A Comprehensive Strategy for Stepwise Design of a Lab PROTOTYPE for the Removal of Emerging Contaminants in Water Using Cyclodextrin Polymers as Adsorbent Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect Contact Time
2.2. Kinetic Analysis
2.3. Adsorption Equilibrium
2.4. Thermodynamic Parameters
2.5. Polymer Reusability
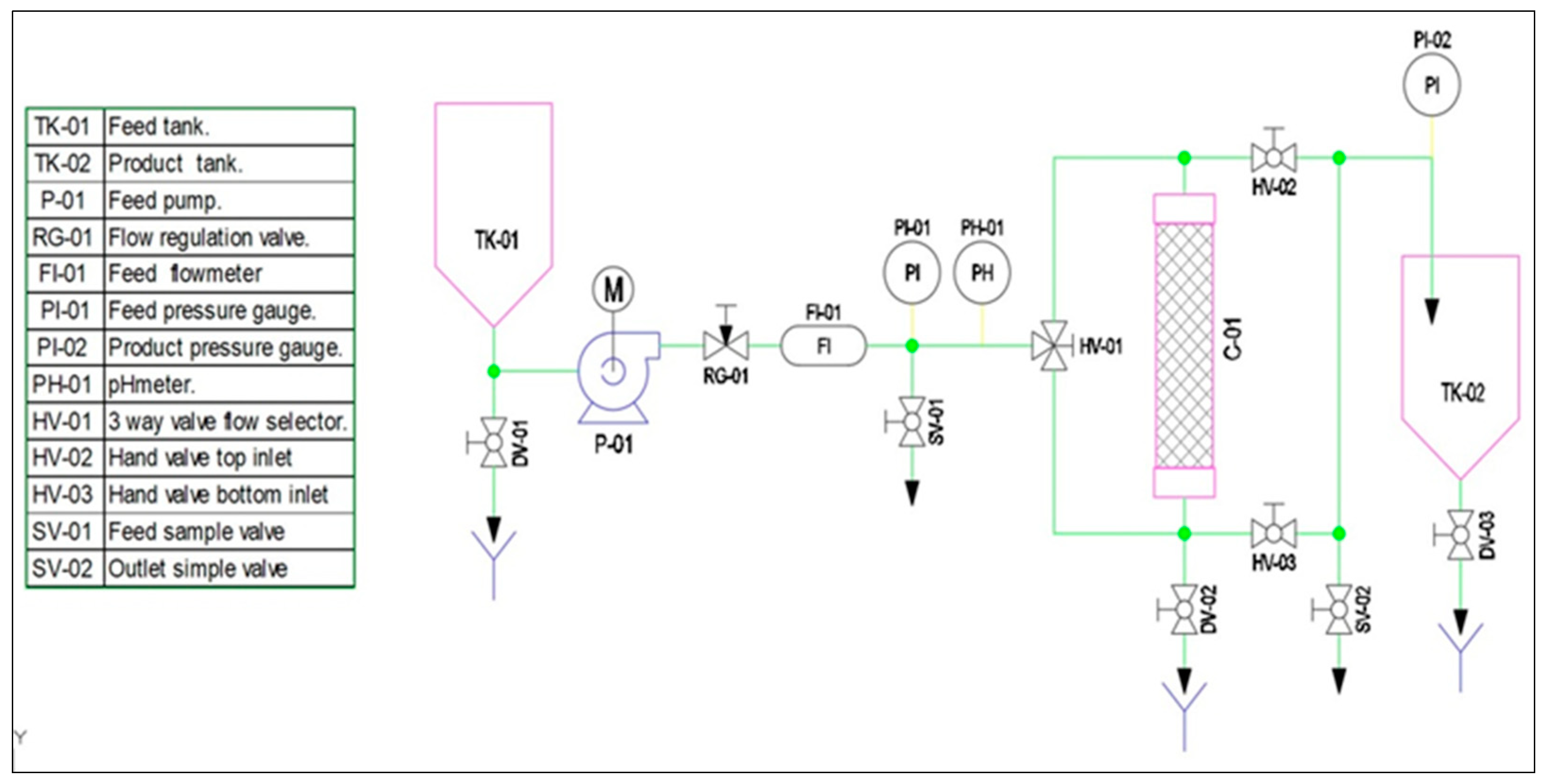
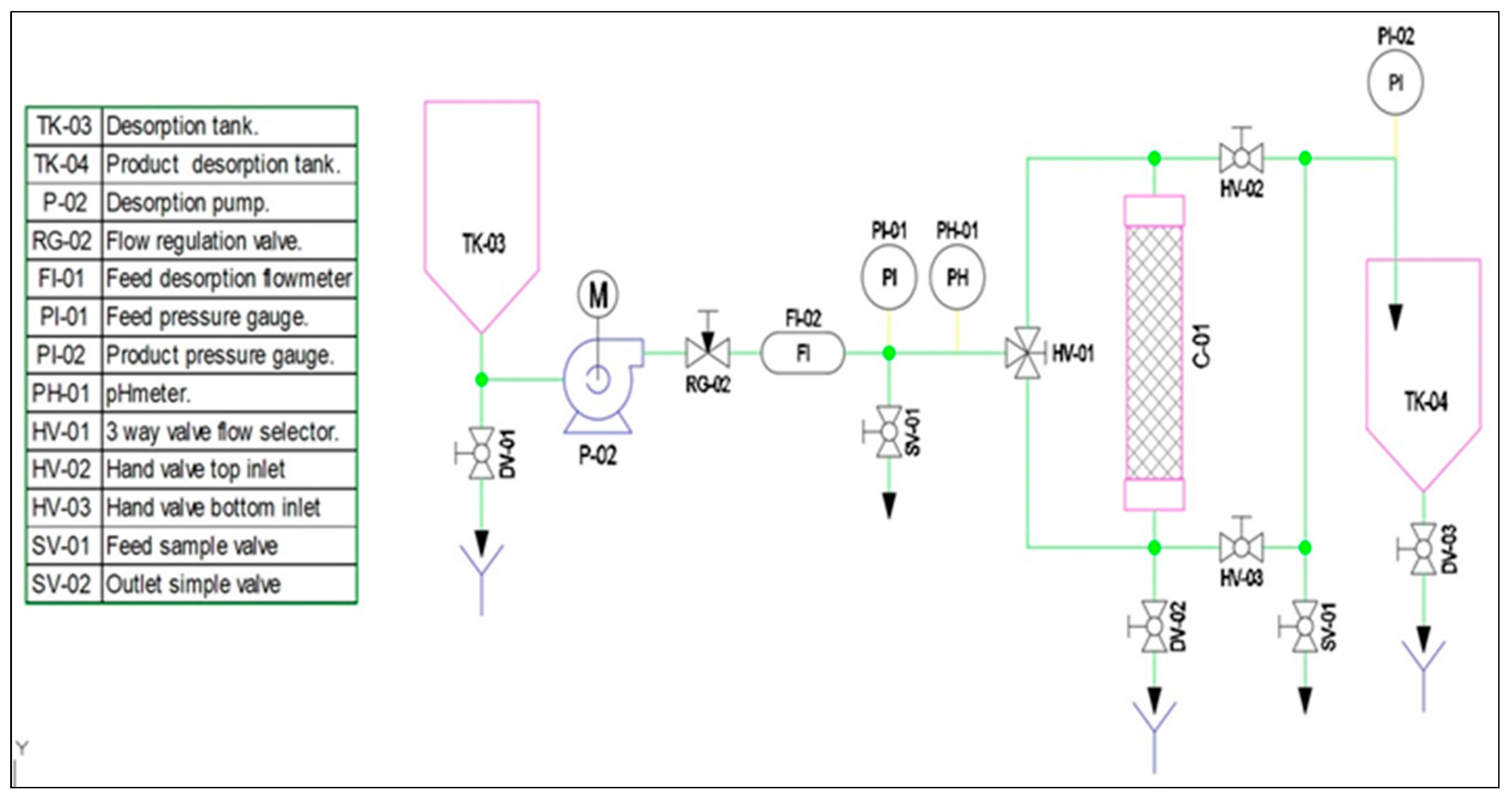
2.6. Design Continuous Flow Prototype Adsorption
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Epichlorohydrin-β-Cyclodextrin Polymer Preparation
3.3. Diuretics Solution Preparation
3.4. Adsorption Experiments
3.5. Kinetics Analysis
3.6. Isotherms Analysis
3.7. Polymer Reusability
3.8. Design of a Pilot-Scale Prototype Cyclodextrin Polymer Adsorption of Pollutants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C.; et al. Water scarcity assessments in the past, present, and future. Earth’s Future 2017, 5, 545–559. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- European Parliament. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 1 October 2023).
