Z.; Luo, G.; Yan, H.; Qian, H.-Z.; Fu, L.; Wang, B.; Huang, R.; Cao, F.; Lin, H.; et al. Global burden of oropharyngeal cancer attributable to human papillomavirus by anatomical subsite and geographic region. Cancer Epidemiol. 2022, 78, 102140. [Google Scholar] [CrossRef] [PubMed]
- Ndon, S.; Singh, A.; Ha, P.K.; Aswani, J.; Chan, J.Y.-K.; Xu, M.J. Human Papillomavirus-Associated Oropharyngeal Cancer: Global Epidemiology and Public Policy Implications. Cancers 2023, 15, 4080. [Google Scholar] [CrossRef]
- Argirion, I.; Zarins, K.R.; McHugh, J.; Cantley, R.L.; Teeramatwanich, W.; Laohasiriwong, S.; Kasemsiri, P.; Naruikon, J.; Srimanta, P.; Chinn, S.B.; et al. Increasing prevalence of HPV in oropharyngeal carcinoma suggests adaptation of p16 screening in Southeast Asia. J. Clin. Virol. 2020, 132, 104637. [Google Scholar] [CrossRef]
- Hwang, T.Z.; Hsiao, J.R.; Tsai, C.R.; Chang, J.S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int. J. Cancer 2015, 137, 395–408. [Google Scholar] [CrossRef]
- Rietbergen, M.M.; van Bokhoven, A.; Lissenberg-Witte, B.I.; Heideman, D.A.M.; Leemans, C.R.; Brakenhoff, R.H.; Bloemena, E. Epidemiologic associations of HPV-positive oropharyngeal cancer and (pre)cancerous cervical lesions. Int. J. Cancer 2018, 143, 283–288. [Google Scholar] [CrossRef]
- Hong, A.; Lee, C.S.; Jones, D.; Veillard, A.S.; Zhang, M.; Zhang, X.; Smee, R.; Corry, J.; Porceddu, S.; Milross, C.; et al. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck 2016, 38, 743–750. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Luu, M.; Rosenberg, P.S.; Elrod, J.K.; Bray, F.; Vaccarella, S.; Gay, C.; Lu, D.J.; Chen, M.M.; Chaturvedi, A.K.; et al. Global epidemiologic patterns of oropharyngeal cancer incidence trends. J. Natl. Cancer Inst. 2023, 115, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2023, 41, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.J.; Shing, J.Z.; Vanegas, J.C.; González, E.; Guillén, D.; Sierra, M.S.; Hildesheim, A.; Porras, C.; Herrero, R.; Torres, G.; et al. Trends in incidence rates of head and neck squamous cell carcinomas overall and by potential relatedness to human papillomavirus, Costa Rica 2006 to 2015. Int. J. Cancer 2023, 152, 2052–2060. [Google Scholar] [CrossRef]
- Wittekindt, C.; Wagner, S.; Bushnak, A.; Prigge, E.S.; von Knebel Doeberitz, M.; Würdemann, N.; Bernhardt, K.; Pons-Kühnemann, J.; Maulbecker-Armstrong, C.; Klussmann, J.P. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev. Res. 2019, 12, 375–382. [Google Scholar] [CrossRef]
- Donà, M.G.; Rollo, F.; Pichi, B.; Spriano, G.; Moretto, S.; Covello, R.; Pellini, R.; Benevolo, M. Evolving Profile of HPV-Driven Oropharyngeal Squamous Cell Carcinoma in a National Cancer Institute in Italy: A 10-Year Retrospective Study. Microorganisms 2020, 8, 1498. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Osazuwa-Peters, N.; Massa, S.T.; Simpson, M.C.; Boakye, E.A.; Varvares, M.A. Survival of human papillomavirus-associated cancers: Filling in the gaps. Cancer 2018, 124, 18–20. [Google Scholar] [CrossRef]
- Rettig, E.M.; Zaidi, M.; Faraji, F.; Eisele, D.W.; El Asmar, M.; Fung, N.; D’Souza, G.; Fakhry, C. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018, 83, 147–153. [Google Scholar] [CrossRef]
- Cline, B.J.; Simpson, M.C.; Gropler, M.; Bukatko, A.R.; Adjei Boakye, E.; Mohammed, K.A.; Osazuwa-Peters, N. Change in Age at Diagnosis of Oropharyngeal Cancer in the United States, 1975–2016. Cancers 2020, 12, 3191. [Google Scholar] [CrossRef]
- Tota, J.E.; Best, A.F.; Zumsteg, Z.S.; Gillison, M.L.; Rosenberg, P.S.; Chaturvedi, A.K. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J. Clin. Oncol. 2019, 37, 1538–1546. [Google Scholar] [CrossRef]
- Lu, D.J.; Luu, M.; Mita, A.; Scher, K.; Shiao, S.L.; Yoshida, E.P.; Sittig, M.P.; Mallen-St Clair, J.; Ho, A.S.; Zumsteg, Z.S. Human papillomavirus–associated oropharyngeal cancer among patients aged 70 and older: Dramatically increased prevalence and clinical implications. Eur. J. Cancer 2018, 103, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Windon, M.J.; D’Souza, G.; Rettig, E.M.; Westra, W.H.; van Zante, A.; Wang, S.J.; Ryan, W.R.; Mydlarz, W.K.; Ha, P.K.; Miles, B.A.; et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer 2018, 124, 2993–2999. [Google Scholar] [CrossRef]
