Deciphering the Mechanism of Action of the Antimicrobial Peptide BP100
Abstract
:1. Introduction
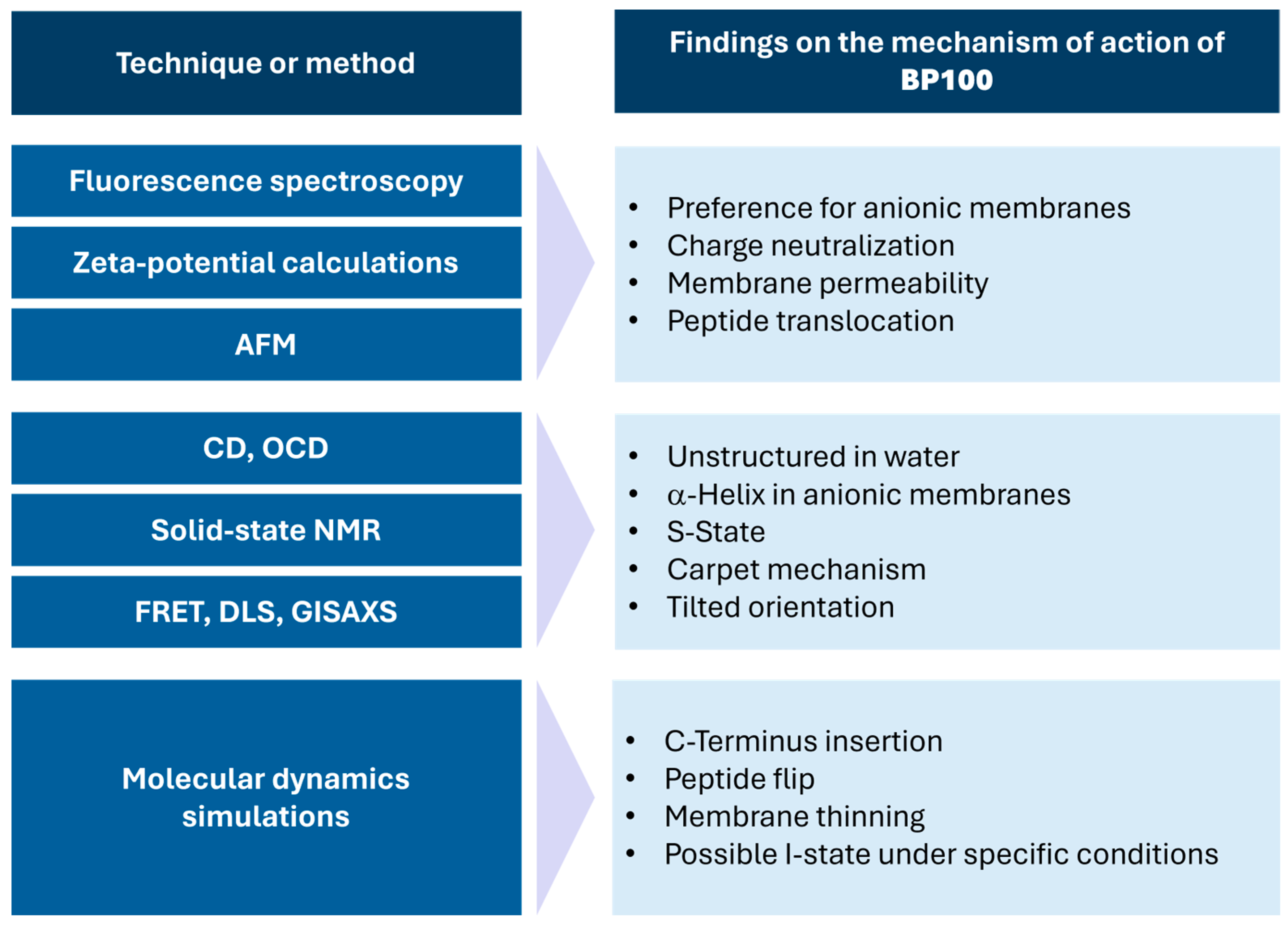
2. Mechanistic Studies on BP100
2.1. Mechanistic Studies Using Biophysical Analysis
2.2. Mechanistic Studies Using NMR Spectroscopy
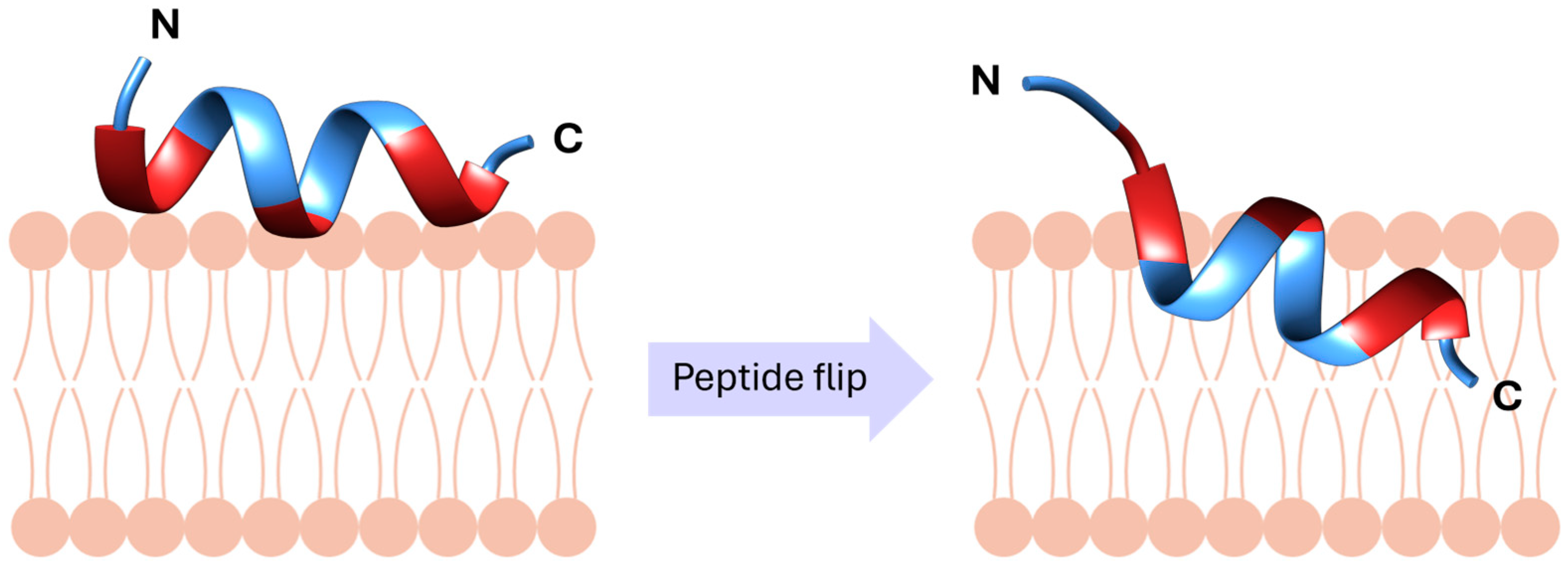
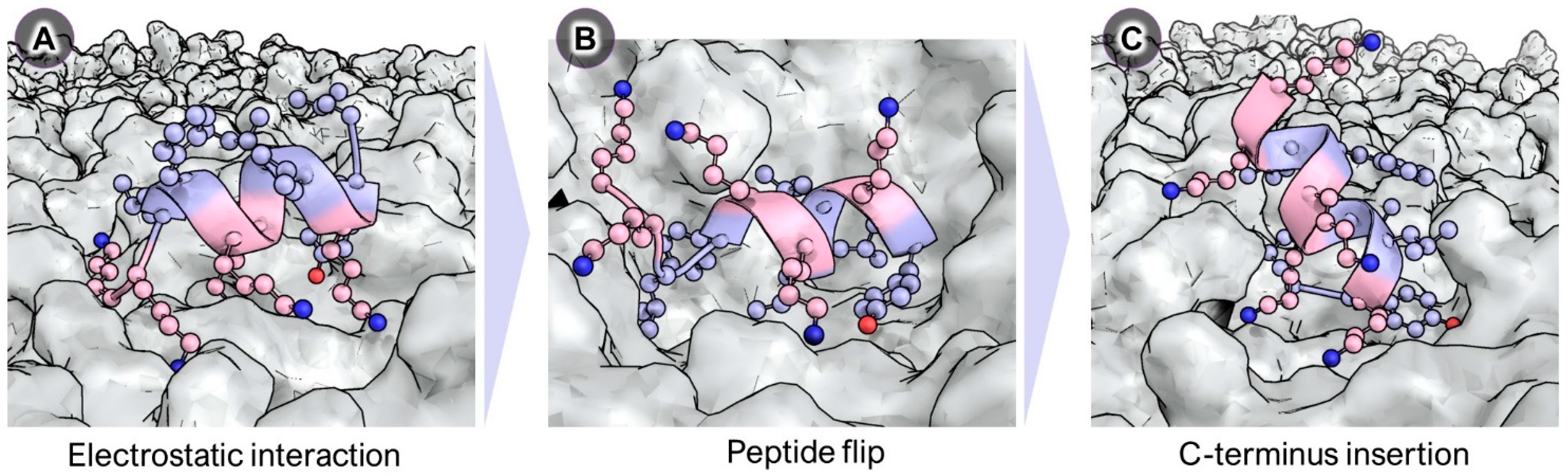
2.3. Mechanistic Studies Using Molecular Dynamics Simulations
3. Mechanistic Studies on BP100 Derivatives
| BP100 Derivative | Method or Technique | System | Main Findings | Refs. |
|---|---|---|---|---|
| RRLFRRILRWL-NH2 (RW-BP100) RRLFRRILRYL-NH2 (R-BP100) |
| LUVs, SUVs:
|
| [38] |
| WKKLFKKILKYL-NH2 (W-BP100) |
| LUVs:
|
| [39,40] |
| BP100-Ala-NH-C16H33 cyclo(1-4)-cILC-BP100 (Figure 4) |
| LUVs
|
| [41] |
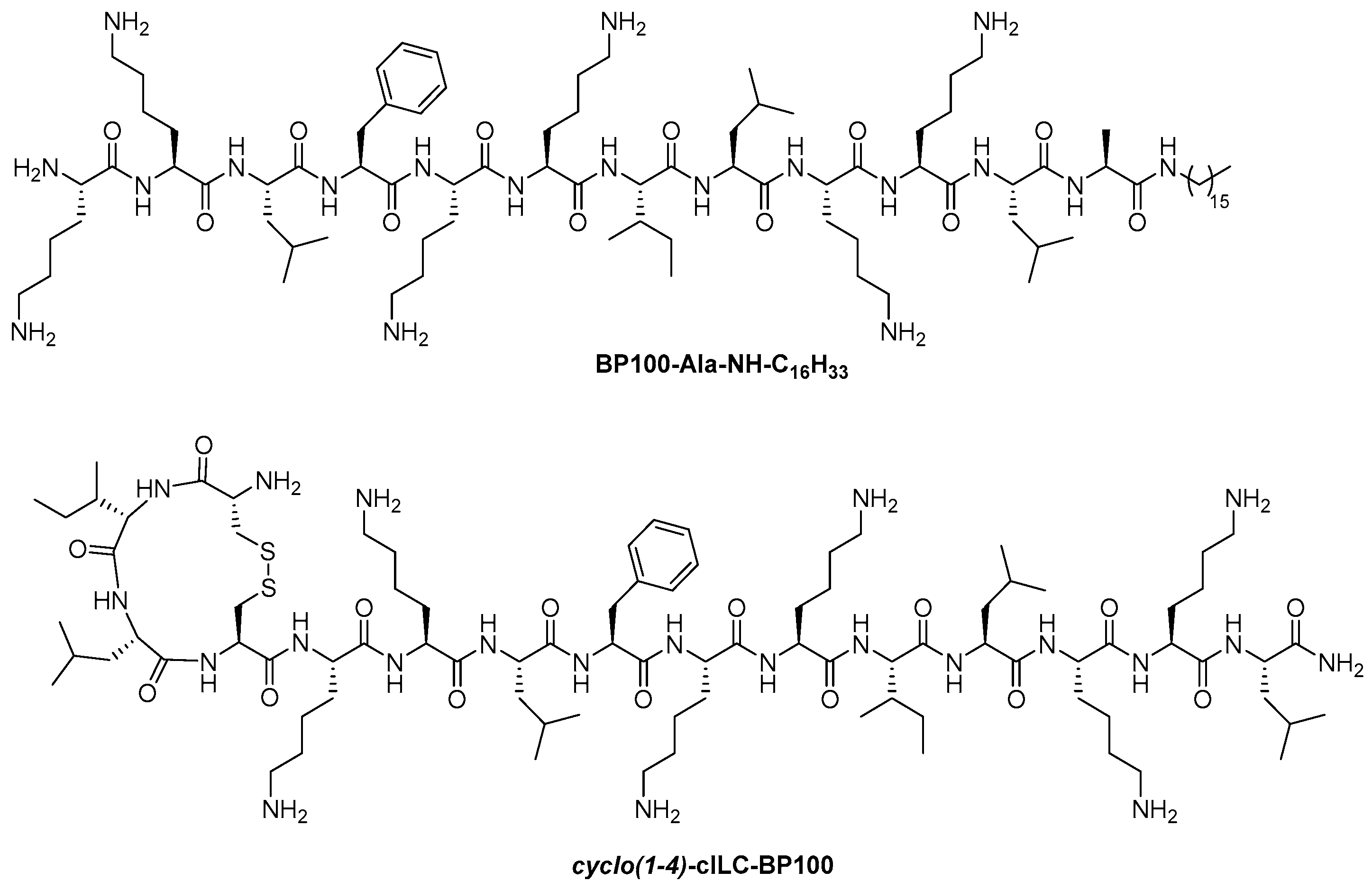
4. Final Remarks and Conclusions
Funding
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| BD | Brownian dynamics |
| CD | circular dichroism |
| CF3-l-Bpg | 3-(trifluoromethyl)-l-bicyclopent-[1.1.1]-1-ylglycine |
| CPP | cell-penetrating peptide |
| DErPC | 1,2-dierucoyl-sn-glycero-3-phosphatidylcholine |
| DErPG | 1,2-dierucoyl-sn-glycero-3-phosphatidylglycerol |
| DLPC | 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine |
| DLPG | 1,2-dilauroyl-sn-glycero-3-phosphoglycerol |
| DLS | dynamic light scattering |
| DMoPC | 1,2-dimyristoleoyl-sn-glycero-3-phosphatidylcholine |
| DMoPG | 1,2-dimyristoleoyl-sn-glycero-3-phosphatidylglycerol |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine |
| DMPG | 1,2-dimyristoyl-sn-glycero-3-phosphatidylglycerol |
| DPhPC | 1,2-diphytanoyl-sn-glycero-3-phosphocholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine |
| DPPG | 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol |
| FRET | Förster resonance energy transfer |