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Community 2013, 1–17. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:en:PDF (accessed on 1 October 2023).
- World Health Organization. State of the World’s Sanitation: An Urgent Call to Transform Sanitation for Better Health, Environments, Economies and Societies. New York (NY): United Nations Children’s Fund (UNICEF) and the World Health Organization. 2020. Available online: https://www.who.int/publications/i/item/9789240014473 (accessed on 1 October 2023).
- Lopez-Pacheco, I.Y.; Silva-Nunez, A.; Salinas-Salazar, C.; Arevalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barcelo, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- Sauve, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 7, 3–27. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Council Directive 91/271/EEC of 21 May 1991 concerning urban waste-water treatment. Off. J. Eur. Communities 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0271 (accessed on 1 October 2023).
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Choudri, B.S.; Al-Awadhi, T.; Charabi, Y.; Al-Nasiri, N. Wastewater treatment, reuse, and disposal-associated effects on environment and health. Water Environ. Res. 2020, 92, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, V.; Jafari, A.; Vahed, F.L.; Su, C.H.; Pirouzfar, V. Polysaccharides as eco-friendly bio-adsorbents for wastewater remediation: Current state and future perspective. J. Water Process Eng. 2023, 54, 103980. [Google Scholar] [CrossRef]
- Murcia-Salvador, A.; Pellicer, J.A.; Fortea, M.I.; Gómez-López, V.M.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A. Adsorption of Direct Blue 78 using chitosan and cyclodextrins as adsorbents. Polymers 2019, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, D.; Bochot, A. Thitry years with cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- González-Louzao, R.; Lucas-Abellán, C.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; Gabaldón, J.A.; López-Miranda, S.; Yañez-Gascón, M.J.; Asín-Lorca, M.; Núñez-Delicado, E. Encapsulation of finasteride with native and modified γ-cyclodextrins. Extensive characterization of the complexes. Int. J. Pharm. 2020, 587, 119619. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Jiayue, L. Cyclodextrin-based adsorbents for the removal of pollutants from wastewater: A review. Environ. Sci. Polut. Res. 2021, 28, 1317–1340. [Google Scholar] [CrossRef]
- Crini, G. Cyclodextrin–epichlorohydrin polymers synthesis, characterization and applications to wastewater treatment: A review. Environ. Chem. Lett. 2021, 19, 2383–2403. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, C.K.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Karpkird, T.; Manaprasertsak, A.; Penkitti, A.; Sinthuvanich, C.; Singchuwong, T.; Leepasert, T. A novel chitosan-citric acid crosslinked beta-cyclodextrin nanocarriers for insoluble drug delivery. Carbohydr. Res. 2020, 498, 108184. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Nasir, M. Effective removal of acetaminophen from aqueous solution using Ca (II)-doped chitosan/β-cyclodextrin composite. J. Mol. Liq. 2020, 301, 112454. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Zhao, H.; Liu, Y.; Long, F.; Pan, X. A versatile β-cyclodextrin and polyethyleneimine bi-functionalized magnetic nanoadsorbent for simultaneous capture of methyl orange and Pb (II) from complex wastewater. Chemosphere 2019, 216, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Subramaniam, K.; Mathimani, T.; Seethappan, S.; Pugazhendhi, A. Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochem. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Semeraro, P.; Gabaldón, J.; Fini, P.; Núñez-Delicado, E.; Pellicer, J.A.; Rizzi, V.; Cosma, P. Removal of an Azo Textile Dye from Wastewater by Cyclodextrin-Epichlorohydrin Polymers; IntechOpen: London, UK, 2018; p. 303. [Google Scholar]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Hernández, J.A.G.; Lucas-Abellán, C.; Mercader-Ros, M.T.; Serrano-Martínez, A.; Núñez-Delicado, E.; Cosma, P.; Ferrándiz, M. Removing of Direct Red 83: 1 using α-and HP-α-CDs polymerized with epichlorohydrin: Kinetic and equilibrium studies. Dye. Pigment. 2018, 149, 736–746. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhang, S.; Yu, H.; Xie, W. A versatile β-cyclodextrin functionalized silver nanoparticle monolayer for capture of methyl orange from complex wastewater. Chin. Chem. Lett. 2020, 31, 539–542. [Google Scholar] [CrossRef]
- Romita, R.; Rizzi, V.; Semeraro, P.; Gubitosa, J.; Gabaldón, J.A.; Gorbe, M.I.F.; Gómez, V.M.; Cosma, P.; Fini, P. Operational parameters affecting the atrazine removal from water by using cyclodextrin based polymers as efficient adsorbents for cleaner technologies. Environ. Technol. Innov. 2019, 16, 100454. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morte, T.; Gómez-López, V.M.; Lucas-Abellán, C.; Martínez-Alcalá, I.; Ayuso, M.; Martínez-López, S.; Montemuro, N.; Pérez, S.; Barceló, D.; Fini, P.; et al. Removal and toxicity evaluation of a diverse group of drugs from water by a cyclodextrin polymer/pulsed light system. J. Hazard. Mater. 2021, 402, 123504. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Barkács, K.; Gruiz, K.; Varga, E.; Kenyeres, I.; Záray, G.; Szente, L. Removal of hazardous micropollutants from treated wastewater using cyclodextrin bead polymer–A pilot demonstration case. J. Hazard. Mater. 2020, 383, 121181. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.; Murtinho, D.; Valente, A.J. Cyclodextrin-based nanosponges: Overview and opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- García-Zubiri, I.X.; González-Gaitano, G.; Isasi, J.R. Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J. Colloid Interface Sci. 2009, 337, 11–18. [Google Scholar] [CrossRef]
- Pellicer, J.; Rodríguez-López, M.; Fortea, M.; Lucas-Abellán, C.; Mercader-Ros, M.; López-Miranda, S.; Gómez-López, V.; Semeraro, P.; Cosma, P.; Fini, P.; et al. Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process. Polymers 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Romita, R.; Gómez-López, V.M.; Gubitosa, J.; Gabaldón, J.A.; Gorbe, M.F.; Gómez, V.M.; Cosma, P.; Fini, P. The synergistic action of cyclodextrin-based adsorbent and advanced oxidation processes for sulfamethoxazole removal from water. Int. J. Environ. Sci. Technol. 2022, 19, 10663–10676. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, E.