- Wyss, A.; Hashibe, M.; Chuang, S.C.; Lee, Y.C.; Zhang, Z.F.; Yu, G.P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, Cigar, and Pipe Smoking and the Risk of Head and Neck Cancers: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; Wünsch-Filho, V. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein–Barr virus in the pathogenesis of oral cancers. Oral Dis. 2017, 24, 497–508. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Andrews, E.; Seaman, W.T.; Webster-Cyriaque, J. Oropharyngeal carcinoma in non-smokers and non-drinkers: A role for HPV. Oral Oncol. 2009, 45, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Valova, V.; HÄnsel, T.; Stromberger, C.; Kofla, G.; Olze, H.; Piwonski, I.; Albers, A.; Ochsenreither, S.; Coordes, A. Impact of Smoking on the Survival of Patients with High-risk HPV-positive HNSCC: A Meta-analysis. Vivo 2021, 35, 1017–1026. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, J.S.; Wagner, S.; Dehlendorff, C.; Friborg, J.; Andersen, E.; Wittekindt, C.; Würdemann, N.; Sharma, S.J.; Gattenlöhner, S.; et al. Impact on survival of tobacco smoking for cases with oropharyngeal squamous cell carcinoma and known human papillomavirus and p16-status: A multicenter retrospective study. Oncotarget 2019, 10, 4655–4663. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Carrillo-Beltrán, D.; Aedo-Aguilera, V.; Calaf, G.M.; León, O.; Maldonado, E.; Tapia, J.C.; Boccardo, E.; Ozbun, M.A.; Aguayo, F. Tobacco Exposure Enhances Human Papillomavirus 16 Oncogene Expression via EGFR/PI3K/Akt/c-Jun Signaling Pathway in Cervical Cancer Cells. Front. Microbiol. 2018, 9, 3022. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.; Dahlstrom, K.R.; Gross, N.; Li, G. Joint effect of human papillomavirus exposure, smoking and alcohol on risk of oral squamous cell carcinoma. BMC Cancer 2023, 23, 457. [Google Scholar] [CrossRef]
- Eldridge, R.C.; Pawlita, M.; Wilson, L.; Castle, P.E.; Waterboer, T.; Gravitt, P.E.; Schiffman, M.; Wentzensen, N. Smoking and subsequent human papillomavirus infection: A mediation analysis. Ann. Epidemiol. 2017, 27, 724–730.e1. [Google Scholar] [CrossRef]
- Arif, R.T.; Mogaddam, M.A.; Merdad, L.A.; Farsi, N.J. Does human papillomavirus modify the risk of oropharyngeal cancer related to smoking and alcohol drinking? A systematic review and meta-analysis. Laryngoscope Investig. Otolaryngol. 2022, 7, 1391–1401. [Google Scholar] [CrossRef]
- Applebaum, K.M.; Furniss, C.S.; Zeka, A.; Posner, M.R.; Smith, J.F.; Bryan, J.; Eisen, E.A.; Peters, E.S.; McClean, M.D.; Kelsey, K.T. Lack of Association of Alcohol and Tobacco with HPV16-Associated Head and Neck Cancer. J. Natl. Cancer Inst. 2007, 99, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Farsi, N.J.; El-Zein, M.; Gaied, H.; Lee, Y.C.; Hashibe, M.; Nicolau, B.; Rousseau, M.C. Sexual behaviours and head and neck cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2015, 39, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Rudolph, J.; Henger, S.; Engel, C.; Wirkner, K.; Wenning, J.R.; Zeynalova, S.; Wiegand, S.; Loeffler, M.; Wald, T.; et al. Is High-Risk Sexual Behavior a Risk Factor for Oropharyngeal Cancer? Cancers 2023, 15, 3356. [Google Scholar] [CrossRef]
- Talamini, R.; Vaccarella, S.; Barbone, F.; Tavani, A.; La Vecchia, C.; Herrero, R.; Muñoz, N.; Franceschi, S. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br. J. Cancer 2000, 83, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, K.; Wennerberg, J.; Schildt, E.B.; Bladström, A.; Göran Hansson, B.; Andersson, G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005, 125, 1327–1336. [Google Scholar] [CrossRef]
- Leiding, J.W.; Holland, S.M. Warts and all: Human papillomavirus in primary immunodeficiencies. J. Allergy Clin. Immunol. 2012, 130, 1030–1048. [Google Scholar] [CrossRef]
- Béziat, V. Human genetic dissection of papillomavirus-driven diseases: New insight into their pathogenesis. Hum. Genet. 2020, 139, 919–939. [Google Scholar] [CrossRef]
- Hewavisenti, R.V.; Arena, J.; Ahlenstiel, C.L.; Sasson, S.C. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front. Immunol. 2023, 14, 1112513. [Google Scholar] [CrossRef]
- Kim, S.Y.; Solomon, D.H. Tumor necrosis factor blockade and the risk of viral infection. Nat. Rev. Rheumatol. 2010, 6, 165–174. [Google Scholar] [CrossRef]
- Kim, S.C.; Glynn, R.J.; Giovannucci, E.; Hernández-Díaz, S.; Liu, J.; Feldman, S.; Karlson, E.W.; Schneeweiss, S.; Solomon, D.H. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: A population-based cohort study. Ann. Rheum. Dis. 2015, 74, 1360–1367. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Madeleine, M.M.; Biggar, R.J.; Engels, E.A. Risk of Human Papillomavirus–Associated Cancers among Persons with AIDS. JNCI J. Natl. Cancer Inst. 2009, 101, 1120–1130. [Google Scholar] [CrossRef]
- Beachler, D.C.; Abraham, A.G.; Silverberg, M.J.; Jing, Y.; Fakhry, C.; Gill, M.J.; Dubrow, R.; Kitahata, M.M.; Klein, M.B.; Burchell, A.N.; et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol. 2014, 50, 1169–1176. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Mikuličić, S.; Strunk, J.; Florin, L. HPV16 Entry into Epithelial Cells: Running a Gauntlet. Viruses 2021, 13, 2460. [Google Scholar] [CrossRef]
- IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses; World Health Organization: Geneva, Switzerland, 2007.
