Lumican, a Multifunctional Cell Instructive Biomarker Proteoglycan Has Novel Roles as a Marker of the Hypercoagulative State of Long Covid Disease
Abstract
:1. Introduction
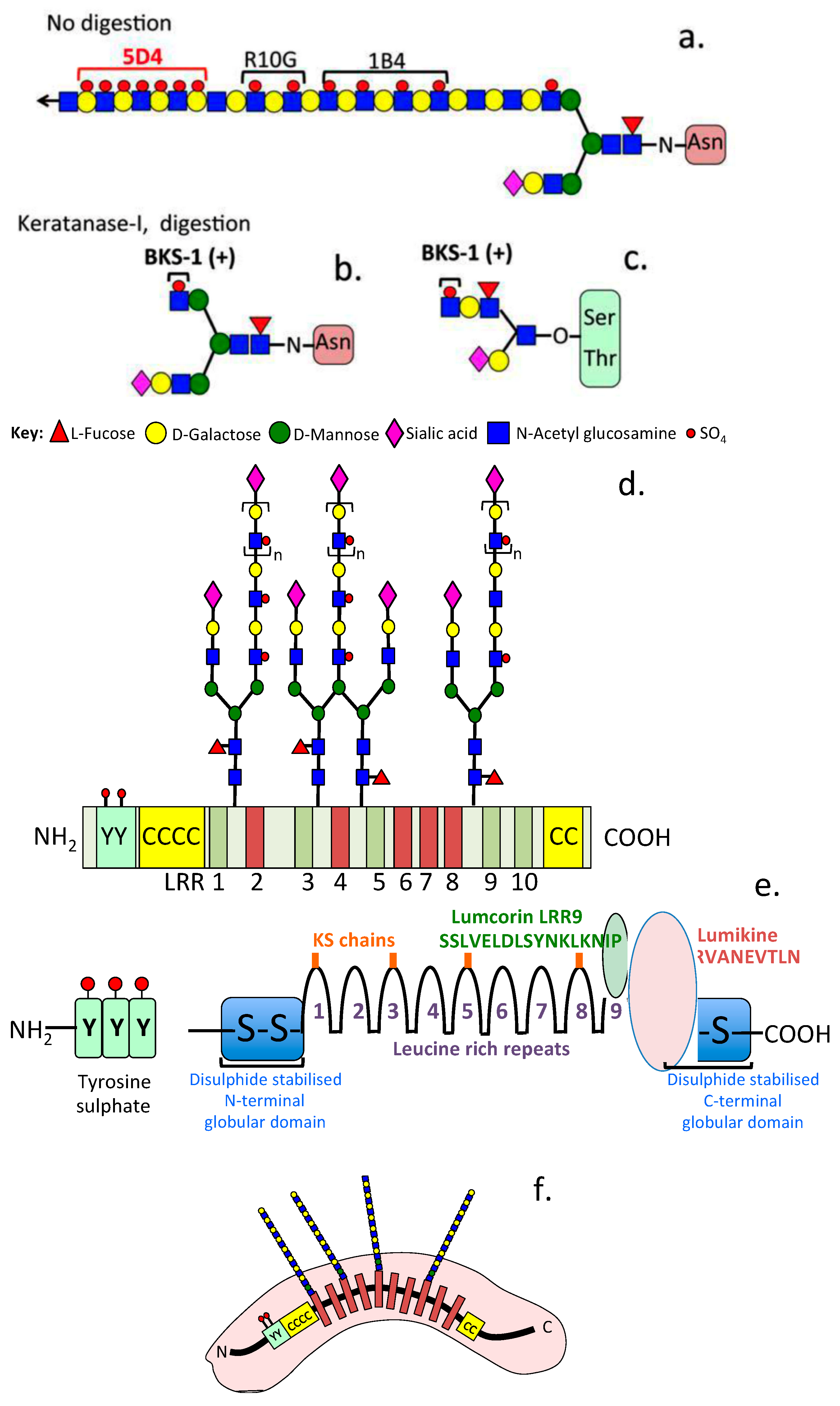
2. Lumican as a Structural Component of the ECM
3. Lumican as a Mediator, Inhibitor and Regulator
4. Lumican in Fibrotic Tissue Pathology
5. Lumican and Lung Pathobiology
6. Lumican and Neurodegenerative Disorders
7. Lumican and Musculoskeletal Disease
8. Lumican and Cancer
9. Lumican and Retinal Homeostasis
10. Lumican and Peridontal Disease
11. Lumican, Liver and Kidney Disease
12. Lumican and Reproductive Processes
13. Lumican and Adipocyte Regulation
14. Lumican and Vascular Disease
15. Lumican in Virally Induced Fibrinaloid Microclots
16. Lumican as a Biomarker
17. Future Research on the Modulatory Cell Instructive Properties of Keratan Sulphate
18. The Potential Significance of SLRPs Containing Low-Sulphation KS Chains
19. The Discriminative Power of the Sulfation Status of the KS Side Chains of KS-SLRPs Is an Untapped Analytical Parameter Yet to Be Fully Utilized in Biomarker Studies
20. Instructive Variably Sulfated Keratocan and Lumican in Embryonic Neural Guidance
21. Pathobiological Information Provided by the KS Chains of PGs Other Than Lumican
22. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAD | Abdominal aortic dissections |
| AD | Aortic dissection |
| AKT | Protein kinase B, refers to spontaneous thymic lymphoma AKR mice |
| ALK5 | Activin receptor like kinase |
| AMI | Acute myocardial infarction |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| ATP | Adenosine triphosphate |
| BBB | Blood brain barrier |
| CA | Coronary artery |
| CAD | Chronic aortic dissection |
| CXCL1 | Chemokine ligand 1 |
| DAMPS | Damage associated molecular patterns |
| ECM | Extracellular matrix |
| ERK | Extracellular signal regulated kinase |
| FAK | Focal adhesion kinase |
| GAG | Glycosaminoglycan |
| GSK | Glycogen synthase kinase |
| IVDD | Intervertebral disc degeneration |
| JNK | c-Jun N-terminal kinase |
| KS | Keratan sulfate |
| LBP | Low back pain |
| LRR | Leucine rich repeat |
| MAb | Monoclonal antibody |
| MAPK | Mitogen activated protein kinase |
| MMP | Matrix metalloprotease |
| OA | Osteoarthritis |
| PAMPS | Pathogen associated molecular patterns |
| PG | Proteoglycan |
| PODXL | Podocalyxin |
| PRP | Platelet rich plasma |
| PSA | Prostate specific antigen |
| SLRP | Small leucine repeat proteoglycan |
| SMC | Smooth muscle cell |
| TLR | Toll-like receptor |
References
- Raouf, A.; Ganss, B.; McMahon, C.; Vary, C.; Roughley, P.J.; Seth, A. Lumican is a major proteoglycan component of the bone matrix. Matrix Biol. 2002, 21, 361–367. [Google Scholar] [CrossRef]
- Ying, S.; Shiraishi, A.; Kao, C.W.-C.; Converse, R.L.; Funderburgh, J.L.; Swiergiel, J.; Roth, M.R.; Conrad, G.W.; Kao, W.W.-Y. Characterization and Expression of the Mouse Lumican Gene. J. Biol. Chem. 1997, 272, 30306–30313. [Google Scholar] [CrossRef]
- Chakravarti, S.; Paul, J.; Roberts, L.; Chervoneva, I.; Oldberg, A.; Birk, D.E. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Corpuz, L.; Funderburgh, J.L.; Funderburgh, M.L.; Bottomley, G.S.; Prakash, S.; Conrad, G.W. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J. Biol. Chem. 1996, 271, 9759–9763. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, S.; Petroll, W.M.; Hassell, J.R.; Jester, J.; Lass, J.H.; Paul, J.; Birk, D.E. Corneal opacity in lumican-null mice: Defects in collagen fibril structure and packing in the posterior stroma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3365–3373. [Google Scholar]
- Quantock, A.; Meek, K.; Chakravarti, S. An X-ray diffraction investigation of corneal structure in lumican-deficient mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1750–1756. [Google Scholar]
- De Wit, M.; Carvalho, B.; Diemen, P.M.D.-V.; Van Alphen, C.; Beliën, J.A.M.; Meijer, G.A.; Fijneman, R.J.A. Lumican and versican protein expression are associated with colorectal adenoma-to-carcinoma progression. PLoS ONE 2017, 12, e0174768. [Google Scholar] [CrossRef]
- Ishiwata, T.; Cho, K.; Kawahara, K.; Yamamoto, T.; Fujiwara, Y.; Uchida, E.; Tajiri, T.; Naito, Z. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol. Rep. 2007, 18, 537–543. [Google Scholar] [CrossRef]
- Kelemen, L.; Couch, F.J.; Ahmed, S.; Dunning, A.M.; Pharoah, P.D.; Easton, D.F.; Fredericksen, Z.S.; Vierkant, R.A.; Pankratz, V.S.; Goode, E.L.; et al. Genetic variation in stromal proteins decorin and lumican with breast cancer: Investigations in two case-control studies. Breast Cancer Res. 2008, 10, R98. [Google Scholar] [CrossRef]
- Yamauchi, N.; Kanke, Y.; Saito, K.; Okayama, H.; Yamada, S.; Nakajima, S.; Endo, E.; Kase, K.; Yamada, L.; Nakano, H.; et al. Stromal expression of cancer-associated fibroblast-related molecules, versican and lumican, is strongly associated with worse relapse-free and overall survival times in patients with esophageal squamous cell carcinoma. Oncol. Lett. 2021, 21, 445. [Google Scholar] [CrossRef]
- Matsuda, Y.; Yamamoto, T.; Kudo, M.; Kawahara, K.; Kawamoto, M.; Nakajima, Y.; Koizumi, K.; Nakazawa, N.; Ishiwata, T.; Naito, Z. Expression and roles of lumican in lung adenocarcinoma and squamous cell carcinoma. Int. J. Oncol. 2008, 33, 1177–1185. [Google Scholar]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Anand, V.; Khandelwal, M.; Saxena, A.; McGranaghan, P.; Odia, Y.; Kotecha, R.; Sharma, A. Lumican, pro-tumorigenic or anti-tumorigenic: A conundrum. Clin. Chim. Acta 2021, 514, 1–7. [Google Scholar] [CrossRef]
- Feng, S.; Gao, Y.; Yin, D.; Lv, L.; Wen, Y.; Li, Z.; Wang, B.; Wu, M.; Liu, B. Identification of Lumican and Fibromodulin as Hub Genes Associated with Accumulation of Extracellular Matrix in Diabetic Nephropathy. Kidney Blood Press. Res. 2021, 46, 275–285. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Lunde, I.G.; Andenæs, K.; Strand, M.E.; Aronsen, J.M.; Skrbic, B.; Marstein, H.S.; Bandlien, C.; Nygård, S.; Gorham, J.; et al. The extracellular matrix proteoglycan lumican improves survival and counteracts cardiac dilatation and failure in mice subjected to pressure overload. Sci. Rep. 2019, 9, 9206. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L. Small leucine-rich proteoglycans in kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, S.; Stallings, R.L.; SundarRaj, N.; Cornuet, P.K.; Hassell, J.R. Primary structure of human lumican (Keratan Sulfate Proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22. Genomics 1995, 27, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, S.; Magnuson, T.; Lass, J.H.; Jepsen, K.J.; LaMantia, C.; Carroll, H. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 1998, 141, 1277–1286. [Google Scholar] [CrossRef]
