Abstract
There is extensive coverage in the existing literature on implant-associated lymphomas like anaplastic large-cell lymphoma, but breast implant-associated squamous cell carcinoma (BIA-SCC) has received limited scholarly attention since its first case in 1992. Thus, this study aims to conduct a qualitative synthesis focused on the underexplored association between breast implants and BIA-SCC. A systematic review was conducted utilizing the PubMed, Web of Science, and Cochrane databases to identify all currently reported cases of BIA-SCC. Additionally, a literature review was performed to identify potential biochemical mechanisms that could lead to BIA-SCC. Studies were vetted for quality using the NIH quality assessment tool. From an initial pool of 246 papers, 11 met the quality criteria for inclusion, examining a total of 14 patients aged between 40 and 81 years. BIA-SCC was found in a diverse range of implants, including those with smooth and textured surfaces, as well as those filled with saline and silicone. The condition notably manifested a proclivity for aggressive clinical progression, as evidenced by a mortality rate approximating 21.4% within a post-diagnostic interval of six months. Our literature review reveals that chronic inflammation, driven by various external factors such as pathogens and implants, can initiate carcinogenesis through epigenetic modifications and immune system alterations. This includes effects from exosomes and macrophage polarization, showcasing potential pathways for the pathogenesis of BIA-SCC. The study highlights the pressing need for further investigation into BIA-SCC, a subject hitherto inadequately addressed in the academic sphere. This necessitates the urgency for early screening and intervention to improve postoperative outcomes. While the review is confined by its reliance on case reports and series, it serves as a valuable reference for future research endeavors.
1. Introduction
On 8 March 2023, the United States Food and Drug Administration (FDA) issued a safety alert, emphasizing the potential risk of squamous cell carcinoma (SCC) following breast implant surgery [1,2]. When the body responds to foreign objects like breast implants, the immune system attempts to isolate the implant by forming a collagenous capsule around it [3,4,5,6]. Within this encapsulated environment, SCC has been identified in a small subset of patients [7]. Cases of SCC localized within these fibrous capsules have been increasingly documented via case reports and series, with initial incidences traced back to the 1990s [8]. Although breast implant-associated squamous cell carcinoma (BIA-SCC) remains relatively rare, its potential for aggressiveness necessitates a rigorous examination of implant-related risks [7]. Therefore, it becomes imperative to provide patients with a comprehensive understanding of the range of risks they might face.
The existing academic literature on the topic has largely focused on the relationship between breast implants and various lymphomas, most notably breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) [9,10,11,12]. The exploration into the link between textured breast implants and the development of BIA-ALCL presents an intriguing look into how foreign body implantation can lead to lymphoma [9,10,11,12]. Despite our early stage of understanding, some theories have emerged from existing research, particularly focusing on the role of chronic inflammatory states in setting the stage for BIA-ALCL [9,10,11,12]. It is suggested that the texture of breast implants is a significant factor in the development of BIA-ALCL [9,10,11,12]. A prevailing theory suggests a connection between the chronic inflammatory state triggered by textured implants and the onset of BIA-ALCL [9,10,11,12]. The precise mechanisms connecting textured breast implants to BIA-ALCL are yet to be fully uncovered, calling for more detailed research to unravel the complex interaction of factors at the cellular and molecular levels. Additionally, the variability in disease presentation, from mild cases appearing as late periprosthetic fluid (seroma) to more severe lymphoma forms, highlights the complexity involved in the pathophysiology of BIA-ALCL [9,10,11,12]. In contrast, the possible connection between breast implants and the development of SCC has garnered comparatively limited empirical attention [8]. This area of inquiry is only recently acquiring prominence, underscoring the need for further research.
Similarly to BIA-ALCL, imaging techniques in BIA-SCC diagnosis often reveal fluid collection or tumor masses around the breast implant, while histopathological confirmation typically follows a biopsy or complete surgical removal, such as capsulectomy, to analyze the affected tissue. This approach ensures accurate diagnosis and informs the subsequent treatment strategy [13]. Unfortunately, unlike its BIA-ALCL counterpart, BIA-SCC tends to be more aggressive, and as such, its diagnosis is more pressing. The diagnostic process could be significantly enhanced by incorporating advanced technologies, such as automated computer-assisted decision-making systems. These systems are capable of detecting subtle asymmetries and other indicators that may suggest the presence of malignancy, thereby increasing the accuracy and speed of diagnosis [14].