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Fehrmann, F.; Laimins, L.A. Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene 2003, 22, 5201–5207. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Jones, R.N.; Zhang, L.; Guram, K.; Sharma, S.; Schoenberger, S.P.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. Human papillomavirus E5 suppresses immunity via inhibition of the immunoproteasome and STING pathway. Cell Rep. 2023, 42, 112508. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Park, D.; Munger, K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA 2013, 110, 16175–16180. [Google Scholar] [CrossRef]
- Mir, B.A.; Ahmad, A.; Farooq, N.; Priya, M.V.; Siddiqui, A.H.; Asif, M.; Manzoor, R.; Ishqi, H.M.; Alomar, S.Y.; Rahaman, P.F. Increased expression of HPV-E7 oncoprotein correlates with a reduced level of pRb proteins via high viral load in cervical cancer. Sci. Rep. 2023, 13, 15075. [Google Scholar] [CrossRef]
- Soto, D.; Song, C.; McLaughlin-Drubin, M.E. Epigenetic Alterations in Human Papillomavirus-Associated Cancers. Viruses 2017, 9, 248. [Google Scholar] [CrossRef]
- Thomas, M.; Banks, L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J. Gen. Virol. 1999, 80 Pt 6, 1513–1517. [Google Scholar] [CrossRef]
- Kazemi, S.; Papadopoulou, S.; Li, S.; Su, Q.; Wang, S.; Yoshimura, A.; Matlashewski, G.; Dever, T.E.; Koromilas, A.E. Control of α Subunit of Eukaryotic Translation Initiation Factor 2 (eIF2α) Phosphorylation by the Human Papillomavirus Type 18 E6 Oncoprotein: Implications for eIF2α-Dependent Gene Expression and Cell Death. Mol. Cell. Biol. 2004, 24, 3415–3429. [Google Scholar] [CrossRef]
- Garnett, T.O.; Filippova, M.; Duerksen-Hughes, P.J. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006, 13, 1915–1926. [Google Scholar] [CrossRef]
- Storey, A.; Thomas, M.; Kalita, A.; Harwood, C.; Gardiol, D.; Mantovani, F.; Breuer, J.; Leigh, I.M.; Matlashewski, G.; Banks, L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 1998, 393, 229–234. [Google Scholar] [CrossRef]
- Nagasaka, K.; Kawana, K.; Osuga, Y.; Fujii, T. PDZ Domains and Viral Infection: Versatile Potentials of HPV-PDZ Interactions in relation to Malignancy. BioMed Res. Int. 2013, 2013, 369712. [Google Scholar] [CrossRef]
- Katzenellenbogen, R. Telomerase Induction in HPV Infection and Oncogenesis. Viruses 2017, 9, 180. [Google Scholar] [CrossRef]
- Schache, A.G.; Liloglou, T.; Risk, J.M.; Jones, T.M.; Ma, X.J.; Wang, H.; Bui, S.; Luo, Y.; Sloan, P.; Shaw, R.J.; et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br. J. Cancer 2013, 108, 1332–1339. [Google Scholar] [CrossRef]
- Fauzi, F.H.; Hamzan, N.I.; Ab Rahman, N.; Suraiya, S.; Mohamad, S. Detection of human papillomavirus in oropharyngeal squamous cell carcinoma. J. Zhejiang Univ. Sci. B 2020, 21, 961–976. [Google Scholar] [CrossRef]
- Prigge, E.-S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16INK4aimmunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.-C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef]
- Nielsen, K.J.; Jakobsen, K.K.; Jensen, J.S.; Grønhøj, C.; Von Buchwald, C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses 2021, 13, 1339. [Google Scholar] [CrossRef]
- Tsentemeidou, A.; Fyrmpas, G.; Stavrakas, M.; Vlachtsis, K.; Sotiriou, E.; Poutoglidis, A.; Tsetsos, N. Human Papillomavirus Vaccine to End Oropharyngeal Cancer. A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2021, 48, 700–707. [Google Scholar] [CrossRef]
- Katz, J. The impact of HPV vaccination on the prevalence of oropharyngeal cancer (OPC) in a hospital-based population: A cross-sectional study of patient’s registry. J. Oral Pathol. Med. 2021, 50, 47–51. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- World Health Organisation. Global Partners Cheer Progress towards Eliminating Cervical Cancer and Underline Challenges. 2023. Available online: https://www.who.int/news/item/17-11-2023-global-partners-cheer-progress-towards-eliminating-cervical-cancer-and-underline-challenges (accessed on 19 January 2024).
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health 2023, 11, 1067299. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomavirus (HPV) Vaccination Coverage; World Health Organization: Geneva, Switzerland, 2023.
- Ferris, R.L.; Spanos, W.C.; Leidner, R.; Gonçalves, A.; Martens, U.M.; Kyi, C.; Sharfman, W.; Chung, C.H.; Devriese, L.A.; Gauthier, H.; et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J. Immunother. Cancer 2021, 9, e002568. [Google Scholar] [CrossRef]
- Zhang, Y.; Fakhry, C.; D’souza, G. Projected Association of Human Papillomavirus Vaccination with Oropharynx Cancer Incidence in the US, 2020–2045. JAMA Oncol. 2021, 7, e212907. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, viii6–viii9. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Stern, P.L. Harnessing immunity for therapy in human papillomavirus driven cancers. Tumour Virus Res. 2021, 11, 200212. [Google Scholar] [CrossRef]
- Nunes, R.A.L.; Morale, M.G.; Silva, G.F.; Villa, L.L.; Termini, L. Innate immunity and HPV: Friends or foes. Clinics 2018, 73, e549s. [Google Scholar] [CrossRef]
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef]
- Manzo-Merino, J.; Del-Toro-Arreola, S.; Rocha-Zavaleta, L.; Peralta-Zaragoza, Ó.; Jiménez-Lima, R.; Madrid-Marina, V. Immunology of cervical cancer. Rev. Investig. Clin. 2020, 72, 188–197. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef]