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Hemine, K.; Łukasik, N.; Gazda, M.; Nowak, I. β-cyclodextrin-containing polymer based on renewable cellulose resources for effective removal of ionic and non-ionic toxic organic pollutants from water. J. Hazard. Mater. 2021, 418, 126286. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Gimbert, F.; Robert, C.; Martel, B.; Adam, O.; Morin-Crini, N.; De Giorgi, F.; Badot, P.-M. The Removal of Basic Blue 3 from Aqueous Solutions by Chitosan-Based Adsorbent: Batch Studies. J. Hazard. Mater. 2008, 153, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Fan, H.-T.; Shi, L.-Q.; Shen, H.; Chen, X.; Xie, K.-P. Equilibrium, Isotherm, Kinetic and Thermodynamic Studies for Removal of Tetracycline Antibiotics by Adsorption onto Hazelnut Shell Derived Activated Carbons from Aqueous Media. RSC Adv. 2016, 6, 109983–109991. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.-X.; Chen, A.W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Murcia-Salvador, A.; Pellicer, J.A.; Rodríguez-López, M.I.; Gómez-López, V.M.; Núñez-Delicado, E.; Gabaldón, J.A. Egg By-Products as a Tool to Remove Direct Blue 78 Dye from Wastewater: Kinetic, Equilibrium Modeling, Thermodynamics and Desorption Properties. Materials 2020, 13, 1262. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of CI Basic Green 4 (Malachite Green) from Aqueous Solutions by Adsorption Using Cyclodextrin-Based Adsorbent: Kinetic and Equilibrium Studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of Second-Order Models for Adsorption Systems. ChemInform 2006, 136, 681–689. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Temkin, M.I. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochimica. URSS 1940, 12, 327–356. [Google Scholar]
- Rodríguez-López, M.I.; Pellicer, J.A.; Gómez-Morte, T.; Auñón, D.; Gómez-López, V.M.; Yáñez-Gascón, M.J.; Gil-Izquierdo, Á.; Cerón-Carrasco, J.P.; Crini, G.; Núñez-Delicado, E.; et al. Removal of an azo dye from wastewater through the use of two technologies: Magnetic cyclodextrin polymers and pulsed light. Int. J. Mol. Sci. 2022, 23, 8406. [Google Scholar] [CrossRef] [PubMed]



| Isotherm | Parameter | Furosemide | Hydrochlorothiazide |
|---|---|---|---|
| Freundlich | KF (L/g) | 0.044 | 0.029 |
| nF | 0.817 | 0.737 | |
| R2 | 0.991 | 0.905 | |
| Langmuir | qmax (mg/g) | 1.282 | 0.844 |
| KL | 0.050 | 0.038 | |
| aL | 0.039 | 0.045 | |
| ∆G | −16,919.810 | −16,730.651 | |
| R2 | 0.516 | 0.514 | |
| RL | 0.838–0.564 | 0.817–0.527 | |
| Tempkin | aT | 0.525 | 0.448 |
| bT (kJ/mol) | 6.890 | 6.79 | |
| R2 | 0.943 | 0.872 |
| Parameter | Value |
|---|---|
| qmax (furosemide) (mg/g) | 1.282 |
| qmax (hydrochlorothiazide) (mg/g) | 0.844 |
| Density (g/cm3) | 1.06 |
| Swelling | 4 ± 1 |
| Particle size (mm) | 0.1→0.3 |
| Stability range (pH) | 2→11 |
| Temperature range (°C) | 5→35 |
| Solubility in H2O | Insoluble |
| Parameter | Value |
|---|---|
| Adsorbent Volume (L) | 1→3 |
| Column diameter | To define |
| Adsorbent bed depth (mm) | 150→550 |
| Adsorbent expansion (%) | Up to 100 |
| Contact time (min) | 1→7.5 |
| Loading flow rate (BV/h) | 8→40 |
| Desorbent flow rate (BV/h) | 2→5 |
| Desorbent contact time (min) | 20→60 |
| Desorbent displacement (BV of water) | 2→4 |
| Final rinse (BV service flow rate) | 2→10 |
| Column Size Design Calculations | ||||
|---|---|---|---|---|
