Astragalus membranaceus Extract Induces Apoptosis via Generation of Reactive Oxygen Species and Inhibition of Heat Shock Protein 27 and Androgen Receptor in Prostate Cancers
Abstract
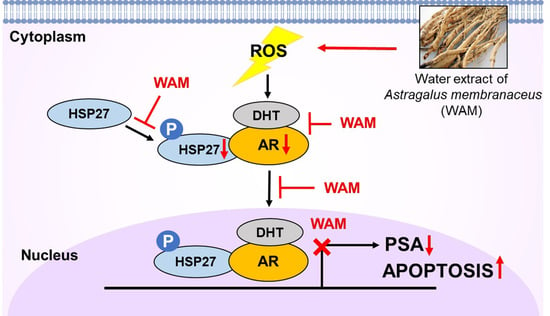
:1. Introduction
2. Results
2.1. WAM Reduced Viability and Increased PARP Cleavage in LNCaP Cells
2.2. WAM Decreased AR and PSA Protein Levels in LNCaP Cells
2.3. WAM Induced Apoptosis in LNCaP Cells
2.4. WAM Regulated Apoptosis through ROS Generation in LNCaP Cells
2.5. WAM Suppressed DHT-Induced AR and PSA and Also Reduced the Protein Stability of AR in LNCaP Cells
2.6. Association with HSP27 and AR Signaling in Prostate Cancer Cells
2.7. WAM Suppressed the Interaction and Colocalization of p-HSP27/AR in LNCaP Cells
3. Discussion
4. Materials and Methods
4.1. High-Performance Liquid Chromatography Analysis
4.2. Cell Line and Culture
4.3. Cell Viability Assay
4.4. Cell Cycle Analysis
4.5. Measurement of ROS Generation
4.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.7. Western Blotting
4.8. Co-Immunoprecipitation
4.9. Immunofluorescence
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Feng, C.; Liu, J.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Jiao, J.; Niu, Y. Androgen receptor and heat shock protein 27 co-regulate the malignant potential of molecular apocrine breast cancer. J. Exp. Clin. Cancer Res. CR 2018, 37, 90. [Google Scholar] [CrossRef]
- Poelaert, F.; Van Praet, C.; Beerens, A.S.; De Meerleer, G.; Fonteyne, V.; Ost, P.; Lumen, N. The role of androgen receptor expression in the curative treatment of prostate cancer with radiotherapy: A pilot study. BioMed Res. Int. 2015, 2015, 812815. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef]
- Zoubeidi, A.; Zardan, A.; Beraldi, E.; Fazli, L.; Sowery, R.; Rennie, P.; Nelson, C.; Gleave, M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007, 67, 10455–10465. [Google Scholar] [CrossRef]
- Azad, A.A.; Zoubeidi, A.; Gleave, M.E.; Chi, K.N. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat. Rev. Urol. 2015, 12, 26–36. [Google Scholar] [CrossRef]
- Li, J.; Fu, X.; Cao, S.; Li, J.; Xing, S.; Li, D.; Dong, Y.; Cardin, D.; Park, H.W.; Mauvais-Jarvis, F.; et al. Membrane-associated androgen receptor (AR) potentiates its transcriptional activities by activating heat shock protein 27 (HSP27). J. Biol. Chem. 2018, 293, 12719–12729. [Google Scholar] [CrossRef]
- Stope, M.B.; Schubert, T.; Staar, D.; Ronnau, C.; Streitborger, A.; Kroeger, N.; Kubisch, C.; Zimmermann, U.; Walther, R.; Burchardt, M. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J. Urol. 2012, 30, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Orahoske, C.M.; Geldenhuys, W.J.; Bhattarai, A.; Sabbagh, A.; Bobba, V.; Salem, F.M.; Zhang, W.; Shukla, G.C.; Lathia, J.D.; et al. Small-Molecule HSP27 Inhibitor Abolishes Androgen Receptors in Glioblastoma. J. Med. Chem. 2021, 64, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, Y.J.; Choi, B.; Lee, N.L.; Lee, H.J.; Kwak, S.Y.; Kwon, Y.; Na, Y.; Lee, Y.S. Overcoming HSP27-mediated resistance by altered dimerization of HSP27 using small molecules. Oncotarget 2016, 7, 53178–53190. [Google Scholar] [CrossRef] [PubMed]
- Mamouni, K.; Zhang, S.; Li, X.; Chen, Y.; Yang, Y.; Kim, J.; Bartlett, M.G.; Coleman, I.M.; Nelson, P.S.; Kucuk, O.; et al. Novel Flavonoid Composition Targets Androgen Receptor Signaling and Inhibits Prostate Cancer Growth in Preclinical Models. Neoplasia 2018, 20, 789–799. [Google Scholar] [CrossRef]