- HPV and Cervical Cancer. Achievements in Prevention and Future Prospects. Anticancer Res. 2012, 32, 3595. [Google Scholar]
- Cai, X.; Chiu, Y.-H.; Chen Zhijian, J. The cGAS-cGAMP-STING Pathway of Cytosolic DNA Sensing and Signaling. Mol. Cell 2014, 54, 289–296. [Google Scholar] [CrossRef]
- Lau, L.; Gray, E.E.; Brunette, R.L.; Stetson, D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 2015, 350, 568–571. [Google Scholar] [CrossRef]
- Lou, M.; Huang, D.; Zhou, Z.; Shi, X.; Wu, M.; Rui, Y.; Su, J.; Zheng, W.; Yu, X.F. DNA virus oncoprotein HPV18 E7 selectively antagonizes cGAS-STING-triggered innate immune activation. J. Med. Virol. 2023, 95, e28310. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Bortnik, V.; McMillan, N.A.; Idris, A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019, 132, 162–165. [Google Scholar] [CrossRef]
- Luo, X.; Donnelly, C.R.; Gong, W.; Heath, B.R.; Hao, Y.; Donnelly, L.A.; Moghbeli, T.; Tan, Y.S.; Lin, X.; Bellile, E.; et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020, 130, 1635–1652. [Google Scholar] [CrossRef]
- Karim, R.; Meyers, C.; Backendorf, C.; Ludigs, K.; Offringa, R.; van Ommen, G.J.; Melief, C.J.; van der Burg, S.H.; Boer, J.M. Human Papillomavirus Deregulates the Response of a Cellular Network Comprising of Chemotactic and Proinflammatory Genes. PLoS ONE 2011, 6, e17848. [Google Scholar] [CrossRef]
- Caberg, J.H.; Hubert, P.; Herman, L.; Herfs, M.; Roncarati, P.; Boniver, J.; Delvenne, P. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: Role of CCL20. Cancer Immunol. Immunother. 2009, 58, 39–47. [Google Scholar] [CrossRef]
- Cicchini, L.; Westrich, J.A.; Xu, T.; Vermeer, D.W.; Berger, J.N.; Clambey, E.T.; Lee, D.; Song, J.I.; Lambert, P.F.; Greer, R.O.; et al. Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14. mBio 2016, 7, e00270-16. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-Risk Human Papillomaviruses Repress Constitutive Kappa Interferon Transcription via E6 To Prevent Pathogen Recognition Receptor and Antiviral-Gene Expression. J. Virol. 2011, 85, 11372–11380. [Google Scholar] [CrossRef]
- Nees, M.; Geoghegan, J.M.; Hyman, T.; Frank, S.; Miller, L.; Woodworth, C.D. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 2001, 75, 4283–4296. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Tosi, A.; Parisatto, B.; Menegaldo, A.; Spinato, G.; Guido, M.; Del Mistro, A.; Bussani, R.; Zanconati, F.; Tofanelli, M.; Tirelli, G.; et al. The immune microenvironment of HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma: A multiparametric quantitative and spatial analysis unveils a rationale to target treatment-naïve tumors with immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 279. [Google Scholar] [CrossRef]
- Mito, I.; Takahashi, H.; Kawabata-Iwakawa, R.; Ida, S.; Tada, H.; Chikamatsu, K. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 16134. [Google Scholar] [CrossRef]
- Matlung, S.E.; van Kempen, P.M.W.; Bovenschen, N.; van Baarle, D.; Willems, S.M. Differences in T-cell infiltrates and survival between HPV+ and HPV− oropharyngeal squamous cell carcinoma. Future Sci. OA 2016, 2, FSO88. [Google Scholar] [CrossRef]
- Gurin, D.; Slavik, M.; Hermanova, M.; Selingerova, I.; Kazda, T.; Hendrych, M.; Shatokhina, T.; Vesela, M. The tumor immune microenvironment and its implications for clinical outcome in patients with oropharyngeal squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 886–896. [Google Scholar] [CrossRef]
- Welters, M.J.P.; Ma, W.; Santegoets, S.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; van Ham, V.J.; van Unen, V.; Koning, F.; van Egmond, S.I.; et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef]
- Whitmarsh, A.; Pring, M.; Thomas, S.J.; Waylen, A.; Ness, A.R.; Dudding, T.; Pawlita, M.; Brenner, N.; Waterboer, T.; Schroeder, L. Survival advantage in patients with human papillomavirus-driven oropharyngeal cancer and variation by demographic characteristics and serologic response: Findings from Head and Neck 5000. Cancer 2021, 127, 2442–2452. [Google Scholar] [CrossRef]
- Kędzierawski, P.; Huruk-Kuchinka, A.; Radowicz-Chil, A.; Mężyk, R.; Rugała, Z.; Sadowski, J. Human papillomavirus infection predicts better survival rate in patients with an oropharyngeal cancer. Arch. Med. Sci. 2021, 17, 1308–1316. [Google Scholar] [CrossRef]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Pokrývková, B.; Grega, M.; Klozar, J.; Vencálek, O.; Nunvář, J.; Tachezy, R. PD1(+)CD8(+) Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer. Biomedicines 2022, 10, 2794. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- Baudouin, R.; Hans, S.; Lisan, Q.; Morin, B.; Adimi, Y.; Martin, J.; Lechien, J.R.; Tartour, E.; Badoual, C. Prognostic Significance of the Microenvironment in Human Papillomavirus Oropharyngeal Carcinoma: A Systematic Review. Laryngoscope, 2023; Early View. [Google Scholar] [CrossRef]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor Infiltrating CD8+ and Foxp3+ Lymphocytes Correlate to Clinical Outcome and Human Papillomavirus (HPV) Status in Tonsillar Cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef]
- Kamila, H.; Vladimír, K.; Jan, B.; Jan, L.; Marek, G.; Miroslav, H.; Michal, Z.; Milan, V.; Kateřina, R.; Hana, V.; et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. ImmunoTherapy Cancer 2019, 7, 261. [Google Scholar]
- Wang, Z.; Wang, Q.; Tao, Y.; Chen, J.; Yuan, Z.; Wang, P. Characterization of immune microenvironment in patients with HPV-positive and negative head and neck cancer. Sci. Data 2023, 10, 694. [Google Scholar] [CrossRef]