At present, increasing evidence is substantiating the association between breast implants and SCC, although substantial gaps in current scientific knowledge still exist. In the event of a confirmed correlation, the responsibility lies in informing potential patients about these hidden risks and, when necessary, implementing effective screening protocols. Therefore, this qualitative systematic review aims to conduct a comprehensive analysis focused on the frequency, etiology, and clinical outcomes of SCC in patients with breast implants.
2. Methods
2.1. Systematic Review
This study protocol was prospectively registered with PROSPERO (Study#: CRD42023420842) [15]. Completion of the study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [16].
2.1.1. Eligibility Criteria
Criteria for included studies were defined as any studies reporting on the occurrence of BIA-SCC. The full eligibility criteria are reported in Table 1.

Table 1.
Eligibility criteria.
2.1.2. Search Strategy
A comprehensive research review using subject headings, controlled vocabulary, and keywords was used to search on PubMed/MEDLINE and Web of Science for studies published until June 2023. Our full-text search strategy is accessible at PROSPERO.
2.1.3. Study Selection
The search results were uploaded to Covidence. A two-stage screening process was conducted for study selection. Two screeners independently reviewed the titles and abstracts in the first step. In situations where disagreement arose between the initial two reviewers, a third reviewer was enlisted to decide on the ultimate inclusion or exclusion of the study, subsequent to a process of moderation. In the second stage, the same two reviewers performed a full-text review and selected studies that fulfilled the eligibility criteria. In situations where disagreement arose between the initial two reviewers, a third reviewer was enlisted to decide on the ultimate inclusion or exclusion of the study, subsequent to a process of moderation.
2.1.4. Data Extraction/Synthesis
The variables extracted were patient characteristics, type of implants, therapy for the treatment of malignancy (chemotherapy, radiation), and outcomes.
2.1.5. Quality Assessment
To assess the risk of bias, the National Institute of Health (NIH) quality assessment tool was utilized. Each article was categorized as follows: “low risk”, “moderate risk”, or “high risk” of bias [17].
2.1.6. Statistical Analysis
Due to the heterogeneity of the topics covered in the studies constituting this systematic review, it was not possible to perform any analysis beyond a qualitative synthesis.
2.2. Literature Review
2.2.1. Search Strategy
A comprehensive research review using subject headings, controlled vocabulary, and keywords was used to search on PubMed/MEDLINE and Web of Science for studies published until December 2023.
2.2.2. Study Selection
Relevant studies highlighting the relationship between molecular pathways of chronic inflammation and carcinogenesis were selected.
2.2.3. Data Extraction/Synthesis
A narrative synthesis was then performed to serve as a preliminary framework for understanding the molecular mechanisms potentially leading to BIA-SCC.
3. Results
3.1. Systematic Review
A total of 246 articles were initially identified, from which duplicates were subsequently removed. A set of 19 articles was subjected to a full-text review, and 11 were eventually selected for data extraction [7,18,19,20,21,22,23,24,25,26,27]. These articles specifically focused on the relationship between breast implants and SCC. Utilizing the NIH quality assessment tool, two of these articles were classified as “good”, five as “fair”, and four as “poor”. See Figure 1 and Table 1.
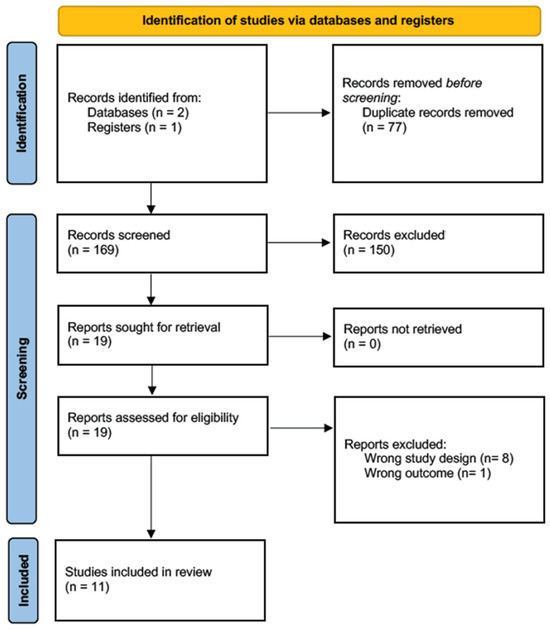
Figure 1.
Prisma flow chart.