| GISAXS | grazing incidence small angle x-ray scattering |
| GUVs | giant unilamellar vesicles |
| LPS | lipopolysaccharides |
| LTA | lipoteichoic acid |
| LUVs | large unilamellar vesicles |
| lyso-LPC | 1-lauroyl-2-hydroxy-sn-glycero-3-phosphatidylcholine |
| lyso-MPC | 1-myristoyl-2-hydroxy-sn-glycero-3-phosphatidylcholine |
| MD | molecular dynamics |
| MIC | minimum inhibitory concentration |
| NMR | nuclear magnetic resonance |
| NTA | nanoparticle tracking analysis |
| OCD | oriented circular dichroism |
| P/L | peptide/lipid ratio |
| PC | phosphatidylcholine |
| PG | phosphatidylglycerol |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3phosphatidylethanolamine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-(phosphor-rac-(1-glycerol)) |
| POPS | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylserine |
| SM | sphingomyelin |
| SPR | surface plasmon resonance |
| SUVs | small unilamellar vesicles |
| TFE | trifluoroethanol |
References
- Ikhimiukor, O.O.; Odih, E.E.; Donado-Godoy, P.; Okeke, I.N. A Bottom-up View of Antimicrobial Resistance Transmission in Developing Countries. Nat. Microbiol. 2022, 7, 757–765. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- FAO. IPPC Secretariat Scientific Review of the Impact of Climate Change on Plant Pests; FAO: Rome, Italy, 2021; ISBN 9789251344354. [Google Scholar]
- Huang, X.; Li, G. Antimicrobial Peptides and Cell-Penetrating Peptides: Non-Antibiotic Membrane-Targeting Strategies against Bacterial Infections. Infect. Drug 2023, 16, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.; Boto, A. Host-Defense Peptides as New Generation Phytosanitaries: Low Toxicity and Low Induction of Antimicrobial Resistance. Agronomy 2022, 12, 1614. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, H.; Song, Y.; Qin, Z.; Xu, L.; He, N.; Tan, Y.; Dessie, W. Advancements, Challenges and Future Perspectives on Peptide-Based Drugs: Focus on Antimicrobial Peptides. Eur. J. Pharm. Sci. 2023, 181, 106363. [Google Scholar] [CrossRef]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial Peptides (AMPs): A Promising Class of Antimicrobial Compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Badosa, E.; Ferre, R.; Planas, M.; Feliu, L.; Besalú, E.; Cabrefiga, J.; Bardají, E.; Montesinos, E. A Library of Linear Undecapeptides with Bactericidal Activity against Phytopathogenic Bacteria. Peptides 2007, 28, 2276–2285. [Google Scholar] [CrossRef]
- Badosa, E.; Planas, M.; Feliu, L.; Montesinos, L.; Bonaterra, A.; Montesinos, E. Synthetic Peptides against Plant Pathogenic Bacteria. Microorganisms 2022, 10, 1784. [Google Scholar] [CrossRef]
- Badosa, E.; Moiset, G.; Montesinos, L.; Talleda, M.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Derivatives of the Antimicrobial Peptide BP100 for Expression in Plant Systems. PLoS ONE 2013, 8, e85515. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, L.; Bundó, M.; Badosa, E.; San Segundo, B.; Coca, M.; Montesinos, E. Production of BP178, a Derivative of the Synthetic Antibacterial Peptide BP100, in the Rice Seed Endosperm. BMC Plant Biol. 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Eales, M.G.; Ferrari, E.; Goddard, A.D.; Lancaster, L.; Sanderson, P.; Miller, C. Mechanistic and Phenotypic Studies of Bicarinalin, BP100 and Colistin Action on Acinetobacter baumannii. Res. Microbiol. 2018, 169, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, K.; Mink, C.; Wadhwani, P.; Ulrich, A.S.; Nick, P. Using the Peptide BP100 as a Cell-Penetrating Tool for the Chemical Engineering of Actin Filaments within Living Plant Cells. ChemBioChem 2011, 12, 132–137. [Google Scholar] [CrossRef]