- Fanetti, G.; Alterio, D.; Marvaso, G.; Gandini, S.; Rojas, D.P.; Gobitti, C.; Minatel, E.; Revelant, A.; Caroli, A.; Francia, C.M.; et al. Prognostic significance of neutrophil-to-lymphocyte ratio in HPV status era for oropharyngeal cancer. Oral Dis. 2020, 26, 1384–1392. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Sánchez-Canteli, M.; Triantafyllou, A.; de Bree, R.; Mäkitie, A.A.; Franchi, A.; Hellquist, H.; Saba, N.F.; Stenman, G.; Takes, R.P.; et al. Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 802. [Google Scholar] [CrossRef]
- Justesen, M.M.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Mordhorst, C.; Carlander, A.F.; Gothelf, A.B.; Grønhøj, C.; von Buchwald, C. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for the Outcome of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Viruses 2023, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Yazdani, H.O.; Morder, K.; Geller, D.A.; Simmons, R.L.; Tohme, S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021, 12, 785222. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.H.; Feng, L.; Su, X.; Brassard, A.; Dhoparee-Doomah, I.; Ferri, L.E.; Spicer, J.D.; Cools-Lartigue, J.J. Neutrophil Extracellular Traps in Cancer Therapy Resistance. Cancers 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Onuma, A.; He, J.; Wang, H.; Xia, Y.; Lal, R.; Cheng, X.; Kasumova, G.; Hu, Z.; et al. Neutrophils Extracellular Traps Inhibition Improves PD-1 Blockade Immunotherapy in Colorectal Cancer. Cancers 2021, 13, 5333. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Peng, Z.P.; Liu, X.C.; Guo, H.F.; Zhou, M.M.; Jiang, D.; Ning, W.R.; Huang, Y.F.; Zheng, L.; Wu, Y. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. OncoImmunology 2022, 11, 2052418. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- Monti, M.; De Rosa, V.; Iommelli, F.; Carriero, M.V.; Terlizzi, C.; Camerlingo, R.; Belli, S.; Fonti, R.; Di Minno, G.; Del Vecchio, S. Neutrophil Extracellular Traps as an Adhesion Substrate for Different Tumor Cells Expressing RGD-Binding Integrins. Int. J. Mol. Sci. 2018, 19, 2350. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020, 13, 3. [Google Scholar] [CrossRef]
- Chen, N.; He, D.; Cui, J. A Neutrophil Extracellular Traps Signature Predicts the Clinical Outcomes and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2022, 9, 833771. [Google Scholar] [CrossRef]
- Yan, B.; Dai, X.; Ma, Q.; Wu, X. Stromal Neutrophil Extracellular Trap Density Is an Independent Prognostic Factor for Cervical Cancer Recurrence. Front. Oncol. 2021, 11, 659445. [Google Scholar] [CrossRef]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journé, F.; Saussez, S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K.-Y. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J. Clin. Med. 2023, 12, 4301. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, L.J.; Livingstone, E.; Zimmer, L.; Schadendorf, D. The Latest Option: Nivolumab and Relatlimab in Advanced Melanoma. Curr. Oncol. Rep. 2023, 25, 647–657. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results from the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Galvis, M.M.; Borges, G.A.; Oliveira, T.B.; Toledo, I.P.; Castilho, R.M.; Guerra, E.N.S.; Kowalski, L.P.; Squarize, C.H. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 150, 102966. [Google Scholar] [CrossRef]
- Kessler, R.; Pandruvada, S. Immune-related adverse events following checkpoint inhibitor treatment in head and neck cancers: A comprehensive review. Oral Oncol. Rep. 2023, 6, 100036. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, A.; Liu, S.; Liu, J.; Lv, D.; Yang, H.; Zou, J. Immune Checkpoint Inhibitor Toxicity in Head and Neck Cancer: From Identification to Management. Front. Pharmacol. 2019, 10, 1254. [Google Scholar] [CrossRef]
- Verma, V.; Sprave, T.; Haque, W.; Simone, C.B., 2nd; Chang, J.Y.; Welsh, J.W.; Thomas, C.R., Jr. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab as a Single Agent in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Mirghani, H.; Amen, F.; Moreau, F.; Guigay, J.; Hartl, D.; Guily, J.L.S. Oropharyngeal cancers: Relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur. J. Cancer 2014, 50, 1100–1111. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, M.; Nie, D.; Xu, L.; Tian, H.; Wang, M.; Liu, W.; Feng, Z.; Han, F. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: A systematic review and meta-analysis. J. Immunother. Cancer 2022, 10, e005158. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Stöhlmacher-Williams, J.; Davidenko, I.; Licitra, L.; Winquist, E.; Villanueva, C.; Foa, P.; Rottey, S.; Skladowski, K.; Tahara, M. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013, 14, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Dobbins, T.; Lee, C.S.; Jones, D.; Jackson, E.; Clark, J.; Armstrong, B.; Harnett, G.; Milross, C.; O’Brien, C.; et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur. J. Cancer 2010, 46, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Narasimhan, B.; Cao, H.; Kwok, S.; Erickson, J.P.; Koong, A.; Pourmand, N.; Le, Q.T. The Relationship Between Human Papillomavirus Status and Other Molecular Prognostic Markers in Head and Neck Squamous Cell Carcinomas. Endocrine 2009, 74, 553–561. [Google Scholar] [CrossRef]