- Fassot, C.; Briet, M.; Rostagno, P.; Barbry, P.; Perret, C.; Laude, D.; Boutouyrie, P.; Bozec, E.; Bruneva, L.P.; Latremouille, C.; et al. Accelerated arterial stiffening and gene expression profile of the aorta in patients with coronary artery disease. J. Hypertens. 2008, 26, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Brézillon, S.; Perreau, C.; Ramont, L.; Maquart, F.X.; Wegrowski, Y. Lumcorin: A leucine-rich repeat 9-derived peptide from human lumican inhibiting melanoma cell migration. FEBS Lett. 2009, 583, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Brézillon, S.; Radwanska, A.; Zeltz, C.; Malkowski, A.; Ploton, D.; Bobichon, H.; Perreau, C.; Malicka-Blaszkiewicz, M.; Maquart, F.X.; Wegrowski, Y. Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion complexes. Cancer Lett. 2009, 283, 92–100. [Google Scholar] [CrossRef]
- Brézillon, S.; Pietraszek, K.; Maquart, F.X.; Wegrowski, Y. Lumican effects in the control of tumour progression and their links with metalloproteinases and integrins. FEBS J. 2013, 280, 2369–2381. [Google Scholar] [CrossRef]
- Gesteira, T.; Coulson-Thomas, V.J.; Yuan, Y.; Zhang, J.; Nader, H.B.; Kao, W.W. Lumican Peptides: Rational Design Targeting ALK5/TGFBRI. Sci. Rep. 2017, 7, 42057. [Google Scholar] [CrossRef]
- Pietraszek, K.; Chatron-Colliet, A.; Brézillon, S.; Perreau, C.; Jakubiak-Augustyn, A.; Krotkiewski, H.; Maquart, F.X.; Wegrowski, Y. Lumican: A new inhibitor of matrix metalloproteinase-14 activity. FEBS Lett. 2014, 588, 4319–4324. [Google Scholar] [CrossRef]
- Pietraszek, K.; Brézillon, S.; Perreau, C.; Malicka-Błaszkiewicz, M.; Maquart, F.X.; Wegrowski, Y. Lumican—Derived peptides inhibit melanoma cell growth and migration. PLoS ONE 2013, 8, e76232. [Google Scholar] [CrossRef]
- Gao, H.; Liu, C.; Ren, Q.; Zhang, L.; Qin, W.; Wang, H.; Zhang, Y. The Novel SLRP Family Member Lumican Suppresses Pancreatic Cancer Cell Growth. Pancreas 2023, 52, e29–e36. [Google Scholar] [CrossRef]
- Itoh, Y.; Sahni, V.; Shnider, S.J.; McKee, H.; Macklis, J.D. Inter-axonal molecular crosstalk via Lumican proteoglycan sculpts murine cervical corticospinal innervation by distinct subpopulations. Cell Rep. 2023, 42, 112182. [Google Scholar] [CrossRef]
- Cho, H.; Lee, Y.S.; Kim, D.A.; Moon, S.A.; Lee, S.E.; Lee, S.H.; Koh, J.M. Lumican, an Exerkine, Protects against Skeletal Muscle Loss. Int. J. Mol. Sci. 2022, 23, 10031. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.S.L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Papoutsidakis, A.; Karamanos, N.; Tzanakakis, G. Lumican affects tumor cell functions, tumor–ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 2014, 35, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, G.; Giatagana, E.-M.; Kuskov, A.; Berdiaki, A.; Tsatsakis, A.; Neagu, M.; Nikitovic, D. Proteoglycans in the Pathogenesis of Hormone-Dependent Cancers: Mediators and Effectors. Cancers 2020, 12, 2401. [Google Scholar] [CrossRef]
- Tzanakakis, G.; Giatagana, E.-M.; Berdiaki, A.; Spyridaki, I.; Hida, K.; Neagu, M.; Tsatsakis, A.; Nikitovic, D. The Role of IGF/IGF-IR-Signaling and Extracellular Matrix Effectors in Bone Sarcoma Pathogenesis. Cancers 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Aggelidakis, J.; Berdiaki, A.; Nikitovic, D.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.M.; Tzanakakis, G.N. Biglycan Regulates MG63 Osteosarcoma Cell Growth Through a LPR6/beta-Catenin/IGFR-IR Signaling Axis. Front. Oncol. 2018, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Papoutsidakis, A.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Spandidos, D.A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican mediates HTB94 chondrosarcoma cell growth via an IGFIR/Erk1/2 axis. Int. J. Oncol. 2020, 57, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Voudouri, K.; Nikitovic, D.; Berdiaki, A.; Kletsas, D.; Karamanos, N.; Tzanakakis, G.N. IGF-I/EGF and E2 signaling crosstalk through IGF-IR conduit point affects breast cancer cell adhesion. Matrix Biol. 2016, 56, 95–113. [Google Scholar] [CrossRef]
- Zafiropoulos, A.; Nikitovic, D.; Katonis, P.; Tsatsakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Decorin-Induced Growth Inhibition Is Overcome through Protracted Expression and Activation of Epidermal Growth Factor Receptors in Osteosarcoma Cells. Mol. Cancer Res. 2008, 6, 785–794. [Google Scholar] [CrossRef]
- Maiti, G.; Ashworth, S.; Choi, T.; Chakravarti, S. Molecular cues for immune cells from small leucine-rich repeat proteoglycans in their extracellular matrix-associated and free forms. Matrix Biol. 2023, 123, 48–58. [Google Scholar] [CrossRef]
- Zeng-Brouwers, J.; Pandey, S.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Communications via the Small Leucine-rich Proteoglycans: Molecular Specificity in Inflammation and Autoimmune Diseases. J. Histochem. Cytochem. 2020, 68, 887–906. [Google Scholar] [CrossRef]
- Frey, H.; Schroeder, N.; Manon-Jensen, T.; Iozzo, R.V.; Schaefer, L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013, 280, 2165–2179. [Google Scholar] [CrossRef]
- Engebretsen, K.; Lunde, I.G.; Strand, M.E.; Waehre, A.; Sjaastad, I.; Marstein, H.S.; Skrbic, B.; Dahl, C.P.; Askevold, E.T.; Christensen, G.; et al. Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli. FEBS J. 2013, 280, 2382–2398. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, S.; Ding, Y.; Li, Y.; Wu, B.; Gao, J.; Li, M.; Xu, L.; Xia, H. Downregulation of B4GALT5 attenuates cardiac fibrosis through Lumican and Akt/GSK-3β/β-catenin pathway. Eur. J. Pharmacol. 2023, 9, 176263. [Google Scholar] [CrossRef]
- Rixon, C.; Andreassen, K.; Shen, X.; Erusappan, P.M.; Almaas, V.M.; Palmero, S.; Dahl, C.P.; Ueland, T.; Sjaastad, I.; Louch, W.E.; et al. Lumican accumulates with fibrillar collagen in fibrosis in hypertrophic cardiomyopathy. ESC Heart Fail. 2023, 10, 858–871. [Google Scholar] [CrossRef]
- Kucukoglu, O.; Sowa, J.-P.; Mazzolini, G.D.; Syn, W.-K.; Canbay, A. Hepatokines and adipokines in nash-related hepatocellular carcinoma. J. Hepatol. 2021, 74, 442–457. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, Z.; Wu, Q.; Liu, H.; Sun, Z.; Wu, Y.; Luo, J. B4GALT5 high expression associated with poor prognosis of hepatocellular carcinoma. BMC Cancer 2022, 22, 392. [Google Scholar] [CrossRef]