- Miyamoto, T.; Toyooka, K.; Chuah, J.-A.; Odahara, M.; Higchi-Takeuchi, M.; Goto, Y.; Motoda, Y.; Kigawa, T.; Kodama, Y.; Numata, K. A Synthetic Multidomain Peptide that Drives a Macropinocytosis-Like Mechanism for Cytosolic Transport of Exogenous Proteins into Plants. JACS Au 2022, 2, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Thomsen, T.T.; Kjelstrup, S.; Gorey, C.; Franzyk, H.; Frimodt-Møller, N.; Løbner-Olesen, A.; Hansen, P.R. An Amphipathic Undecapeptide with All D-Amino Acids Shows Promising Activity against Colistin-Resistant Strains of Acinetobacter Baumannii and a Dual Mode of Action. Antimicrob. Agents Chemother. 2016, 60, 592–599. [Google Scholar] [CrossRef]
- Yilmaz, N.; Kodama, Y.; Numata, K. Lipid Membrane Interaction of Peptide/DNA Complexes Designed for Gene Delivery. Langmuir 2021, 37, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, W.; Li, J.; Liao, C.; Yang, L.; Huang, W.; Qian, H. Synthesis and Biological Evaluation of Novel Peptides Based on Antimicrobial Peptides as Potential Agents with Antitumor and Multidrug Resistance-Reversing Activities. Chem. Biol. Drug Des. 2017, 90, 972–980. [Google Scholar] [CrossRef]
- Devi, N.; Singh, P.; Sharma, R.; Kumar, M.; Pandey, S.K.; Sharma, R.K.; Wangoo, N. A Lysine-Rich Cell Penetrating Peptide Engineered Multifunctional Gold Nanoparticle-Based Drug Delivery System with Enhanced Cellular Penetration and Stability. J. Mater. Sci. 2022, 57, 16842–16857. [Google Scholar] [CrossRef]
- Souza, F.R.; Fornasier, F.; Carvalho, A.S.; Silva, B.M.; Lima, M.C.; Pimentel, A.S. Polymer-Coated Gold Nanoparticles and Polymeric Nanoparticles as Nanocarrier of the BP100 Antimicrobial Peptide through a Lung Surfactant Model. J. Mol. Liq. 2020, 314, 113661. [Google Scholar] [CrossRef]