- Reimers, N.; Kasper, H.U.; Weissenborn, S.J.; Stützer, H.; Preuss, S.F.; Hoffmann, T.K.; Speel, E.J.M.; Dienes, H.P.; Pfister, H.J.; Guntinas-Lichius, O.; et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer 2007, 120, 1731–1738. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Lingen, M.W.; Martin, L.E.; Harris, P.L.; Brannigan, B.W.; Haserlat, S.M.; Okimoto, R.A.; Sgroi, D.C.; Dahiya, S.; Muir, B.; et al. Response of Some Head and Neck Cancers to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors May Be Linked to Mutation of ERBB2 rather than EGFR. Clin. Cancer Res. 2005, 11, 8105–8108. [Google Scholar] [CrossRef]
- Licitra, L.; Störkel, S.; Kerr, K.M.; Van Cutsem, E.; Pirker, R.; Hirsch, F.R.; Vermorken, J.B.; Von Heydebreck, A.; Esser, R.; Celik, I.; et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: Analysis of data from the EXTREME and CRYSTAL studies. Eur. J. Cancer 2012, 49, 1161–1168. [Google Scholar] [CrossRef]
- Pollock, N.I.; Wang, L.; Wallweber, G.; Gooding, W.E.; Huang, W.; Chenna, A.; Winslow, J.; Sen, M.; DeGrave, K.A.; Li, H.; et al. Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies. Clin. Cancer Res. 2015, 21, 4597–4606. [Google Scholar] [CrossRef]
- Mazibrada, J.; Longo, L.; Vatrano, S.; Cappia, S.; Giorcelli, J.; Pentenero, M.; Gandolfo, S.; Volante, M.; dell’Oste, V.; Lo Cigno, I.; et al. Differential expression of HER2, STAT3, SOX2, IFI16 and cell cycle markers during HPV-related head and neck carcinogenesis. New Microbiol. 2014, 37, 129–143. [Google Scholar]
- Brand, T.M.; Hartmann, S.; Bhola, N.E.; Peyser, N.D.; Li, H.; Zeng, Y.; Isaacson Wechsler, E.; Ranall, M.V.; Bandyopadhyay, S.; Duvvuri, U.; et al. Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV(+) Patients. Clin. Cancer Res. 2017, 23, 3072–3083. [Google Scholar] [CrossRef]
- Baro, M.; Lopez Sambrooks, C.; Burtness, B.A.; Lemmon, M.A.; Contessa, J.N. Neuregulin Signaling Is a Mechanism of Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2019, 18, 2124–2134. [Google Scholar] [CrossRef]
- Wang, D.; Qian, G.; Zhang, H.; Magliocca, K.R.; Nannapaneni, S.; Amin, A.R.; Rossi, M.; Patel, M.; El-Deiry, M.; Wadsworth, J.T.; et al. HER3 Targeting Sensitizes HNSCC to Cetuximab by Reducing HER3 Activity and HER2/HER3 Dimerization: Evidence from Cell Line and Patient-Derived Xenograft Models. Clin. Cancer Res. 2017, 23, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, U.; George, J.; Kim, S.; Alvarado, D.; Neumeister, V.M.; Chenna, A.; Gedrich, R.; Hawthorne, T.; LaVallee, T.; Grandis, J.R.; et al. Molecular and Clinical Activity of CDX-3379, an Anti-ErbB3 Monoclonal Antibody, in Head and Neck Squamous Cell Carcinoma Patients. Clin. Cancer Res. 2019, 25, 5752–5758. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Bauer, T.M.; LoRusso, P.; McLaughlin, J.F.; LaVallee, T.; Peck, R.A.; Eder, J.P. Safety, pharmacokinetics (PK), pharmacodynamics (Pd), and antitumor activity in a phase 1b study evaluating anti-ErbB3 antibody KTN3379 in adults with advanced tumors alone and with targeted therapies. J. Clin. Oncol. 2016, 34, 2501. [Google Scholar] [CrossRef]
- Bauman, J.E.; Julian, R.; Saba, N.F.; Wise-Draper, T.M.; Adkins, D.R.; O’Brien, P.; Fidler, M.J.; Gibson, M.K.; Duvvuri, U.; Heath-Chiozzi, M.; et al. Phase II Trial of CDX-3379 and Cetuximab in Recurrent/Metastatic, HPV-Negative, Cetuximab-Resistant Head and Neck Cancer. Cancers 2022, 14, 2355. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, T.; Yonemori, K.; Masuda, N.; Takahashi, S.; Takahashi, M.; Iwase, H.; Nakayama, T.; Saeki, T.; Toyama, T.; Takano, T. Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in breast cancer patients: Phase 1 dose escalation study. J. Clin. Oncol. 2018, 36, 2512. [Google Scholar] [CrossRef]
- Janne, P.A.; Yu, H.A.; Johnson, M.L.; Steuer, C.E.; Vigliotti, M.; Iacobucci, C.; Chen, S.; Yu, C.; Sellami, D.B. Safety and preliminary antitumor activity of U3-1402: A HER3-targeted antibody drug conjugate in EGFR TKI-resistant, EGFRm NSCLC. J. Clin. Oncol. 2019, 37, 9010. [Google Scholar] [CrossRef]
- Li, Y.-J.; Zhang, C.; Martincuks, A.; Herrmann, A.; Yu, H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer 2023, 23, 115–134. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Geiger, J.L.; Grandis, J.R.; Bauman, J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol. 2016, 56, 84–92. [Google Scholar] [CrossRef]
- Moreira, D.; Sampath, S.; Won, H.; White, S.V.; Su, Y.L.; Alcantara, M.; Wang, C.; Lee, P.; Maghami, E.; Massarelli, E.; et al. Myeloid cell–targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell–mediated immunity. J. Clin. Investig. 2021, 131, e137001. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Eckols, T.K.; Xu, X.; Kasembeli, M.M.; Chen, Y.; Adachi, M.; Song, Y.; Mo, Q.; Lai, S.Y.; Tweardy, D.J. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget 2016, 7, 26307–26330. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Cui, C.; Dodge, C.T.; Bhayani, M.K.; Lai, S.Y. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2012, 48, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.L.; Zhao, Z.L.; Liu, J.F.; Ma, S.R.; Huang, C.F.; Liu, B.; Zhang, W.F.; Sun, Z.J. STAT3 blockade enhances the efficacy of conventional chemotherapeutic agents by eradicating head neck stemloid cancer cell. Oncotarget 2015, 6, 41944–41958. [Google Scholar] [CrossRef]