- Hannun, Y.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Zhu, C.-S.; Wang, Y.-M.; Xie, X.-X.; Xiao, L.-L.; Zhang, Z.-C.; Tang, Q.-Q.; Li, X. Downregulation of β1, 4-galactosyltransferase 5 improves insulin resistance by promoting adipocyte commitment and reducing inflammation. Cell Death Dis. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, M.; Qi, X.; Li, J. β1, 4-galactosyltransferase v modulates breast cancer stem cells through wnt/β-catenin signaling pathway. Cancer Res. Treat Off. J. Korean Cancer Assoc. 2020, 52, 1084. [Google Scholar]
- Coats, C.; Heywood, W.E.; Virasami, A.; Ashrafi, N.; Syrris, P.; Dos Remedios, C.; Treibel, T.A.; Moon, J.C.; Lopes, L.R.; McGregor, C.G.A.; et al. Proteomic Analysis of the Myocardium in Hypertrophic Obstructive Cardiomyopathy. Circ. Genom. Precis. Med. 2018, 11, e001974. [Google Scholar] [CrossRef]
- Das, T.; Prodhan, C.; Patsa, S.; Ray, J.G.; Chaudhuri, K. Identification of over expressed proteins in oral submucous fibrosis by proteomic analysis. J. Cell Biochem. 2018, 119, 4361–4371. [Google Scholar] [CrossRef]
- Zhou, B.; Tu, T.; Gao, Z.; Wu, X.; Wang, W.; Liu, W. Impaired collagen fibril assembly in keloids with enhanced expression of lumican and collagen V. Arch. Biochem. Biophys. 2021, 697, 108676. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Xu, X.; He, Z.; Cui, L.; Lv, X. Lumican alleviates hypertrophic scarring by suppressing integrin-FAK signaling. Biochem. Biophys. Res. Commun. 2016, 480, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Dolhnikoff, M.; Morin, J.; Roughley, P.J.; Ludwig, M.S. Expression of lumican in human lungs. Am. J. Respir. Cell Mol. Biol. 1998, 19, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Cao, Y.; Wang, H.; Zhou, Y.; Gao, L.; Zeng, Z.; Cheng, M.; Jin, X.; Chen, J.; et al. Lumican is elevated in the lung in human and experimental acute respiratory distress syndrome and promotes early fibrotic responses to lung injury. J. Transl. Med. 2022, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Pilling, D.; Vakil, V.; Cox, N.; Gomer, R.H. TNF-α-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, 11929–11934. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Kao, W.W.; Yeh, Y.H.; Chen, W.J.; Chu, P.H. Lumican deficiency promotes pulmonary arterial remodeling. Transl. Res. 2021, 237, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.J.H.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mikael, T.M.; Salih, R.Q.; Salh, A.M.; Hussein, D.A. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar]
- Long, K.; Newland, B.; Florio, M.; Kalebic, N.; Langen, B.; Kolterer, A.; Wimberger, P.; Huttner, W.B. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron 2018, 99, 702–719.e6. [Google Scholar] [CrossRef]
- Kolb, J.; Tsata, V.; John, N.; Kim, K.; Möckel, C.; Rosso, G.; Kurbel, V.; Parmar, A.; Sharma, G.; Karandasheva, K.; et al. Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment. Nat. Commun. 2023, 14, 6814. [Google Scholar] [CrossRef]
- Bouhrira, N.; DeOre, B.J.; Tran, K.A.; Galie, P.A. Transcriptomic analysis of a 3D blood-brain barrier model exposed to disturbed fluid flow. Fluids Barriers CNS 2022, 19, 94. [Google Scholar] [CrossRef]
- Friden, V.; Oveland, E.; Tenstad, O.; Ebefors, K.; Nystrom, J.; Nilsson, U.A.; Haraldsson, B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011, 79, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; Fernández-Palleiro, P.; Iglesias-Martínez-Almeida, M.; Freiría-Martínez, L.; Jarmardo-Rodriguez, C.; Del Carmen Vallejo-Curto, M.; Álvarez-Ariza, M.; López-García, M.; De Las Heras, E.; et al. Changes in the Brain Extracellular Matrix Composition in schizophrenia: A Pathophysiological Dysregulation and a Potential Therapeutic Target. Cell. Mol. Neurobiol. 2022, 42, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.; Hu, D.; George, S.Z.; Schwartz, T.A.; Kraus, V.B.; Huebner, J.L.; Cleveland, R.J.; Taylor, K.A.; Jordan, J.M.; Golightly, Y.M. Biomarker clusters differentiate phenotypes of lumbar spine degeneration and low back pain: The Johnston County Osteoarthritis Project. Osteoarthr. Cartil. Open. 2022, 4, 100270. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.; Cleveland, R.J.; Kraus, V.B.; Taylor, K.A.; George, S.Z.; Schwartz, T.A.; Renner, J.; Huebner, J.L.; Jordan, J.M.; Golightly, Y.M. Biomarkers and longitudinal changes in lumbar spine degeneration and low back pain: The Johnston County Osteoarthritis Project. Osteoarthr. Cartil. 2023, 31, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Djuric, N.; Rajasekaran, S.; Tangavel, C.; Raveendran, M.; Soundararajan, D.C.R.; Nayagam, S.M.; Matchado, M.S.; Anand, K.S.S.V.; Shetty, A.P.; Kanna, R.M. Influence of endplate avulsion and Modic changes on the inflammation profile of herniated discs: A proteomic and bioinformatic approach. Eur. Spine J. 2022, 31, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, C.; Chen, M.; Wang, B. Lumican silencing alleviates tumor necrosis factor-α-induced nucleus pulposus cell inflammation and senescence by inhibiting apoptosis signal regulating kinase 1/p38 signaling pathway via inactivating Fas ligand expression. Bioengineered 2021, 12, 6891–6901. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.A.; Kim, E.Y.; Chang, E.J.; Park, S.J.; Kim, B.J. Lumican Inhibits Osteoclastogenesis and Bone Resorption by Suppressing Akt Activity. Int. J. Mol. Sci. 2021, 22, 4717. [Google Scholar] [CrossRef]
- Rada, J.A.; Cornuet, P.K.; Hassell, J.R. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp. Eye Res. 1993, 56, 635–648. [Google Scholar] [CrossRef]
- Ezura, Y.; Chakravarti, S.; Oldberg, A.; Chervoneva, I.; Birk, D.E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000, 151, 779–788. [Google Scholar] [CrossRef]
- Giatagana, E.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Chu, P.Y.; Chang, G.C.; Liu, K.J. Elevated Expression of Lumican in Lung Cancer Cells Promotes Bone Metastasis through an Autocrine Regulatory Mechanism. Cancers 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Hu, X.; Jiang, F.; Shen, Y.; Xu, R.; Wu, L.; Wei, P.; Shen, X. LUM Expression and Its Prognostic Significance in Gastric Cancer. Front. Oncol. 2020, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Q.; Yu, Z.; Wu, X.; Chen, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; Liu, B.; et al. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin β1-FAK signaling pathway. Int. J. Cancer 2017, 141, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Suhovskih, A.; Aidagulova, S.V.; Kashuba, V.I.; Grigorieva, E.V. Proteoglycans as potential microenvironmental biomarkers for colon cancer. Cell Tissue Res. 2015, 361, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Truty, M.A.; Kang, Y.; Chopin-Laly, X.; Zhang, R.; Roife, D.; Chatterjee, D.; Lin, E.; Thomas, R.M.; Wang, H.; et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin. Cancer Res. 2014, 20, 6529–6540. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, Y.; Kawahara, K.; Ishiwata, T.; Naito, Z. Secreted 70kDa lumican stimulates growth and inhibits invasion of human pancreatic cancer. Cancer Lett. 2012, 320, 31–39. [Google Scholar] [CrossRef]