The studies cumulatively reported on 14 patients who were diagnosed with SCC post-breast implant insertion. The age at diagnosis ranged from 40 to 81 years, with the follow-up periods extending from one month to eight years. Importantly, the latency period from implant insertion to diagnosis for all patients exceeded 11 years, with a range of 11 to 42 years. See Table 2.

Table 2.
Study characteristics.
Among the patients, three had no recorded personal or family history of cancer. One patient had a history of metastatic colon cancer, one had a history of invasive ductal carcinoma status post-mastectomy, and one had a history of right breast carcinoma. For the remaining nine patients, this information was not available. Ten patients had undergone implantation for the purpose of cosmetic breast augmentation, and four patients had undergone implantation due to reconstruction. Typical clinical presentations of the BIA-SCC in our cohort included breast swelling, erythema, enlargement, and localized pain. See Table 3.

Table 3.
Patient characteristics.
Out of the 14 patients, 3 had expired at the time of the last follow-up. Of these three, one individual exhibited distant metastases to the liver, lungs, and retroperitoneum. The second patient developed distant metastases within five months of the diagnosis. The third patient had been given palliative care one month into follow-up. Of the eleven remaining patients, six patients were reported as disease-free, one patient was in remission at 12 months after the initiation of adjuvant therapy, and one was alive but with ongoing disease (AWD). Follow-up information for the remaining three patients was unavailable.
As for implant types, 10 of the 14 patients initially received silicone implants, while the remaining 4 had saline implants. The majority of patients were treated with radical mastectomy, while the use of neoadjuvant or postoperative chemotherapy, chemoradiation, or radiotherapy was poorly recorded in the included studies. See Table 4.

Table 4.
Patient presentation and treatment.
3.2. Literature Review
3.2.1. Inflammation and Carcinogenesis
Chronic inflammation is increasingly recognized as a significant catalyst in the development of various cancer types. The relationship between chronic inflammation and cancer development posits a complex interplay of cytokines, growth factors, and immune cells, creating an environment conducive to tumorigenesis [28,29,30]. This section delves into the underlying mechanisms of this relationship, laying the groundwork for understanding specific cancer types, including BIA-SCC.
Research by Xiong et al. and Nicolò et al. underscores the role of chronic inflammation in eliciting cancer [31,32]. They highlight how long-term exposure to inflammatory stimuli, such as pathogenic infections and persistent immune response, can lead to genetic mutations and cellular transformations. This process is a critical element in the development of lung cancer, as demonstrated by Xiong et al., where Mycobacterium tuberculosis infection leads to chronic inflammation, significantly contributing to lung cancer progression [31]. Similarly, Nicolò et al. discuss how bacterial infections, distinct from Lactobacillus, can induce a chronic inflammatory state, facilitating the neoplastic progression in human papillomavirus (HPV)-infected epithelial cells [32].
3.2.2. Breast Implant Material
Historically, breast implants utilized problematic materials like polyurethane and Teflon, leading to various complications. Saline-filled implants, though minimally invasive, failed to mimic the natural feel of breast tissue [33]. Innovations introduced smooth silicone elastomer shells to replicate the breast’s anatomy, yet faced challenges like displacement and capsular contracture, prompting the use of Dacron patches and textured surfaces to address these issues [33]. However, textured implants brought new problems, such as seroma and BIA-ALCL. Modern silicone-based implants feature a gel filler and elastomer shell, designed with varying viscosities and textures to optimize outcomes. Continuous advancements aim to refine implant surfaces, balancing technological improvements with persistent challenges [33].
3.2.3. Chronic Inflammation: A Catalyst for Tumorigenesis
Chronic inflammation can drive tumorigenesis through various mechanisms, primarily involving immune cell recruitment and the release of pro-inflammatory cytokines [28,29,30]. Gubernatorova et al. emphasize the dual role of tumor necrosis factor (TNF) and lymphotoxin (LTα) in cancer. In their study, different mouse models showed that TNF can drive lung metastasis, providing another example of inflammation-induced carcinogenesis [34,35].
Other specialized immune cells have been implicated in the connection between inflammation and cancer, including macrophages [34]. The endocytosis of mycobacterium tuberculosis by alveolar macrophages and the subsequent downstream immune response have been shown to cause sustained inflammation and tissue damage, driving cancer development [31]. T cells also play a central role in the inflammatory milieu. Exhausted T cell phenotypes, enriched in granulomas, can contribute to the development of tumors. This is further affected by the expression of immune checkpoints like PD-1 and LAG-3 in these T cells, which inhibit effective immune response and facilitate tumor progression [35].