- Yu, G.T.; Mao, L.; Wu, L.; Deng, W.W.; Bu, L.L.; Liu, J.F.; Chen, L.; Yang, L.L.; Wu, H.; Zhang, W.F.; et al. Inhibition of SRC family kinases facilitates anti-CTLA4 immunotherapy in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 2018, 75, 4223–4234. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Harrington, K.J.; Hong, D.S.; Mesia, R.; Brana, I.; Perez Segura, P.; Wise-Draper, T.; Scott, M.L.; Mitchell, P.D.; Mugundu, G.M.; et al. A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Ann. Oncol. 2018, 29, viii372. [Google Scholar] [CrossRef]
- Bonner, J.A.; Yang, E.S.; Trummell, H.Q.; Nowsheen, S.; Willey, C.D.; Raisch, K.P. Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother. Oncol. 2011, 99, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Di, J.-X.; Zhang, H.-Y. C188-9, a small-molecule STAT3 inhibitor, exerts an antitumor effect on head and neck squamous cell carcinoma. Anti-Cancer Drugs 2019, 30, 846–853. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. Manipulation of JAK/STAT Signalling by High-Risk HPVs: Potential Therapeutic Targets for HPV-Associated Malignancies. Viruses 2020, 12, 977. [Google Scholar] [CrossRef]
- Kohut, A.; Martincuks, A.; Santiago, N.L.; Austria, T.; Zhao, Q.; Lee, S.; Tergas, A.; Dellinger, T.; Han, E.; Rodriguez-Rodriguez, L.; et al. STAT3 is a potential therapeutic target in cervical cancer (257). Gynecol. Oncol. 2022, 166, S137–S138. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Huang, X.; Huo, L.; Xiao, B.; Ouyang, Y.; Chen, F.; Li, J.; Zheng, X.; Wei, D.; Wu, Y.; Zhang, R.; et al. Activating STING/TBK1 suppresses tumor growth via degrading HPV16/18 E7 oncoproteins in cervical cancer. Cell Death Differ. 2023, 31, 78–89. [Google Scholar] [CrossRef]
- Chin, E.N.; Yu, C.; Vartabedian, V.F.; Jia, Y.; Kumar, M.; Gamo, A.M.; Vernier, W.; Ali, S.H.; Kissai, M.; Lazar, D.C.; et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 2020, 369, 993–999. [Google Scholar] [CrossRef]
- Huang, R.; Ning, Q.; Zhao, J.; Zhao, X.; Zeng, L.; Yi, Y.; Tang, S. Targeting STING for cancer immunotherapy: From mechanisms to translation. Int. Immunopharmacol. 2022, 113, 109304. [Google Scholar] [CrossRef] [PubMed]
- Dorta-Estremera, S.; Hegde, V.L.; Slay, R.B.; Sun, R.; Yanamandra, A.V.; Nicholas, C.; Nookala, S.; Sierra, G.; Curran, M.A.; Sastry, K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer. J. Immunother. Cancer 2019, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Concha-Benavente, F.; Shayan, G.; Srivastava, R.M.; Gibson, S.P.; Wang, L.; Gooding, W.E.; Ferris, R.L. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncol. 2018, 78, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Katoch, S.; Sharma, V.; Patial, V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: Experimental and clinical scenarios. World J. Gastroenterol. 2022, 28, 3535–3554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.-W.; Lin, D.-Q.; Cao, L.-Q. Peroxisome proliferator-activated receptor-γ inhibits pancreatic cancer cell invasion and metastasis via regulating MMP-2 expression through PTEN. Mol. Med. Rep. 2015, 12, 6255–6260. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Banno, A.; Reddy, R.C. PPAR Agonists for the Prevention and Treatment of Lung Cancer. PPAR Res. 2017, 2017, 8252796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xin, Z.; Ren, P.; Wu, H. The Role of PPARs in Breast Cancer. Cells 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.I.; Baek, W.K.; Suh, S.I.; Jang, B.C.; Song, D.K.; Bae, J.H.; Kwon, K.Y.; Bae, J.H.; Cha, S.D.; Bae, I.; et al. Down-regulation of peroxisome proliferator-activated receptor gamma in human cervical carcinoma. Gynecol. Oncol. 2005, 97, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zhang, D.G.; Wu, J.X.; Pei, D.S.; Zheng, J.N. Ubiquitination of p53 is Involved in Troglitazone Induced Apoptosis in Cervical Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 2313–2318. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Mao, X.; Huang, J.; Yang, J.; Yin, X.; Wu, L.; Zheng, L.; Wang, Q. Elevation of miR-27b by HPV16 E7 inhibits PPARγ expression and promotes proliferation and invasion in cervical carcinoma cells. Int. J. Oncol. 2015, 47, 1759–1766. [Google Scholar] [CrossRef]
- O’Neill, W.Q.; Xie, X.; Gui, S.; Yu, H.; Davenport, J.; Cartwright, T.; Storl-Desmond, M.; Ryu, E.; Chan, E.R.; Cao, S.; et al. Repositioning Fenofibrate to Reactivate p53 and Reprogram the Tumor-Immune Microenvironment in HPV+ Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 282. [Google Scholar] [CrossRef]
- John, K.; Lahoti, T.S.; Wagner, K.; Hughes, J.M.; Perdew, G.H. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol. Carcinog. 2014, 53, 765–776. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Smith, K.; John, K.; Krishnegowda, G.; Amin, S.G.; Perdew, G.H. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol. Cancer Res. 2012, 10, 1369–1379. [Google Scholar] [CrossRef]
- Stanford, E.A.; Ramirez-Cardenas, A.; Wang, Z.; Novikov, O.; Alamoud, K.; Koutrakis, P.; Mizgerd, J.P.; Genco, C.A.; Kukuruzinska, M.; Monti, S.; et al. Role for the Aryl Hydrocarbon Receptor and Diverse Ligands in Oral Squamous Cell Carcinoma Migration and Tumorigenesis. Mol. Cancer Res. 2016, 14, 696–706. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, J.W.; Yang, L.Y.; Qiao, P.P.; Lu, D. AHRR contributes to inflammatory lymphangiogenesis by activating the EPAS1/VEGFD signaling axis in head and neck cancer. Am. J. Cancer Res. 2022, 12, 537–548. [Google Scholar]
- Kenison, J.E.; Wang, Z.; Yang, K.; Snyder, M.; Quintana, F.J.; Sherr, D.H. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012692118. [Google Scholar] [CrossRef]