- Stasiak, M.; Boncela, J.; Perreau, C.; Karamanou, K.; Chatron-Colliet, A.; Proult, I.; Przygodzka, P.; Chakravarti, S.; Maquart, F.X.; Kowalska, M.A.; et al. Lumican Inhibits SNAIL-Induced Melanoma Cell Migration Specifically by Blocking MMP-14 Activity. PLoS ONE 2016, 11, e0150226. [Google Scholar] [CrossRef]
- Dauvé, J.; Belloy, N.; Rivet, R.; Etique, N.; Nizet, P.; Pietraszek-Gremplewicz, K.; Karamanou, K.; Dauchez, M.; Ramont, L.; Brézillon, S.; et al. Differential MMP-14 Targeting by Lumican-Derived Peptides Unraveled by In Silico Approach. Cancers 2021, 13, 4930. [Google Scholar] [CrossRef]
- Brézillon, S.; Venteo, L.; Ramont, L.; D’Onofrio, M.F.; Perreau, C.; Pluot, M.; Maquart, F.X.; Wegrowski, Y. Expression of lumican, a small leucine-rich proteoglycan with antitumour activity, in human malignant melanoma. Clin. Exp. Dermatol. 2007, 32, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Vuillermoz, B.; Khoruzhenko, A.; D’Onofrio, M.F.; Ramont, L.; Venteo, L.; Perreau, C.; Antonicelli, F.; Maquart, F.X.; Wegrowski, Y. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp. Cell Res. 2004, 296, 294–306. [Google Scholar] [CrossRef]
- Brezillon, S.; Zeltz, C.; Schneider, L.; Terryn, C.; Vuillermoz, B.; Ramont, L.; Perrau, C.; Pluot, M.; Diebold, M.D.; Radwanska, A.; et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J. Physiol. Pharmacol. 2009, 60 (Suppl. S4), 15–22. [Google Scholar]
- Coulson-Thomas, V.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Andrade de Paula, C.A.; Carneiro, C.R.; Ortiz, V.; Toma, L.; Kao, W.W.; Nader, H.B. Lumican expression, localization and antitumor activity in prostate cancer. Exp. Cell Res. 2013, 319, 967–981. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Vynios, D.; Brézillon, S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. In Semin Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–133. [Google Scholar]
- Linke, F.; Johnson, J.; Kern, S.; Bennett, C.; Lourdusamy, A.; Lea, D.; Clifford, S.; Merry, C.; Stolnik, S.; Alexander, M.; et al. Identifying new biomarkers of aggressive Group 3 and SHH medulloblastoma using 3D hydrogel models, single cell RNA sequencing and 3D OrbiSIMS imaging. Acta Neuropathol. Commun. 2023, 11, 6. [Google Scholar] [CrossRef]
- Low, S.; Connor, T.B.; Kassem, I.S.; Costakos, D.M.; Chaurasia, S.S. Small Leucine-Rich Proteoglycans (SLRPs) in the Retina. Int. J. Mol. Sci. 2021, 22, 7293. [Google Scholar] [CrossRef]
- Keenan, T.; Clark, S.J.; Unwin, R.D.; Ridge, L.A.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7528–7538. [Google Scholar] [CrossRef]
- Amjadi, S.; Mai, K.; McCluskey, P.; Wakefield, D. The role of lumican in ocular disease. ISRN Ophthalmol. 2013, 2013, 632302. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Lee, C.H.; Chiou, S.H.; Liao, C.C.; Cheng, C.W. Aqueous Lumican Correlates with Central Retinal Thickness in Patients with Idiopathic Epiretinal Membrane: A Proteome Study. Dis. Markers 2022, 2022, 9886846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.-C.; Liu, H.-Y.; Chu, H.-S.; Chen, W.-L.; Hu, F.-R.; Kao, W.W.-Y.; Wang, I.-J. The versatile roles of lumican in eye diseases: A review. Ocul. Surf. 2023, 29, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guan, Q.; Han, X.; Bai, D.; Li, D.; Tian, Y. Proteoglycans in the periodontium: A review with emphasis on specific distributions, functions, and potential applications. J. Periodontal Res. 2021, 56, 617–632. [Google Scholar] [CrossRef]
- Matheson, S.; Larjava, H.; Häkkinen, L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J. Periodontal Res. 2005, 40, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Embery, G.; Lloyd, D. Immunochemical localization of the small leucine-rich proteoglycan lumican in human predentine and dentine. Arch. Oral. Biol. 1997, 42, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Hasegawa, M.; Yamato, M.; Takagi, R.; Okano, T.; Ishikawa, I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J. Periodontal. Res. 2008, 43, 364–371. [Google Scholar] [CrossRef]
- Saito, M.; Tsuji, T. Extracellular matrix administration as a potential therapeutic strategy for periodontal ligament regeneration. Expert. Opin. Biol. Ther. 2012, 12, 299–309. [Google Scholar] [CrossRef]
- Bülow, R.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014, 7, 4. [Google Scholar] [CrossRef]
- Krishnan, A.; Li, X.; Kao, W.Y.; Viker, K.; Butters, K.; Masuoka, H.; Knudsen, B.; Gores, G.; Charlton, M. Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis. Lab. Investig. 2012, 92, 1712–1725. [Google Scholar] [CrossRef]
- Nastase, M.; Janicova, A.; Roedig, H.; Hsieh, L.T.; Wygrecka, M.; Schaefer, L. Small Leucine-Rich Proteoglycans in Renal Inflammation: Two Sides of the Coin. J. Histochem. Cytochem. 2018, 66, 261–272. [Google Scholar] [CrossRef]
- Schaefer, L.; Gröne, H.J.; Raslik, I.; Robenek, H.; Ugorcakova, J.; Budny, S.; Schaefer, R.M.; Kresse, H. Small proteoglycans of normal adult human kidney: Distinct expression patterns of decorin, biglycan, fibromodulin, and lumican. Kidney Int. 2000, 58, 1557–1568. [Google Scholar] [CrossRef]
- Soylemezoglu, O.; Wild, G.; Dalley, A.J.; MacNeil, S.; Milford Ward, A.; Brown, C.B.; el Nahas, A.M. Urinary and serum type III collagen: Markers of renal fibrosis. Nephrol. Dial. Transpl. 1997, 12, 1883–1889. [Google Scholar] [CrossRef]
- Sharma, A.; Mauer, S.M.; Kim, Y.; Michael, A.F. Interstitial fibrosis in obstructive nephropathy. Kidney Int. 1993, 44, 774–788. [Google Scholar] [CrossRef]
- Colon-Caraballo, M.; Lee, N.; Nallasamy, S.; Myers, K.; Hudson, D.; Iozzo, R.V.; Mahendroo, M. Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy. Matrix Biol. 2022, 105, 53–71. [Google Scholar] [CrossRef]
- Kedem, A.; Ulanenko-Shenkar, K.; Yung, Y.; Youngster, M.; Avraham, S.; Yerushalmi, G.M.; Hourvitz, A. The Involvement of Lumican in Human Ovulatory Processes. Reprod. Sci. 2022, 29, 366–373. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.; Wang, Z.; Pan, H.; Ren, Y.; Li, X.; Liu, Z.; Gao, H. The Downregulation of Placental Lumican Promotes the Progression of Preeclampsia. Reprod. Sci. 2021, 28, 3147–3154. [Google Scholar] [CrossRef]