These studies collectively underscore the critical role of chronic inflammation in cancer. The recruitment of immune cells, combined with the sustained release of inflammatory cytokines and growth factors, creates an environment that promotes the proliferation and survival of cancer cells [28]. This understanding provides a crucial foundation for examining specific cancer types, particularly BIA-SCC, in the context of inflammation-induced carcinogenesis.
3.2.4. Inflammatory Mediators and Tumor Microenvironment
The tumor microenvironment (TME), heavily influenced by inflammatory mediators, plays a pivotal role in cancer progression [35,36]. Chronic inflammation within the TME facilitates cellular transformations leading to cancer. It has been demonstrated that macrophage-secreted cytokines like tumor necrosis factor (TNF) and interleukins activate cancer-associated pathways such as NF-κB in pulmonary epithelial cells. LTα in the TME has also been shown to promote tumor growth and metastasis [28]. This highlights the complexity of the inflammatory response in the TME and its impact on cancer development [35,36]. Apart from cytokines, exosomes derived from macrophage-processed cancer cells may play a role in the TME. As discussed in the work of Xiang et al., breast cancer cell-derived exosomes can induce macrophages into an M2 polarization state, which is typically associated with tissue repair and tumor progression [36]. These exosomes alter the TME, promoting conditions favorable for cancer growth [35,36].
3.2.5. Pathways Leading to BIA-SCC
In the context of BIA-SCC, the chronic inflammatory response to breast implants may serve as a critical factor in carcinogenesis. Doloff et al. demonstrate that different surface topographies of breast implants can induce varying immune responses, potentially leading to fibrosis and other chronic inflammatory states [37]. Their study shows that smoother implant surfaces can reduce fibrosis and immune cell recruitment, suggesting that implant surface characteristics significantly influence the local immune response.
The research by Ghosh et al. further supports this notion. Their immunopeptidomic analysis reveals that biomaterial contact can alter the immune system’s response, showing potential pathways for immune system dysregulation leading to cancer [38]. Materials used in breast implants might induce specific immune responses that could contribute to the development of BIA-SCC.
Furthermore, the studies by Xiong et al., Nicolò et al., and Gubernatorova et al. provide insights into how chronic inflammation and immune dysregulation, potentially caused by breast implants, can lead to carcinogenesis [31,32,35]. The prolonged inflammatory state, combined with the release of cytokines and growth factors, and changes in the immune cell profiles within the TME, may create conditions that promote the development of BIA-SCC.
3.2.6. Chronic Inflammation and Epigenetic Changes in Cancer
Chronic inflammation is known to induce epigenetic changes that can lead to cancer. The studies by Xiong et al. and Nicolò et al. highlight how chronic inflammatory conditions, such as those caused by infections or the presence of foreign bodies, can lead to alterations in DNA methylation and histone modification [31,32]. These epigenetic changes are crucial in the transition from a normal cell to a cancer cell. The epigenetic changes associated with chronic inflammation have been shown to be implicated in disease progression in breast cancer and cervical intraepithelial neoplasia in the context of bacterial or viral infection [32]. These mechanisms can be extrapolated to understand the development of BIA-SCC, where chronic inflammation around the implant site could lead to epigenetic modifications in the surrounding breast tissue, predisposing it to malignant transformation.
3.2.7. The Role of Exosomes and Macrophage Polarization in Tumor Progression
The studies by Xiang et al. and Doloff et al. provide insights into the role of exosomes and macrophage polarization in tumor progression [36,37]. Exosomes driving breast cancer progression, as shown by Xiang et al. in the setting of chronic infection, may also have a role in the setting of patients with breast implants [36]. Here, chronic inflammation or the implant material itself could alter the local immune response, promoting exosome production conducive to cancer development.
Doloff et al. further elucidate how different implant surface topographies can modulate the immune response, including the behavior of macrophages [37]. They demonstrate that smoother implant surfaces can lead to a reduced fibrotic and inflammatory response, suggesting that the physical characteristics of breast implants can significantly influence the local immune environment. This could have implications for the development of BIA-SCC, as a pro-inflammatory and fibrotic microenvironment around rougher implant surfaces might facilitate the transformation of adjacent breast tissue into squamous cell carcinoma.
These studies collectively suggest a complex interplay of factors in the development of BIA-SCC, involving chronic inflammation, exosome-mediated communication, macrophage polarization, and the physical properties of breast implants. Understanding these mechanisms is essential for improving the design and safety of breast implants to reduce the risk of BIA-SCC.