- Arellano-Gutiérrez, C.V.; Quintas-Granados, L.I.; Cortés, H.; González Del Carmen, M.; Leyva-Gómez, G.; Bustamante-Montes, L.P.; Rodríguez-Morales, M.; López-Reyes, I.; Padilla-Mendoza, J.R.; Rodríguez-Páez, L.; et al. Indole-3-Carbinol, a Phytochemical Aryl Hydrocarbon Receptor-Ligand, Induces the mRNA Overexpression of UBE2L3 and Cell Proliferation Arrest. Curr. Issues Mol. Biol. 2022, 44, 2054–2068. [Google Scholar] [CrossRef]
- Meara, C.H.O.; Coupland, L.A.; Kordbacheh, F.; Quah, B.J.C.; Chang, C.W.; Simon Davis, D.A.; Bezos, A.; Browne, A.M.; Freeman, C.; Hammill, D.J.; et al. Neutralizing the pathological effects of extracellular histones with small polyanions. Nat. Commun. 2020, 11, 6408. [Google Scholar] [CrossRef]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Stürzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef]
- Vaguliene, N.; Zemaitis, M.; Lavinskiene, S.; Miliauskas, S.; Sakalauskas, R. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol. 2013, 14, 366. [Google Scholar] [CrossRef]
- Kistowski, M.; Dębski, J.; Karczmarski, J.; Paziewska, A.; Olędzki, J.; Mikula, M.; Ostrowski, J.; Dadlez, M. A Strong Neutrophil Elastase Proteolytic Fingerprint Marks the Carcinoma Tumor Proteome. Mol. Cell. Proteom. 2017, 16, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, M.; Fukutomi, T.; Takasugi, M.; Takahashi, S.; Sato, T.; Harao, M.; Mizumoto, T.; Yamashita, J. Prognostic Significance of Immunoreactive Neutrophil Elastase in Human Breast Cancer: Long-Term Follow-Up Results in 313 Patients. Neoplasia 2007, 9, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Llobat, P.; Dovizio, M.; Bruno, A.; Ricciotti, E.; Cufino, V.; Sacco, A.; Grande, R.; Alberti, S.; Arena, V.; Cirillo, M.; et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 2016, 7, 32462–32477. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dovizio, M.; Tacconelli, S.; Patrignani, P. Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2012, 26, e1–e13. [Google Scholar] [CrossRef]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, H.; Dai, S.; Li, M.; Gao, Y.; Yin, L.; Zhang, K.; Zhang, J.; Jiang, K.; Miao, Y.; et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice. Cancer Lett. 2023, 562, 216155. [Google Scholar] [CrossRef]
- Yang, L.Y.; Shen, X.T.; Sun, H.T.; Zhu, W.W.; Zhang, J.B.; Lu, L. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic. J. Cancer 2022, 13, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, C.C.; Chang, C.Y.; Li, J.R.; Ou, Y.C.; Chen, W.Y.; Liao, S.L.; Wang, J.D. Metformin Mitigated Obesity-Driven Cancer Aggressiveness in Tumor-Bearing Mice. Int. J. Mol. Sci. 2022, 23, 9134. [Google Scholar] [CrossRef] [PubMed]

| Phase | Trial | Population | Therapy | Objectives | Status |
|---|---|---|---|---|---|
| 2 | NCT03799445 | Advanced HPV+ HNSCC | Concurrent ipilimumab + nivolumab + RT |
| Recruiting |
| 2 | NCT03383094 | Intermediate/high-risk HPV+ locoregionally-advanced HNSCC | Concurrent and adjuvant pembrolizumab + RT vs. RT + cisplatin |
| Recruiting |
| 2 | NCT04988074 | Advanced HPV+OPSCC | Neoadjuvant cemiplimab + TORS/RT +/− chemotherapy |
| Recruiting |
| 2 | NCT04867330 | HPV+OPSCC | Toripalimab + docetaxel/cisplatin |
| Recruiting |
| 3 | NCT04116047 | Intermediate/high-risk HPV+OPSCC | Durvalumab vs. chemoradiotherapy |
| Recruiting |
| 2 | NCT03410615 | Intermediate-risk HPV+ locoregionally-advanced OPSCC | Durvalumab + RT + adjuvant Durvalumab vs. Durvalumab + RT + adjuvant Tremelimumab and Durvalumab vs. Cisplatin + RT |
| Active, not recruiting |
| 2 | NCT03829722 | High-risk HPV+OPSCC | Concurrent nivolumab + RT + carboplatin |
| Active, not recruiting |
| 2 | NCT03107182 | Locoregionally-advanced HPV+OPSCC | Nivolumab/Nab-paclitaxel/Carboplatin Induction Chemotherapy followed by Response-stratified Locoregional Therapy |
| Active, not recruiting |
| 2 | NCT03838263 | High-risk HPV+OPSCC | Neoadjuvant nivolumab + chemoradiotherapy vs. chemoradiotherapy alone |
| Active, not recruiting |
| 2/3 | NCT03952585 | Early-stage HPV+OPSCC | Concurrent reduced-dose RT + either nivolumab or cisplatin |
| Suspended |
| 3 | NCT03811015 | Intermediate risk locally-advanced HPV+OPSCC | Definitive chemoradiotherapy followed by maintenance nivolumab |
| Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoo, A.; Boyer, M.; Jafri, Z.; Makeham, T.; Pham, T.; Khachigian, L.M.; Floros, P.; Dowling, E.; Fedder, K.; Shonka, D., Jr.; et al. Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2798. https://doi.org/10.3390/ijms25052798
Khoo A, Boyer M, Jafri Z, Makeham T, Pham T, Khachigian LM, Floros P, Dowling E, Fedder K, Shonka D Jr., et al. Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(5):2798. https://doi.org/10.3390/ijms25052798
Chicago/Turabian StyleKhoo, A., M. Boyer, Z. Jafri, T. Makeham, T. Pham, L. M. Khachigian, P. Floros, E. Dowling, K. Fedder, D. Shonka, Jr., and et al. 2024. "Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives" International Journal of Molecular Sciences 25, no. 5: 2798. https://doi.org/10.3390/ijms25052798
APA StyleKhoo, A., Boyer, M., Jafri, Z., Makeham, T., Pham, T., Khachigian, L. M., Floros, P., Dowling, E., Fedder, K., Shonka, D., Jr., Garneau, J., & O’Meara, C. H. (2024). Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives. International Journal of Molecular Sciences, 25(5), 2798. https://doi.org/10.3390/ijms25052798