- Simões, R.; Soares, J.M., Jr.; Simões, M.J.; Nader, H.B.; Baracat, M.C.P.; Maciel, G.A.R.; Serafini, P.C.; Azziz, R.; Baracat, E.C. Small leucine-rich proteoglycans (SLRPs) in the endometrium of polycystic ovary syndrome women: A pilot study. J. Ovarian Res. 2017, 10, 54. [Google Scholar] [CrossRef]
- Salgado, R.; Favaro, R.R.; Zorn, T.M. Modulation of small leucine-rich proteoglycans (SLRPs) expression in the mouse uterus by estradiol and progesterone. Reprod. Biol. Endocrinol. 2011, 9, 22. [Google Scholar] [CrossRef]
- Salgado, R.; Favaro, R.R.; Martin, S.S.; Zorn, T.M. The estrous cycle modulates small leucine-rich proteoglycans expression in mouse uterine tissues. Anat. Rec. 2009, 292, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Lucariello, A.; Trabucco, E.; Boccia, O.; Perna, A.; Sellitto, C.; Castaldi, M.A.; De Falco, M.; De Luca, A.; Cobellis, L. Small leucine rich proteoglycans are differently distributed in normal and pathological endometrium. In Vivo 2015, 29, 217–222. [Google Scholar] [PubMed]
- San Martin, S.; Soto-Suazo, M.; De Oliveira, S.F.; Aplin, J.D.; Abrahamsohn, P.; Zorn, T.M. Small leucine-rich proteoglycans (SLRPs) in uterine tissues during pregnancy in mice. Reproduction 2003, 125, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Strieder-Barboza, C.; Flesher, C.G.; Geletka, L.M.; Eichler, T.; Akinleye, O.; Ky, A.; Ehlers, A.P.; Lumeng, C.N.; O’Rourke, R.W. Lumican modulates adipocyte function in obesity-associated type 2 diabetes. Adipocyte 2022, 11, 665–675. [Google Scholar] [CrossRef]
- Guzmán-Ruiz, R.; Tercero-Alcázar, C.; Rabanal-Ruiz, Y.; Díaz-Ruiz, A.; El Bekay, R.; Rangel-Zuñiga, O.A.; Navarro-Ruiz, M.C.; Molero, L.; Membrives, A.; Ruiz-Rabelo, J.F.; et al. Adipose tissue depot-specific intracellular and extracellular cues contributing to insulin resistance in obese individuals. FASEB J. 2020, 34, 7520–7539. [Google Scholar] [CrossRef]
- Wolff, G.; Taranko, A.E.; Meln, I.; Weinmann, J.; Sijmonsma, T.; Lerch, S.; Heide, D.; Billeter, A.T.; Tews, D.; Krunic, D.; et al. Diet-dependent function of the extracellular matrix proteoglycan Lumican in obesity and glucose homeostasis. Mol. Metab. 2019, 19, 97–106. [Google Scholar] [CrossRef]
- Kirankaya, A.; Tugrul, S.; Ozcan, S.; Ince, O.; Donmez, E.; Atici, A.; Hancioglu, E.; Okuyan, E.; Sahin, I. Correlation between the serum lumican level and the severity of coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2350–2357. [Google Scholar]
- Yang, Y.; Wu, Q.H.; Li, Y.; Gao, P.J. Association of SLRPs with carotid artery atherosclerosis in essential hypertensive patients. J. Hum. Hypertens. 2018, 32, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Hansmeier, N.; Buttigieg, J.; Kumar, P.; Pelle, S.; Choi, K.Y.; Kopriva, D.; Chao, T.C. Identification of Mature Atherosclerotic Plaque Proteome Signatures Using Data-Independent Acquisition Mass Spectrometry. J. Proteome Res. 2018, 17, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Cheng, W.; Yao, C.; Yin, J.; Tong, C.; Rao, A.; Yen, L.; Ku, M.; Rao, J. Quantitative proteomics analysis by isobaric tags for relative and absolute quantitation identified Lumican as a potential marker for acute aortic dissection. J. Biomed. Biotechnol. 2011, 2011, 920763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xue, Y.; Yao, C.; Gu, G.; Zhang, Y.; Zhang, J.; Fan, F.; Luan, X.; Deng, Z.; Tao, Z.; et al. Acute Aortic Dissection Biomarkers Identified Using Isobaric Tags for Relative and Absolute Quantitation. Biomed. Res. Int. 2016, 2016, 6421451. [Google Scholar] [CrossRef] [PubMed]
- Hultgårdh-Nilsson, A.; Borén, J.; Chakravarti, S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J. Intern. Med. 2015, 278, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Talusan, P.; Bedri, S.; Yang, S.; Kattapuram, T.; Silva, N.; Roughley, P.J.; Stone, J.R. Analysis of intimal proteoglycans in atherosclerosis-prone and atherosclerosis-resistant human arteries by mass spectrometry. Mol. Cell Proteom. 2005, 4, 1350–1357. [Google Scholar] [CrossRef]
- Baba, H.; Ishiwata, T.; Takashi, E.; Xu, G.; Asano, G. Expression and localization of lumican in the ischemic and reperfused rat heart. Jpn. Circ. J. 2001, 65, 445–450. [Google Scholar] [CrossRef]
- Funderburgh, J.L.; Mitschler, R.R.; Funderburgh, M.L.; Roth, M.R.; Chapes, S.K.; Conrad, G.W. Macrophage receptors for lumican. A corneal keratan sulfate proteoglycan. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1159–1167. [Google Scholar]
- Lee, S.; Bowrin, K.; Hamad, A.R.; Chakravarti, S. Extracellular Matrix Lumican Deposited on the Surface of Neutrophils Promotes Migration by Binding to β2 Integrin. J. Biol. Chem. 2009, 284, 23662–23669. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Mann, M.M.; Conrad, G.W. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J. Biol. Chem. 1991, 266, 24773–24777. [Google Scholar] [CrossRef] [PubMed]
- Önnerfjord, P.; Heathfield, T.F.; Heinegård, D. Identification of Tyrosine Sulfation in Extracellular Leucine-rich Repeat Proteins Using Mass Spectrometry. J. Biol. Chem. 2004, 279, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hoffhines, A.J.; Moore, K.L.; Leary, J.A. Determination of the sites of tyrosine O-sulfation in peptides and proteins. Nat. Methods 2007, 4, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Bubenek, S.; Nastase, A.; Niculescu, A.M.; Baila, S.; Herlea, V.; Lazar, V.; Paslaru, L.; Botezatu, A.; Tomescu, D.; Popescu, I. Assessment of gene expression profiles in peripheral occlusive arterial disease. Can. J. Cardiol. 2012, 28, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Liu, Y.; Gael, A.; Fu, X.; Deng, X.; Liu, Y.; Wu, Y.; Wu, Y.; Wang, H.; Deng, Y.; et al. Study on Proteomics-Based Aortic Dissection Molecular Markers Using iTRAQ Combined With Label Free Techniques. Front. Physiol. 2022, 13, 862732. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Chou, S.-H.; Tung, Y.-C.; Hsiao, F.-C.; Ho, C.-T.; Chan, Y.-H.; Wu, V.C.-C.; Chou, A.-H.; Hsu, M.-E.; Lin, P.-J.; et al. Expression and role of lumican in acute aortic dissection: A human and mouse study. PLoS ONE 2021, 16, e0255238. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc. Diabetol. 2022, 21, 190. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.Y.; Collins, J.; Krastins, B.; Sarracino, D.; Lopez, M.; Losina, E.; Aliprantis, A.O. Mass spectrometry assays of plasma biomarkers to predict radiographic progression of knee osteoarthritis. Arthritis Res. Ther. 2014, 16, 456. [Google Scholar] [CrossRef] [PubMed]