4. Discussion
Breast implantation, a procedure conducted for both reconstructive and esthetic enhancement of the breast, is not without its associated risks and complications [39,40]. While existing research has shed light on the relationship between breast implants and ALCL, the intersection between breast implants and SCC is relatively underexplored [41]. This dearth of data is even more significant given that SCC usually emerges around the implant capsule and appears to affect both smooth and textured as well as both silicone and saline implants.
Given the limited understanding of the pathophysiology of BIA-SCC, it is reasonable to consider chronic inflammation as a plausible underpinning [41,42,43]. Indeed, chronic inflammation has been implicated in the development of various types of cancers, and the literature on the topic posits that SCC may be mediated by sustained inflammatory processes [41,42,43,44,45,46]. Notably, inflammation could arise from mechanical factors, such as implant movement, or, even less likely, microbiological factors [41,42,43,44,45,46]. Chronic inflammation can exacerbate the production of reactive oxygen species and other pro-inflammatory cytokines, creating an environment conducive to neoplastic transformations. In other malignancies associated with breast implants like BIA-ALCL, hypotheses have already been advanced regarding the role of inflammation [46]. Indeed, BIA-ALCL disease emerges in an inflammatory microenvironment rich in lymphocyte and plasma cell infiltration, showing a pronounced Th1/Th17 phenotype in advanced stages [41,42,43,46]. Genetic alterations affecting the JAK/STAT signaling pathway are also commonly observed. This possibly provides a template for understanding BIA-SCC [41,42,43].
As we navigate further into this intricate web of potential associations between breast implants and squamous cell carcinoma, it is prudent to recognize the etiological complexity of this pathology. Both genetic predisposition and environmental triggers may synergistically contribute to the genesis of BIA-SCC, implicating a multifactorial causation model. Adding a layer of complexity is the finding that BIA-SCC is not entirely specific to any particular type of implant. This suggests that the mere presence of a foreign body within the breast may itself act as a catalyst, instigating aberrant cell proliferation irrespective of the implant’s material or textural composition. This is notably different from BIA-ALCL, which has a strong association with macro-textured implants and tissue expanders [47]. Such a supposition further intensifies the need for a multifaceted risk-assessment strategy that transcends the physical properties of the implant to encapsulate a broader range of biological, mechanical, and environmental factors.
It is noteworthy that the onset of SCC often manifests more than a decade post-implantation with a high degree of clinical heterogeneity as seen in our cohort [7,18,19,20,21,22,23,24,25,26,27]. While most patients present with swelling and pain, these symptoms are non-specific and can easily be attributed to more benign conditions, thereby introducing the risk of diagnostic inertia [7,18,19,20,21,22,23,24,25,26,27]. Therefore, heightened clinical acumen, supplemented by advanced diagnostic modalities, is integral to ensuring early detection and intervention. Furthermore, our cohort exhibited a distinctively aggressive clinical course, with a high mortality rate and resistance to conventional therapies like chemotherapy and radiotherapy [7,18,19,20,21,22,23,24,25,26,27]. Despite the aggressive course of BIA-SCC, en bloc capsulectomy appears to yield superior outcomes, highlighting the importance of complete surgical resection [7,18,19,20,21,22,23,24,25,26,27].
The ASPS/PSF offers limited recommendations that include preoperative workups and particular surgical protocols designed to facilitate the diagnosis and management of BIA-SCC and other lymphomas [1,2]. Consequently, there is a pronounced need for the assembly of an expert panel to standardize treatment protocols, ensuring data collection and effective patient management. Continuous patient education and vigilant, long-term monitoring are also pivotal in optimizing patient outcomes, given the prolonged indolent nature of BIA-SCC and its often-late presentation.
While mortality rates in our cohort were indeed alarming, it is crucial to realize that these statistics may not be universally generalizable due to the restricted scope and size of our study sample. Therefore, these findings should act as an impetus for more expansive research initiatives, incorporating a wider demographic and clinical spectrum to facilitate a more nuanced understanding of BIA-SCC. The establishment of dedicated sub-registries for BIA-SCC would serve as invaluable repositories of clinical data, aiding in the formulation of more evidence-based diagnostic and therapeutic algorithms.