- Toman, J.; Ostry, V.; Grosse, Y.; Roubal, T.; Malir, F. Occurrence of ochratoxin A in Astragalus propinquus root and its transfer to decoction. Mycotoxin Res. 2018, 34, 223–227. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsay, H.J.; Lu, M.K.; Lin, C.H.; Yeh, C.W.; Liu, H.K.; Shiao, Y.J. Astragalus membranaceus-Polysaccharides Ameliorates Obesity, Hepatic Steatosis, Neuroinflammation and Cognition Impairment without Affecting Amyloid Deposition in Metabolically Stressed APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2017, 18, 2746. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-kappaB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Bamodu, O.A.; Kuo, K.T.; Wang, C.H.; Huang, W.C.; Wu, A.T.H.; Tsai, J.T.; Lee, K.Y.; Yeh, C.T.; Wang, L.S. Astragalus polysaccharides (PG2) Enhances the M1 Polarization of Macrophages, Functional Maturation of Dendritic Cells, and T Cell-Mediated Anticancer Immune Responses in Patients with Lung Cancer. Nutrients 2019, 11, 2264. [Google Scholar] [CrossRef]
- Huang, W.C.; Kuo, K.T.; Bamodu, O.A.; Lin, Y.K.; Wang, C.H.; Lee, K.Y.; Wang, L.S.; Yeh, C.T.; Tsai, J.T. Astragalus polysaccharide (PG2) Ameliorates Cancer Symptom Clusters, as well as Improves Quality of Life in Patients with Metastatic Disease, through Modulation of the Inflammatory Cascade. Cancers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Y.; He, D.; Tan, W.; Lv, F.; Liang, B.; Xia, T.; Li, J. Astragalus Polysaccharide Promotes Adriamycin-Induced Apoptosis in Gastric Cancer Cells. Cancer Manag. Res. 2020, 12, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, S.; Xu, W.; Yu, B.; Wang, G.; Wang, H. Astragalus polysaccharide inhibits breast cancer cell migration and invasion by regulating epithelialmesenchymal transition via the Wnt/betacatenin signaling pathway. Mol. Med. Rep. 2020, 21, 1819–1832. [Google Scholar]
- Zhai, Q.L.; Hu, X.D.; Xiao, J.; Yu, D.Q. Astragalus polysaccharide may increase sensitivity of cervical cancer HeLa cells to cisplatin by regulating cell autophagy. China J. Chin. Mater. Medica 2018, 43, 805–812. [Google Scholar]
- Zhao, L.; Zhong, Y.; Liang, J.; Gao, H.; Tang, N. Effect of Astragalus Polysaccharide on the Expression of VEGF and EGFR in Mice with Lewis Transplantable Lung Cancer. J. Coll. Physicians Surg. Pak. 2019, 29, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; He, B. Androgen Receptor Signaling in the Development of Castration-Resistant Prostate Cancer. Front. Oncol. 2019, 9, 858. [Google Scholar] [CrossRef]
- Jamroze, A.; Chatta, G.; Tang, D.G. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett. 2021, 518, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Okumura, F.; Tsukiyama, T.; Watanabe, M.; Miyajima, N.; Tanaka, J.; Imamura, M.; Hatakeyama, S. TRIM24 mediates ligand-dependent activation of androgen receptor and is repressed by a bromodomain-containing protein, BRD7, in prostate cancer cells. Biochim. Biophys. Acta 2009, 1793, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Eftekharzadeh, B.; Banduseela, V.C.; Chiesa, G.; Martinez-Cristobal, P.; Rauch, J.N.; Nath, S.R.; Schwarz, D.M.C.; Shao, H.; Marin-Argany, M.; Di Sanza, C.; et al. Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor. Nat. Commun. 2019, 10, 3562. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, S.; Sarkar, F.H. Advances in androgen receptor targeted therapy for prostate cancer. J. Cell. Physiol. 2014, 229, 271–276. [Google Scholar] [CrossRef]