- Maiti, G.; Frikeche, J.; Lam, C.Y.-M.; Biswas, A.; Shinde, V.; Samanovic, M.; Kagan, J.C.; Mulligan, M.J.; Chakravarti, S. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc. Nat. Acad. Sci. USA 2021, 118, e2100999118. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Pan, Y.Y.; Kopylov, A.T.; Zgoda, V.; Ma, M.C.; Wang, C.H.; Su, W.C.; Lai, W.W.; Cheng, P.N.; Liao, P.C. Assessment of Serological Early Biomarker Candidates for Lung Adenocarcinoma by using Multiple Reaction Monitoring-Mass Spectrometry. PROTEOMICS Clin. Appl. 2020, 14, 1900095. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Zhang, M.; Nogueras-Gonzalez, G.M.; Xiao, L.; Zurita, A.J.; Subudhi, S.K.; Tu, S.-M.; Aparicio, A.M.; Coarfa, C.; Rajapakshe, K. A phase II study of cabozantinib and androgen ablation in patients with hormone-naïve metastatic prostate cancer. Clin. Cancer Res. 2020, 26, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Chen, R.; Brand, R.E.; Hawley, S.; Tamura, Y.; Gafken, P.R.; Milless, B.P.; Goodlett, D.R.; Rush, J.; Brentnall, T.A. Multiplex Targeted Proteomic Assay for Biomarker Detection in Plasma: A Pancreatic Cancer Biomarker Case Study. J. Proteome Res. 2012, 11, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Brocca, L.; Monti, E.; Franchi, M.V.; Zwiebel, M.; Steigerwald, S.; Giacomello, E.; Sartori, R.; Zampieri, S.; Capovilla, G.; et al. Plasma proteome profiling of healthy subjects undergoing bed rest reveals unloading-dependent changes linked to muscle atrophy. J. Cachexia Sarcopenia Muscle 2023, 14, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Puente, P.; Mateos, J.; Fernández-Costa, C.; Oreiro, N.; Fernández-López, C.; Ruiz-Romero, C.; Blanco, F.J. Identification of a Panel of Novel Serum Osteoarthritis Biomarkers. J. Proteome Res. 2011, 10, 5095–5101. [Google Scholar] [CrossRef]
- Hsu, M.-E.; Cheng, Y.-T.; Chang, C.-H.; Chan, Y.H.; Wu, V.C.-C.; Hung, K.-C.; Lin, C.-P.; Liu, K.-S.; Chu, P.-H.; Chen, S.-W. Level of serum soluble lumican and risks of perioperative complications in patients receiving aortic surgery. PLoS ONE 2021, 16, e0247340. [Google Scholar] [CrossRef]
- Bălănescu, A.; Codreanu, I.F.; Comanici, V.D.; Stan, I.V.; Bălănescu, E.; Bălănescu, P. Endocan and Lumican in Relation to Cardiometabolic Risk in a Pediatric Overweight and Obese Cohort: A Cross-Sectional Study. BioMed Res. Int. 2020, 2020, 2102401. [Google Scholar] [CrossRef]
- Xue, D.; Li, M.; Xiang, D.; Sun, J.; Zhang, W.; Duan, J.; Cheng, X.; Wang, C. Serum Proteomic Analysis by Nanoflow LC-MS/MS-Based Proteomics in IgA Chronic Kidney Disease. Clin. Lab. 2023; 69, in press. [Google Scholar]
- Athanasiou, A.; Tennstedt, P.; Wittig, A.; Huber, R.; Straub, O.; Schiess, R.; Steuber, T. A novel serum biomarker quintet reveals added prognostic value when combined with standard clinical parameters in prostate cancer patients by predicting biochemical recurrence and adverse pathology. PLoS ONE 2021, 16, e0259093. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.; Xu, G.; Chu, N.; Xu, N.; Wen, H.; Gu, B.; Liu, J.; Mao, S.; Na, R.; et al. iTRAQ-based quantitative proteomic analysis reveals potential early diagnostic markers of clear-cell Renal cell carcinoma. BioSci. Trends 2016, 10, 210–219. [Google Scholar] [CrossRef]
- Appunni, S.; Anand, V.; Khandelwal, M.; Seth, A.; Mathur, S.; Sharma, A. Altered expression of small leucine-rich proteoglycans (Decorin, Biglycan and Lumican): Plausible diagnostic marker in urothelial carcinoma of bladder. Tumor Biol. 2017, 39, 101042831769911. [Google Scholar] [CrossRef] [PubMed]
- Sahar, T.; Nigam, A.; Anjum, S.; Gupta, N.; Wajid, S. Secretome Profiling and Computational Biology of Human Leiomyoma Samples Unravel Molecular Signatures with Potential for Diagnostic and Therapeutic Interventions. Reprod. Sci. 2021, 28, 2672–2684. [Google Scholar] [CrossRef]
- Lee, K.N.; Park, K.H.; Ahn, K.; Im, E.M.; Oh, E.; Cho, I. Extracellular matrix-related and serine protease proteins in the amniotic fluid of women with early preterm labor: Association with spontaneous preterm birth, intra-amniotic inflammation, and microbial invasion of the amniotic cavity. Am. J. Reprod. Immunol. 2023, 90, e13736. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-Y.; Tsai, K.-W.; Lan, K.-C.; Hung, H.-N.; Lai, Y.-J.; Cheng, H.-H.; Tsai, C.-C.; Li, S.-C. Identifying the potential protein biomarkers of preterm birth in amniotic fluid. Taiwan. J. Obstet. Gynecol. 2020, 59, 366–371. [Google Scholar] [CrossRef]
- Shultz, S.R.; Shah, A.D.; Huang, C.; Dill, L.K.; Schittenhelm, R.B.; Morganti-Kossmann, M.C.; Semple, B.D. Temporal proteomics of human cerebrospinal fluid after severe traumatic brain injury. J. Neuroinflamm. 2022, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.; Liu, C.Y.; Chikama, T.; Hayashi, Y.; Kao, C.W.; Birk, D.E.; Funderburgh, J.L.; Jester, J.V.; Kao, W.W. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J. Biol. Chem. 2005, 280, 25541–25547. [Google Scholar] [CrossRef]
- Dunlevy, J.; Neame, P.J.; Vergnes, J.P.; Hassell, J.R. Identification of the N-linked oligosaccharide sites in chick corneal lumican and keratocan that receive keratan sulfate. J. Biol. Chem. 1998, 273, 9615–9621. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.; Funderburgh, M.L.; Brown, S.J.; Vergnes, J.P.; Hassell, J.R.; Mann, M.M.; Conrad, G.W. Sequence and structural implications of a bovine corneal keratan sulfate proteoglycan core protein. Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J. Biol. Chem. 1993, 268, 11874–11880. [Google Scholar] [CrossRef]
- Krull, N.; Gressner, A.M. Differential expression of keratan sulphate proteoglycans fibromodulin, lumican and aggrecan in normal and fibrotic rat liver. FEBS Lett. 1992, 312, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J. Keratan sulfate biosynthesis. IUBMB Life 2002, 54, 187–194. [Google Scholar] [CrossRef]
- Dunlevy, J.; Beales, M.P.; Berryhill, B.L.; Cornuet, P.K.; Hassell, J.R. Expression of the keratan sulfate proteoglycans lumican, keratocan and osteoglycin/mimecan during chick corneal development. Exp. Eye Res. 2000, 70, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Hutchison, J.C.; Hughes, M.E.; Kellogg, D.R.; Murray, R.W. Electrical characterization of gel collected from shark electrosensors. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2002, 65, 061903. [Google Scholar] [CrossRef]
- Josberger, E.; Hassanzadeh, P.; Deng, Y.; Sohn, J.; Rego, M.J.; Amemiya, C.T.; Rolandi, M. Proton conductivity in ampullae of Lorenzini jelly. Sci. Adv. 2016, 2, e1600112. [Google Scholar] [CrossRef]