5. Limitations
While this paper serves as a comprehensive compilation of available data on BIA-SCC, its intrinsic limitations lie in the nature of the included studies, which are mostly case reports and series. Therefore, further robust, multicentric research is essential to bridge the existing knowledge gap and to refine the clinical guidelines for the effective management of BIA-SCC. We must exercise caution when extrapolating the pathogenetic mechanisms identified in other malignancies and SCC instances to BIA-SCC, as discussed in this review. These mechanisms are presented as theoretical possibilities, offering insight into potential biological pathways that could be relevant to BIA-SCC’s pathogenesis.
6. Conclusions
In conclusion, our understanding of BIA-SCC remains in its nascent stages. It is an amalgam of incomplete data, emerging theories, and clinical observations that warrant rigorous scientific investigation. By adopting a methodical, multidisciplinary approach that integrates advancements in molecular biology, surgical techniques, and patient care protocols, we have the potential to unravel the complexities of BIA-SCC. These collective efforts are not only academic endeavors but also ethical imperatives, with the aim of protecting the wellbeing of the numerous individuals who opt for breast implantation each year.
Author Contributions
A.C., J.A.F. and O.R. were instrumental in conceptualizing and initiating the study. A.C. and M.M. assisted significantly in drafting the research protocol. In the screening process, J.A.F., A.C., I.C.T., A.H.A., S.N, D.L. and R.W. were primarily responsible. M.Z. and M.M. offered their expertise as subject-matter experts, contributing substantially to both the initial draft and its subsequent revisions. K.A.S. played a crucial role in refining the research methodology and in the comprehensive literature review. S.J.L. oversaw the project, providing essential guidance and oversight. J.A.F., G.J.L. and T.C.L. conceptualized and generated the literature review portion of the manuscript. All authors, including A.C., J.A.F., O.R., A.C., M.M., I.C.T., A.H.A., D.L., R.W., G.J.L., T.C.L., M.Z., S.N. and K.A.S. were actively engaged in the revision process and gave their approval to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article. All information relevant to this systematic review is part of the manuscript, figures, tables, and/or digital supplemental content. Additional information can be found within the publicly available PROSPERO protocol for this study. If any further information is required, the reader may contact the corresponding author for clarifications.
Acknowledgments
We sincerely thank Victor Joao, affiliated with the Universidad De La Republica Uruguay, for his help as the active librarian for this project. He helped with creating search strategies and gathering the search results for this manuscript.
Conflicts of Interest
All authors declared that there are no conflicts of interest.
References
- Jewell, M.L.; Walden, J.L.; Fontbona, M.; Triana, L. US FDA Safety Communication on Breast Implant Associated Squamous Cell Carcinoma BIA-SCC). Aesthetic Plast. Surg. 2023, 47, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S. How to Respond to the US Food and Drug Administration’s Report on Squamous Cell Carcinoma in Breast Implant Capsules. Aesthetic Surg. J. 2023, 43, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Chien, P.N.; Trinh, X.-T.; Nam, S.-Y.; Heo, C.-Y. Comparison of Formation of Capsule Among Different Breast Silicone Implants. In Vivo 2022, 36, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Bayston, R. Capsule formation around breast implants. JPRAS Open 2022, 31, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Headon, H.; Kasem, A.; Mokbel, K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch. Plast. Surg. 2015, 42, 532–543. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Whaley, R.D.; Aldrees, R.; Dougherty, R.E.; Prieto Granada, C.; Badve, S.S.; Al Diffalha, S. Breast Implant Capsule-Associated Squamous Cell Carcinoma: Report of 2 Patients. Int. J. Surg. Pathol. 2022, 30, 900–907. [Google Scholar] [CrossRef]