- Obinata, D.; Lawrence, M.G.; Takayama, K.; Choo, N.; Risbridger, G.P.; Takahashi, S.; Inoue, S. Recent Discoveries in the Androgen Receptor Pathway in Castration-Resistant Prostate Cancer. Front. Oncol. 2020, 10, 581515. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.Y.; Shyu, B.C.; Chen, T.M.; Shieh, J.Y.; Sun, W.Z. Protein synthesis inhibitor cycloheximide dose-dependently decreases formalin-induced c-Fos protein and behavioral hyperalgesia in rats. Neurosci. Lett. 1997, 227, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Sim, D.Y.; Lee, H.J.; Im, E.; Choi, J.B.; Park, J.E.; Park, W.Y.; Jung, J.H.; Shim, B.S.; Kim, S.H. MicroRNA216b mediated downregulation of HSP27/STAT3/AKT signaling is critically involved in lambertianic acid induced apoptosis in human cervical cancers. Phytother. Res. PTR 2021, 35, 898–907. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014, 5, 346. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef]
- Choi, S.K.; Kam, H.; Kim, K.Y.; Park, S.I.; Lee, Y.S. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef]
- Nelson, W.G.; Yegnasubramanian, S. Resistance emerges to second-generation antiandrogens in prostate cancer. Cancer Discov. 2013, 3, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Mohler, J. Role of 5alpha-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012, 15, 222–230. [Google Scholar] [CrossRef]
- Szabo, N.J. Dietary safety of cycloastragenol from Astragalus spp.: Subchronic toxicity and genotoxicity studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 64, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Shin, W.C.; Lee, H.J.; Yoon, D.; Sim, D.Y.; Ahn, C.H.; Park, S.Y.; Shim, B.S.; Park, S.J.; Kim, K.S.; et al. SH-PRO extract alleviates benign prostatic hyperplasia via ROS-mediated activation of PARP/caspase 3 and inhibition of FOXO3a/AR/PSA signaling in vitro and in vivo. Phytother. Res. PTR 2023, 37, 452–463. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Park, J.E.; Lee, H.-J.; Sim, D.Y.; Ahn, C.-H.; Park, S.-Y.; Shim, B.-S.; Kim, B.; Lee, D.Y.; Kim, S.-H. Astragalus membranaceus Extract Induces Apoptosis via Generation of Reactive Oxygen Species and Inhibition of Heat Shock Protein 27 and Androgen Receptor in Prostate Cancers. Int. J. Mol. Sci. 2024, 25, 2799. https://doi.org/10.3390/ijms25052799
Kim S-Y, Park JE, Lee H-J, Sim DY, Ahn C-H, Park S-Y, Shim B-S, Kim B, Lee DY, Kim S-H. Astragalus membranaceus Extract Induces Apoptosis via Generation of Reactive Oxygen Species and Inhibition of Heat Shock Protein 27 and Androgen Receptor in Prostate Cancers. International Journal of Molecular Sciences. 2024; 25(5):2799. https://doi.org/10.3390/ijms25052799
Chicago/Turabian StyleKim, Seok-Young, Ji Eon Park, Hyo-Jung Lee, Deok Yong Sim, Chi-Hoon Ahn, Su-Yeon Park, Bum-Sang Shim, Bonglee Kim, Dae Young Lee, and Sung-Hoon Kim. 2024. "Astragalus membranaceus Extract Induces Apoptosis via Generation of Reactive Oxygen Species and Inhibition of Heat Shock Protein 27 and Androgen Receptor in Prostate Cancers" International Journal of Molecular Sciences 25, no. 5: 2799. https://doi.org/10.3390/ijms25052799