- Torres, L.M.F.C.; Almeida, M.T.; Santos, T.L.; Marinho, L.E.S.; de Mesquita, J.P.; da Silva, L.M.; dos Santos, W.T.P.; Martins, H.R.; Kato, K.C.; Alves, E.S.F.; et al. Antimicrobial Alumina Nanobiostructures of Disulfide- and Triazole-Linked Peptides: Synthesis, Characterization, Membrane Interactions and Biological Activity. Colloids Surf. B Biointerfaces 2019, 177, 94–104. [Google Scholar] [CrossRef]
- Güell, I.; Ferre, R.; Sørensen, K.K.; Badosa, E.; Ng-Choi, I.; Montesinos, E.; Bardají, E.; Feliu, L.; Jensen, K.J.; Planas, M. Multivalent Display of the Antimicrobial Peptides BP100 and BP143. Beilstein J. Org. Chem. 2012, 8, 2106–2117. [Google Scholar] [CrossRef]
- Ferre, R.; Melo, M.N.; Correia, A.D.; Feliu, L.; Bardaji, E.; Planas, M.; Castanho, M. Synergistic Effects of the Membrane Actions of Cecropin-Melittin Antimicrobial Hybrid Peptide BP100. Biophys. J. 2009, 96, 1815–1827. [Google Scholar] [CrossRef]
- Alves, C.S.; Melo, M.N.; Franquelim, H.G.; Ferre, R.; Planas, M.; Feliu, L.; Bardají, E.; Kowalczyk, W.; Andreu, D.; Santos, N.C.; et al. Escherichia Coli Cell Surface Perturbation and Disruption Induced by Antimicrobial Peptides BP100 and PepR. J. Biol. Chem. 2010, 285, 27536–27544. [Google Scholar] [CrossRef] [PubMed]
- Manzini, M.C.; Perez, K.R.; Riske, K.A.; Bozelli, J.C.; Santos, T.L.; Da Silva, M.A.; Saraiva, G.K.V.; Politi, M.J.; Valente, A.P.; Almeida, F.C.L.; et al. Peptide:Lipid Ratio and Membrane Surface Charge Determine the Mechanism of Action of the Antimicrobial Peptide BP100. Conformational and Functional Studies. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1985–1999. [Google Scholar] [CrossRef]
- Mink, C.; Strandberg, E.; Wadhwani, P.; Melo, M.N.; Reichert, J.; Wacker, I.; Castanho, M.A.R.B.; Ulrich, A.S. Overlapping Properties of the Short Membrane-Active Peptide BP100 with (i) Polycationic TAT and (ii) α-Helical Magainin Family Peptides. Front. Cell. Infect. Microbiol. 