- Glasberg, S.B.; Sommers, C.A.; McClure, G.T. Breast Implant-associated Squamous Cell Carcinoma: Initial Review and Early Recommendations. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5072. [Google Scholar] [CrossRef]
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Gilmour, A.; Jones, G.; O’Donoghue, J.M.; Clemens, M.W. A Systematic Review of Outcomes Following Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). JPRAS Open 2022, 34, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Tevis, S.E.; Hunt, K.K.; Miranda, R.N.; Lange, C.; Butler, C.E.; Clemens, M.W. Differences in Human Leukocyte Antigen Expression Between Breast Implant-Associated Anaplastic Large Cell Lymphoma Patients and the General Population. Aesthetic Surg. J. 2019, 39, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Tan, Y.; Lv, W.; Zhao, C.; Xiong, M.; Hou, K.; Wu, M.; Ren, Y.; Zeng, N.; et al. Current Progress in Breast Implant-Associated Anaplastic Large Cell Lymphoma. Front. Oncol. 2021, 11, 785887. [Google Scholar] [CrossRef] [PubMed]
- Niraula, S.; Katel, A.; Barua, A.; Weiss, A.; Strawderman, M.S.; Zhang, H.; Manrique, O.; O’Connell, A.; Pandey, S.R.; Dhakal, A. A Systematic Review of Breast Implant-Associated Squamous Cell Carcinoma. Cancers 2023, 15, 4516. [Google Scholar] [CrossRef] [PubMed]
- Bayareh-Mancilla, R.; Medina-Ramos, L.A.; Toriz-Vázquez, A.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Automated Computer-Assisted Medical Decision-Making System Based on Morphological Shape and Skin Thickness Analysis for Asymmetry Detection in Mammographic Images. Diagnostics 2023, 13, 3440. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Shamseer, L.; Tricco, A.C. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 2018, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Goldberg, M.T.; Llaneras, J.; Willson, T.D.; Boyd, J.B.; Venegas, R.J.; Dauphine, C.; Kalantari, B.N. Squamous Cell Carcinoma Arising in Breast Implant Capsules. Ann. Plast. Surg. 2021, 86, 268–272. [Google Scholar] [CrossRef]
- Paletta, C.; Paletta, F.X., Jr.; Paletta, F.X., Sr. Squamous cell carcinoma following breast augmentation. Ann. Plast. Surg. 1992, 29, 425–429; discussion 429–432. [Google Scholar] [CrossRef]
- Kitchen, S.B.; Paletta, C.E.; Shehadi, S.I.; Bauer, W.C. Epithelialization of the lining of a breast implant capsule. Possible origins of squamous cell carcinoma associated with a breast implant capsule. Cancer 1994, 73, 1449–1452. [Google Scholar] [CrossRef]
- Olsen, D.L.; Keeney, G.L.; Chen, B.; Visscher, D.W.; Carter, J.M. Breast implant capsule-associated squamous cell carcinoma: A report of 2 cases. Hum. Pathol. 2017, 67, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zomerlei, T.A.; Samarghandi, A.; Terando, A.M. Primary Squamous Cell Carcinoma Arising from a Breast Implant Capsule. Plast. Reconstr. Surg. Glob. Open 2015, 3, e586. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, P.J.; Chopra, V.K.; Walker, K.L.; Rudolph, R.; Greco, R.J. Primary Squamous Cell Carcinoma Arising From a Breast Implant Capsule: A Case Report and Review of the Literature. Aesthetic Surg. J. 2018, 38, NP 97–NP 102. [Google Scholar] [CrossRef]
- Soni, S.E.; Laun, J.C.; Beard, A.S.; Kuykendall, L.V. Breast Implant Capsule-Associated Squamous Cell Carcinoma during Pregnancy: A Mimicker of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast. Reconstr. Surg. 2022, 150, 926e–928e. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Chaudhry, H.E.; Shah, A.; Andrews, J. Breast Squamous Cell Carcinoma Following Breast Augmentation. Cureus 2018, 10, e3405. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, M.B.; Nassar, A.; Mansoor, I. Squamous metaplasia on the breast implant capsule. Int. J. Surg. Pathol. 2010, 18, 570–574. [Google Scholar] [CrossRef]
- Satgunaseelan, L.; Cheung, D.; Reddy, J. Breast implant-associated squamous cell carcinoma—A rare long term complication. Pathology 2015, 47, S72–S73. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Kalisperati, P.; Spanou, E.; Pateras, I.S.; Korkolopoulou, P.; Varvarigou, A.; Karavokyros, I.; Gorgoulis, V.G.; Vlachoyiannopoulos, P.G.; Sougioultzis, S. Inflammation, DNA Damage, Helicobacter pylori and Gastric Tumorigenesis. Front. Genet. 2017, 8, 20. [Google Scholar] [CrossRef]