- Melrose, J. Mucin-like glycopolymer gels in electrosensory tissues generate cues which direct electrolocation in amphibians and neuronal activation in mammals. Neural Regen. Res. 2019, 14, 1191–1195. [Google Scholar] [CrossRef]
- Melrose, J. Functional Consequences of Keratan Sulfate Sulfation in Electrosensory Tissues and in Neuronal Regulation. Adv. Biosyst. 2019, 3, e1800327. [Google Scholar] [CrossRef]
- Melrose, J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: The importance of KS-glycodynamics and interactive capability with neuroregulatory ligands. J. Neurochem. 2019, 149, 170–194. [Google Scholar] [CrossRef]
- Manger, P.; Pettigrew, J.D. Ultrastructure, number, distribution and innervation of electroreceptors and mechanoreceptors in the bill skin of the platypus, Ornithorhynchus anatinus. Brain Behav. Evol. 1996, 48, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.; Fields, K.D.; Fields, M.C. Semiconductor gel in shark sense organs? Neurosci. Lett. 2007, 426, 166–170. [Google Scholar] [CrossRef]
- Proske, U.; Gregory, J.E.; Iggo, A. Sensory receptors in monotremes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.; Leitch, D.B.; Julius, D. Molecular tuning of electroreception in sharks and skates. Nature 2018, 558, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Haueisen, M.; Reis, R.E. High resolution in turbid waters: Ampullae of lorenzini in the daggernose shark Carcharhinus oxyrhynchus (Valenciennes, 1839) (Elasmobranchii: Carcharhinidae). J. Fish Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, K.; Lin, L.; Zhang, F.; Yu, Y.; St Ange, K.; Han, X.; Edsinger, E.; Sohn, J.; Linhardt, R.J. Structural and Functional Components of the Skate Sensory Organ Ampullae of Lorenzini. ACS Chem. Biol. 2018, 13, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Selberg, J.; Jia, M.; Rolandi, M. Proton conductivity of glycosaminoglycans. PLoS ONE 2019, 14, e0202713. [Google Scholar] [CrossRef]
- Jia, M.; Kim, J.; Nguyen, T.; Duong, T.; Rolandi, M. Natural biopolymers as proton conductors in bioelectronics. Biopolymers 2021, 112, e23433. [Google Scholar] [CrossRef]
- Cavallo, F.; Lagally, G.M. Nano-origami: Art and function. Nanotoday 2015, 10, 538–541. [Google Scholar] [CrossRef]
- Rogers, J.; Huang, Y.; Schmidt, O.G.; Gracias, D.H. Origami MEMS and NEMS. MRS Bull. 2016, 41, 123–129. [Google Scholar] [CrossRef]
- Huang, J.; Huang, G.; Zhao, Z.; Wang, C.; Cui, J.; Song, E.; Mei, Y. Nanomembrane-assembled nanophotonics and optoelectronics: From materials to applications. J. Phys. Condens. Matter 2023, 35, 093001. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, B.; Wang, L.; Huang, G.; Mei, Y. Tubular micro/nanomachines: From the basics to recent advances. Adv. Funct. Mater. 2017, 28, 1705872. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, B.; Zhang, Z.; Ke, S.; Jin, Y.; Wen, X.; Ye, C. A review of memristor: Material and structure design, device performance, applications and prospects. Sci. Technol. Adv. Mater. 2023, 24, 2162323. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gao, Z.; Fu, J.; Ren, W.; Yang, C.; Wen, J.; Wan, X.; Ren, Q.; Gu, S.; Liu, X.; et al. Overview of Memristor-Based Neural Network Design and Applications. Front. Phys. 2022, 10, 839243. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Lwigale, P. Corneal Development: Different Cells from a Common Progenitor. Prog. Mol. Biol. Transl. Sci. 2015, 134, 43–59. [Google Scholar] [PubMed]
- Schwend, T.; Deaton, R.J.; Zhang, Y.; Caterson, B.; Conrad, G.W. Corneal sulfated glycosaminoglycans and their effects on trigeminal nerve growth cone behavior in vitro: Roles for ECM in cornea innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8118–8137. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Conrad, G.W. The keratocan gene is expressed in both ocular and non-ocular tissues during early chick development. Matrix Biol. 2003, 22, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Birk, D.E.; Hassell, J.R.; Kane, B.; Kao, W.W. Keratocan-deficient mice display alterations in corneal structure. J. Biol. Chem. 2003, 278, 21672–21677. [Google Scholar] [CrossRef]
- Hayes, A.; Melrose, J. Immunolocalization of Keratan Sulfate in Rat Spinal Tissues Using the Keratanase Generated BKS-1(+) Neoepitope: Correlation of Expression Patterns with the Class II SLRPs, Lumican and Keratocan. Cells 2020, 9, 826. [Google Scholar] [CrossRef]
- Akhtar, S.; Kerr, B.C.; Hayes, A.J.; Hughes, C.E.; Meek, K.M.; Caterson, B. Immunochemical localization of keratan sulfate proteoglycans in cornea, sclera, and limbus using a keratanase-generated neoepitope monoclonal antibody. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2424–2431. [Google Scholar] [CrossRef]
- Conrad, A.; Zhang, Y.; Tasheva, E.S.; Conrad, G.W. Proteomic analysis of potential keratan sulfate, chondroitin sulfateA, and hyaluronic acid molecular interactions.Invest. Ophthalmol. Vis. Sci. 2010, 51, 4500–4515. [Google Scholar] [CrossRef]
- Melrose, J. Keratan Sulfate, An Electrosensory Neurosentient Bioresponsive Cell Instructive Glycosaminoglycan. Glycobiology 2024, cwae014. [Google Scholar] [CrossRef]
- Kawabe, K.; Tateyama, D.; Toyoda, H.; Kawasaki, N.; Hashii, N.; Nakao, H.; Matsumoto, S.; Nonaka, M.; Matsumura, H.; Hirose, Y.; et al. A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology 2013, 23, 322–336. [Google Scholar] [CrossRef]
- Nagai, Y.; Nakao, H.; Kojima, A.; Komatsubara, Y.; Ohta, Y.; Kawasaki, N.; Kawasaki, N.; Toyoda, H.; Kawasaki, T. Glycan Epitopes on 201B7 Human-Induced Pluripotent Stem Cells Using R-10G and R-17F Marker Antibodies. Biomolecules 2021, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Nagai, Y.; Kojima, A.; Kinoshita-Toyoda, A. Podocalyxin as a major pluripotent marker and novel keratan sulfate proteoglycan in human embryonic and induced pluripotent stem cells. Glycoconj. J. 2017, 34, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Binder, Z.; Siu, I.M.; Eberhart, C.G.; Ap Rhys, C.; Bai, R.Y.; Staedtke, V.; Zhang, H.; Smoll, N.R.; Piantadosi, S.; Piccirillo, S.G.; et al. Podocalyxin-like protein is expressed in glioblastoma multiforme stem-like cells and is associated with poor outcome. PLoS ONE 2013, 8, e75945. [Google Scholar] [CrossRef] [PubMed]
- Cait, J.; Hughes, M.R.; Zeglinski, M.R.; Chan, A.W.; Osterhof, S.; Scott, R.W.; Canals Hernaez, D.; Cait, A.; Vogl, A.W.; Bernatchez, P.; et al. Podocalyxin is required for maintaining blood-brain barrier function during acute inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 4518–4527. [Google Scholar] [CrossRef]