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, P.; Strandberg, E.; van den Berg, J.; Mink, C.; Bürck, J.; Ciriello, R.A.M.; Ulrich, A.S. Dynamical Structure of the Short Multifunctional Peptide BP100 in Membranes. Biochim. Biophys. Acta Biomembr. 2014, 1838, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, J.; Afonin, S.; Grage, S.L.; Van Den Berg, J.; Strandberg, E.; Wadhwani, P.; Ulrich, A.S. Action of the Multifunctional Peptide BP100 on Native Biomembranes Examined by Solid-State NMR. J. Biomol. NMR 2015, 61, 287–298. [Google Scholar] [CrossRef]
- Grage, S.L.; Afonin, S.; Kara, S.; Buth, G.; Ulrich, A.S. Membrane Thinning and Thickening Induced by Membrane-Active Amphipathic Peptides. Front. Cell Dev. Biol. 2016, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Carreras, H.; Strandberg, E.; Mühlhäuser, P.; Bürck, J.; Wadhwani, P.; Jiménez, M.Á.; Bruix, M.; Ulrich, A.S. Alanine Scan and 2H NMR Analysis of the Membrane-Active Peptide BP100 Point to a Distinct Carpet Mechanism of Action. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1328–1338. [Google Scholar] [CrossRef]
- Santisteban, N.P.; Morrow, M.R.; Booth, V. Effect of AMPs MSI-78 and BP100 on the Lipid Acyl Chains of 2H-Labeled Intact Gram Positive Bacteria. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183199. [Google Scholar] [CrossRef]
- Strandberg, E.; Wadhwani, P.; Bürck, J.; Anders, P.; Mink, C.; van den Berg, J.; Ciriello, R.A.M.; Melo, M.N.; Castanho, M.A.R.B.; Bardají, E.; et al. Temperature-Dependent Re-alignment of the Short Multifunctional Peptide BP100 in Membranes Revealed by Solid-State NMR Spectroscopy and Molecular Dynamics Simulations. ChemBioChem 2023, 24, e202200602. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.S.; Kairys, V.; Castanho, M.A.R.B.; Fernandes, M.X. Interaction of Antimicrobial Peptides, BP100 and PepR, with Model Membrane Systems as Explored by Brownian Dynamics Simulations on a Coarse-Grained Model. Biopolymers 2012, 98, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, T.; Wei, D.; Strandberg, E.; Ulrich, A.S.; Ulmschneider, J.P. How Reliable are Molecular Dynamics Simulations of Membrane Active Antimicrobial Peptides? Biochim. Biophys. Acta Biomembr. 2014, 1838, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Franco, L.R.; Chaimovich, H.; Coutinho, K.; Cuccovia, I.M.; Lima, F.S. Binding and Flip as Initial Steps for BP100 Antimicrobial Actions. Sci. Rep. 2019, 9, 8622. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.R.; Park, P.; Chaimovich, H.; Coutinho, K.; Cuccovia, I.M.; Lima, F.S. Simulations Reveal that Antimicrobial BP100 Induces Local Membrane Thinning, Slows Lipid Dynamics and Favors Water Penetration. RSC Adv. 2022, 12, 4573–4588. [Google Scholar] [CrossRef] [PubMed]
- Torcato, I.M.; Huang, Y.-H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.R.B.; Troeira Henriques, S. Design and Characterization of Novel Antimicrobial Peptides, R-BP100 and RW-BP100, with Activity against Gram-Negative and Gram-Positive Bacteria. Biochim. Biophys. Acta Biomembr. 2013, 1828, 944–955. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Teixeira, C.; Sousa, C.F.; Bessa, L.J.; Gomes, P.; Gameiro, P. How Insertion of a Single Tryptophan in the N-Terminus of a Cecropin A-Melittin Hybrid Peptide Changes its Antimicrobial and Biophysical Profile. Membranes 2021, 11, 48. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Ferreira, M.; Nunes, C.; Reis, S.; Teixeira, C.; Gomes, P.; Gameiro, P. The Unusual Aggregation and Fusion Activity of the Antimicrobial Peptide W-BP100 in Anionic Vesicles. Membranes 2023, 13, 138. [Google Scholar] [CrossRef]