- Heredia-Torres, T.G.; Rincón-Sánchez, A.R.; Lozano-Sepúlveda, S.A.; Galan-Huerta, K.; Arellanos-Soto, D.; García-Hernández, M.; Garza-Juarez, A.J.; Rivas-Estilla, A.M. Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis. Viruses 2022, 14, 2762. [Google Scholar] [CrossRef]
- Xiong, K.; Sun, W.; He, Y.; Fan, L. Advances in molecular mechanisms of interaction between Mycobacterium tuberculosis and lung cancer: A narrative review. Transl. Lung Cancer Res. 2021, 10, 4012–4026. [Google Scholar] [CrossRef]
- Nicolò, S.; Antonelli, A.; Tanturli, M.; Baccani, I.; Bonaiuto, C.; Castronovo, G.; Rossolini, G.M.; Mattiuz, G.; Torcia, M.G. Bacterial Species from Vaginal Microbiota Differently Affect the Production of the E6 and E7 Oncoproteins and of p53 and p-Rb Oncosuppressors in HPV16-Infected Cells. Int. J. Mol. Sci. 2023, 24, 7173. [Google Scholar] [CrossRef]
- Kang, S.H.; Bengtson, B.P.; Heo, C.Y. Various Properties of Silicone Breast Implant Surfaces and Multimodal Techniques for the Functional Surface Modification. Clin. Plast. Surg. 2021, 48, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Parris, B.A.; O’Farrell, H.E.; Fong, K.M.; Yang, I.A. Chronic obstructive pulmonary disease (COPD) and lung cancer: Common pathways for pathogenesis. J. Thorac. Dis. 2019, 11, S2155–S2172. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Polinova, A.I.; Petropavlovskiy, M.M.; Namakanova, O.A.; Medvedovskaya, A.D.; Zvartsev, R.V.; Telegin, G.B.; Drutskaya, M.S.; Nedospasov, S.A. Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers 2021, 13, 1775. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, X.; Ma, Z.; Shi, Q.; Panteleev, M.; Ataullakhanov, F.I. Bioactive engineered scaffolds based on PCL-PEG-PCL and tumor cell-derived exosomes to minimize the foreign body reaction. Biomater. Biosyst. 2022, 7, 100055. [Google Scholar] [CrossRef]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Ghosh, M.; Hartmann, H.; Jakobi, M.; März, L.; Bichmann, L.; Freudenmann, L.K.; Mühlenbruch, L.; Segan, S.; Rammensee, H.G.; Schneiderhan-Marra, N.; et al. The Impact of Biomaterial Cell Contact on the Immunopeptidome. Front. Bioeng. Biotechnol. 2020, 8, 571294. [Google Scholar] [CrossRef] [PubMed]
- Codner, M.A.; Mejia, J.D.; Locke, M.B.; Mahoney, A.; Thiels, C.; Nahai, F.R.; Hester, T.R.; Nahai, F. A 15-year experience with primary breast augmentation. Plast. Reconstr. Surg. 2011, 127, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.B.; Packowski, K.; Horick, N.; Rosado, N.; Chinta, S.; Koh, D.J.; Sobti, N.; Specht, M.C.; Liao, E.C. The Timing of Acute and Late Complications Following Mastectomy and Implant-based Reconstruction. Ann. Surg. 2023, 278, e203–e208. [Google Scholar] [CrossRef]
- Prothe, J.; Rozovics, P.; Sykes, R.; Taccona, M. Breast Implant Surgery: An Overview of the Risks and Health Complications. Plast Aesthetic Nurs. 2023, 43, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.D.; Inghirami, G.; Miranda, R.N.; Kadin, M.E. Cell of Origin and Immunologic Events in the Pathogenesis of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Am. J. Pathol. 2020, 190, 2–10. [Google Scholar] [CrossRef]
- Deva, A.K.; Turner, S.D.; Kadin, M.E.; Magnusson, M.R.; Prince, H.M.; Miranda, R.N.; Inghirami, G.G.; Adams, W.P., Jr. Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research. Cancers 2020, 12, 3861. [Google Scholar] [CrossRef]
- Tang, L.; Wang, K. Chronic Inflammation in Skin Malignancies. J. Mol. Signal 2016, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis. Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef] [PubMed]
- Fracol, M.; Miranda, R.; Rodriguez, M.; Santanelli di Pompeo, F.; Hunt, K.; Clemens, M. 9. Breast Implant Associated Chronic Inflammatory Malignancies. Plast. Reconstr. Surg. Glob. Open. 2023, 11, 5. [Google Scholar] [CrossRef]
- Nelson, J.A.; McCarthy, C.; Dabic, S.; Polanco, T.; Chilov, M.; Mehrara, B.J.; Disa, J.J. BIA-ALCL and Textured Breast Implants: A Systematic Review of Evidence Supporting Surgical Risk Management Strategies. Plast. Reconstr. Surg. 2021, 147, 7s–13s. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).