| Parameters | Ø90 mm | Ø63 mm | ||
| Flow (L/h) | 8 | 40 | 8 | 40 |
| Flow rate (m/h) | 1.43 | 7.17 | 3.10 | 15.51 |
| Ad volume (L) | 1 | 1 | 1 | 1 |
| BV (BV/h) | 8 | 40 | 8 | 40 |
| Area (m²) | 0.0056 | 0.0056 | 0.0026 | 0.0026 |
| Bed depth (m) | 0.18 | 0.18 | 0.39 | 0.39 |
| Expansion (%) | 100 | 100 | 100 | 100 |
| Column height (m) | 0.36 | 0.36 | 0.78 | 0.78 |
| Contact time (min) | 7.5 | 1.5 | 7.5 | 1.5 |
| Flow (L/h) | 16 | 80 | 16 | 80 |
| Flow rate (m/h) | 2.87 | 14.33 | 6.20 | 31.02 |
| Ad volume (L) | 2 | 2 | 2 | 2 |
| BV (BV/h) | 8 | 40 | 8 | 40 |
| Area (m²) | 0.0056 | 0.0056 | 0.0026 | 0.0026 |
| Bed depth (m) | 0.36 | 0.36 | 0.78 | 0.78 |
| Expansion (%) | 100 | 100 | 100 | 100 |
| Column height (m) | 0.72 | 0.72 | 1.55 | 1.55 |
| Contact time (min) | 7.5 | 1.5 | 7.5 | 1.5 |
| Flow (L/h) | 24 | 120 | 24 | 120 |
| Flow rate (m/h) | 4.30 | 21.50 | 9.31 | 46.54 |
| Ad volume (L) | 3 | 3 | 3 | 3 |
| BV (BV/h) | 8 | 40 | 8 | 40 |
| Area (m²) | 0.0056 | 0.0056 | 0.0026 | 0.0026 |
| Bed depth (m) | 0.54 | 0.54 | 1.16 | 1.16 |
| Expansion (%) | 100 | 100 | 100 | 100 |
| Column height (m) | 1.07 | 1.07 | 2.33 | 2.33 |
| Contact time (min) | 7.5 | 1.5 | 7.5 | 1.5 |
| Parameter | Value |
|---|---|
| PhACs concentration (mg/L) | 5→20 |
| Tank volume of PhACs solution (L) | 50 |
| Amount PhACs concentration (mg) | 250→1000 |
| β-CDs-EPI qmax (furosemide) (mg/g) | 1.282 |
| β-CDs-EPI qmax (hydrochlorothiazide) (mg/g) | 0.844 |
| β-CDs-EPI volume (L) | 1→3 |
| β-CDs-EPI weight (g/column) | 1060→3180 |
| Amount β-CDs-EPI qmax (furosemide) (mg) | 1358→3846 |
| Amount β-CDs-EPI qmax (hydrochlorothiazide) (mg) | 894→2683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Cegarra, A.T.; Gómez-Morte, T.; Pellicer, J.A.; Vela, N.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A. A Comprehensive Strategy for Stepwise Design of a Lab PROTOTYPE for the Removal of Emerging Contaminants in Water Using Cyclodextrin Polymers as Adsorbent Material. Int. J. Mol. Sci. 2024, 25, 2829. https://doi.org/10.3390/ijms25052829
Hernández Cegarra AT, Gómez-Morte T, Pellicer JA, Vela N, Rodríguez-López MI, Núñez-Delicado E, Gabaldón JA. A Comprehensive Strategy for Stepwise Design of a Lab PROTOTYPE for the Removal of Emerging Contaminants in Water Using Cyclodextrin Polymers as Adsorbent Material. International Journal of Molecular Sciences. 2024; 25(5):2829. https://doi.org/10.3390/ijms25052829
Chicago/Turabian StyleHernández Cegarra, Antonio Tomás, Teresa Gómez-Morte, José Antonio Pellicer, Nuria Vela, María Isabel Rodríguez-López, Estrella Núñez-Delicado, and José Antonio Gabaldón. 2024. "A Comprehensive Strategy for Stepwise Design of a Lab PROTOTYPE for the Removal of Emerging Contaminants in Water Using Cyclodextrin Polymers as Adsorbent Material" International Journal of Molecular Sciences 25, no. 5: 2829. https://doi.org/10.3390/ijms25052829
APA StyleHernández Cegarra, A. T., Gómez-Morte, T., Pellicer, J. A., Vela, N., Rodríguez-López, M. I., Núñez-Delicado, E., & Gabaldón, J. A. (2024). A Comprehensive Strategy for Stepwise Design of a Lab PROTOTYPE for the Removal of Emerging Contaminants in Water Using Cyclodextrin Polymers as Adsorbent Material. International Journal of Molecular Sciences, 25(5), 2829. https://doi.org/10.3390/ijms25052829