- Carretero, G.P.B.; Saraiva, G.K.V.; Cauz, A.C.G.; Rodrigues, M.A.; Kiyota, S.; Riske, K.A.; dos Santos, A.A.; Pinatto-Botelho, M.F.; Bemquerer, M.P.; Gueiros-Filho, F.J.; et al. Synthesis, Biophysical and Functional Studies of Two BP100 Analogues Modified by a Hydrophobic Chain and a Cyclic Peptide. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1502–1516. [Google Scholar] [CrossRef]

| Method or Technique | System | Main Findings | Refs. |
|---|---|---|---|
| LUVs:
|
| [24] |
| Bacterium E. coli |
| [25] |
| LUVs:
|
| [26] |
| SUVs:
|
| [27] |
| SUVs and oriented bilayers:
|
| [28] |
| Oriented bilayers
|
| [29] |
| Oriented bilayers
|
| [30] |
| Oriented bilayers
|
| [31] |
| 2H-Enriched membrane of bacterium B. subtilis |
| [32] |
| Oriented bilayers:
|
| [33] |
| Model membranes:
|
| [34] |
| Model bilayers:
|
| [35] |
| Model bilayers:
|
| [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riesco-Llach, G.; Llanet-Ferrer, S.; Planas, M.; Feliu, L. Deciphering the Mechanism of Action of the Antimicrobial Peptide BP100. Int. J. Mol. Sci. 2024, 25, 3456. https://doi.org/10.3390/ijms25063456
Riesco-Llach G, Llanet-Ferrer S, Planas M, Feliu L. Deciphering the Mechanism of Action of the Antimicrobial Peptide BP100. International Journal of Molecular Sciences. 2024; 25(6):3456. https://doi.org/10.3390/ijms25063456
Chicago/Turabian StyleRiesco-Llach, Gerard, Sergi Llanet-Ferrer, Marta Planas, and Lidia Feliu. 2024. "Deciphering the Mechanism of Action of the Antimicrobial Peptide BP100" International Journal of Molecular Sciences 25, no. 6: 3456. https://doi.org/10.3390/ijms25063456
APA StyleRiesco-Llach, G., Llanet-Ferrer, S., Planas, M., & Feliu, L. (2024). Deciphering the Mechanism of Action of the Antimicrobial Peptide BP100. International Journal of Molecular Sciences, 25(6), 3456. https://doi.org/10.3390/ijms25063456