- Hayes, A.; Melrose, J. Keratan Sulphate in the Tumour Environment. Adv. Exp. Med. Biol. 2020, 1245, 39–66. [Google Scholar] [PubMed]
- Caterson, B.; Christner, J.E.; Baker, J.R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 1983, 258, 8848–8854. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, H.; Scudder, P.; Tang, P.W.; Hounsell, E.F.; Caterson, B.; Feizi, T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur. J. Biochem. 1986, 157, 385–391. [Google Scholar] [CrossRef]
- Tang, P.; Scudder, P.; Mehmet, H.; Hounsell, E.F.; Feizi, T. Sulphate groups are involved in the antigenicity of keratan sulphate and mask i antigen expression on their poly-N-acetyllactosamine backbones. An immunochemical and chromatographic study of keratan sulphate oligosaccharides after desulphation or nitrosation. Eur. J. Biochem. 1986, 160, 537–545. [Google Scholar]
- Suzuki, H.; Chikada, M.; Yokoyama, M.K.; Kurokawa, M.S.; Ando, T.; Furukawa, H.; Arito, M.; Miyairi, T.; Kato, T. Aberrant Glycosylation of Lumican in Aortic Valve Stenosis Revealed by Proteomic Analysis. Int. Heart J. 2016, 57, 104–111. [Google Scholar] [CrossRef]
- Imagama, S.; Sakamoto, K.; Tauchi, R.; Shinjo, R.; Ohgomori, T.; Ito, Z.; Zhang, H.; Nishida, Y.; Asami, N.; Takeshita, S.; et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J. Neurosci. 2011, 31, 17091–17102. [Google Scholar] [CrossRef]
- Ito, Z.; Sakamoto, K.; Imagama, S.; Matsuyama, Y.; Zhang, H.; Hirano, K.; Ando, K.; Yamashita, T.; Ishiguro, N.; Kadomatsu, K. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 5937–5947. [Google Scholar] [CrossRef]
- Jones, L.; Tuszynski, M.H. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J. Neurosci. 2002, 22, 4611–4624. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Ohgomori, T.; Natori, T.; Miyamoto, K.; Kusunoki, S.; Sakamoto, K.; Ishiguro, N.; Imagama, S.; Kadomatsu, K. Keratan sulfate expression in microglia is diminished in the spinal cord in experimental autoimmune neuritis. Cell Death Dis. 2013, 4, e946. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R. Systematic reviews of treatment for inflammatory demyelinating neuropathy. J. Anat. 2002, 200, 331–339. [Google Scholar] [CrossRef]
- Kieseier, B.; Kiefer, R.; Gold, R.; Hemmer, B.; Willison, H.J.; Hartung, H.P. Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve 2004, 30, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.Y.; Schluesener, H.J. Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J. Immunol. 2009, 183, 3081–3091. [Google Scholar] [CrossRef]
| Condition | Population (n) | Change in Lumican | Reference |
|---|---|---|---|
| In plasma by proteomics and/or ELISA | |||
| Knee osteoarthritis (OA) | Patients (173) | Positively associated with joint space narrowing | [134] |
| Sepsis | Patients (11) vs. healthy (17) | Higher in sepsis than healthy p < 0.01 | [135] |
| Lung adenocarcinoma | Patients (102) | Higher with poorer prognosis | [136] |
| Metastatic prostate cancer | Patients (62) | Higher with poorer prognosis | [137] |
| Pancreatic cancer | Patients (40) vs. healthy controls (20) | Higher in cancer | [138] |
| Bed rest | Healthy males (10) | Decreased with rest | [139] |
| In serum by proteomics and/or ELISA | |||
| Back pain | OA patients (731) | Higher in group with more pain and inflammation | [64] |
| IVDD and disc space narrowing | OA patients (723) | Higher with advancing IVDD | [65] |
| Knee and hip OA | Patients with no (50), moderate (50) or severe OA (50) | Higher in severe OA | [140] |
| Coronary artery disease | Stable angina pectoris (255) | Higher in advanced disease 0.6 ng/mL vs. 0.4 ng/mL, p < 0.001 | [115] |
| Aortic dissection (AD) | Aortic or aneurysm surgery patients (58) | Positively associated with unfavourable p.o. outcome | [141] |
| Acute aortic dissection (AAD) | AAD patients (14) vs. chronic AD (CAD; 3) | Higher in AAD vs. CAD. | [130] |
| Acute aortic dissection (AAD) | AAD patients (26) vs. non-AAD (144) | Higher in AAD vs. non-AAD. | [129] |
| Acute aortic dissection (AAD) | AAD patients (20) vs. healthy (20) | Higher in AAD vs. healthy. | [119] |
| Acute aortic dissection (AAD) | AAD patients (60) vs. AMI * (30) vs. healthy (30) | Higher in AAD vs. AMI or healthy. | |
| Carotid artery (CA) atherosclerosis | Patients with (105) or without (71) CA plaque | Higher with CA plaque | [116] |
| Arterial pressure in obese children | Patients (n = 68) | Positively correlated with higher pressure | [142] |
| Chronic kidney disease (IgN nephropathy) | Patients (60) vs. controls (43) | Downregulated in advanced disease | [143] |
| Prostate cancer | Men undergoing radical prostatectomy (557) | In a multivariate model with other biomarkers, predicted recurrence | [144] |
| Renal cell carcinoma | Patients (99) vs. healthy controls (18) | Positively associated with tumour grade | [145] |
| Urothelial carcinoma | Patients (30) vs. healthy controls (30) | Higher in carcinoma than healthy p < 0.001 | [146] |
| Uterine leiomyoma | Patients (6) vs. healthy (6) | Higher in leiomyoma than healthy | [147] |
| In amniotic fluid by ELISA | |||
| Preterm birth | Pregancies (252) | Positively associated with inflammation and/or microbial invasion; lower in preterm births | [148] |
| Preterm birth | Pre-term (36) vs. full-term births (21) | Lower in preterm births | [149] |
| In aqueous humor by proteomics and ELISA | |||
| Idiopathic epiretinal membrane | Patients (10) vs. age-matched controls (10) | Correlated with central retinal thickness (r = 0.655; p = 0.002) | [89] |
| In CSF by mass spectrometry | |||
| Traumatic brain injury | Patients (16) vs. controls (11) | Negatively associated with favourable outcome | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, M.M.; Melrose, J. Lumican, a Multifunctional Cell Instructive Biomarker Proteoglycan Has Novel Roles as a Marker of the Hypercoagulative State of Long Covid Disease. Int. J. Mol. Sci. 2024, 25, 2825. https://doi.org/10.3390/ijms25052825
Smith MM, Melrose J. Lumican, a Multifunctional Cell Instructive Biomarker Proteoglycan Has Novel Roles as a Marker of the Hypercoagulative State of Long Covid Disease. International Journal of Molecular Sciences. 2024; 25(5):2825. https://doi.org/10.3390/ijms25052825
Chicago/Turabian StyleSmith, Margaret M., and James Melrose. 2024. "Lumican, a Multifunctional Cell Instructive Biomarker Proteoglycan Has Novel Roles as a Marker of the Hypercoagulative State of Long Covid Disease" International Journal of Molecular Sciences 25, no. 5: 2825. https://doi.org/10.3390/ijms25052825





