Genome-Wide Identification of LOX Gene Family and Its Expression Analysis under Abiotic Stress in Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
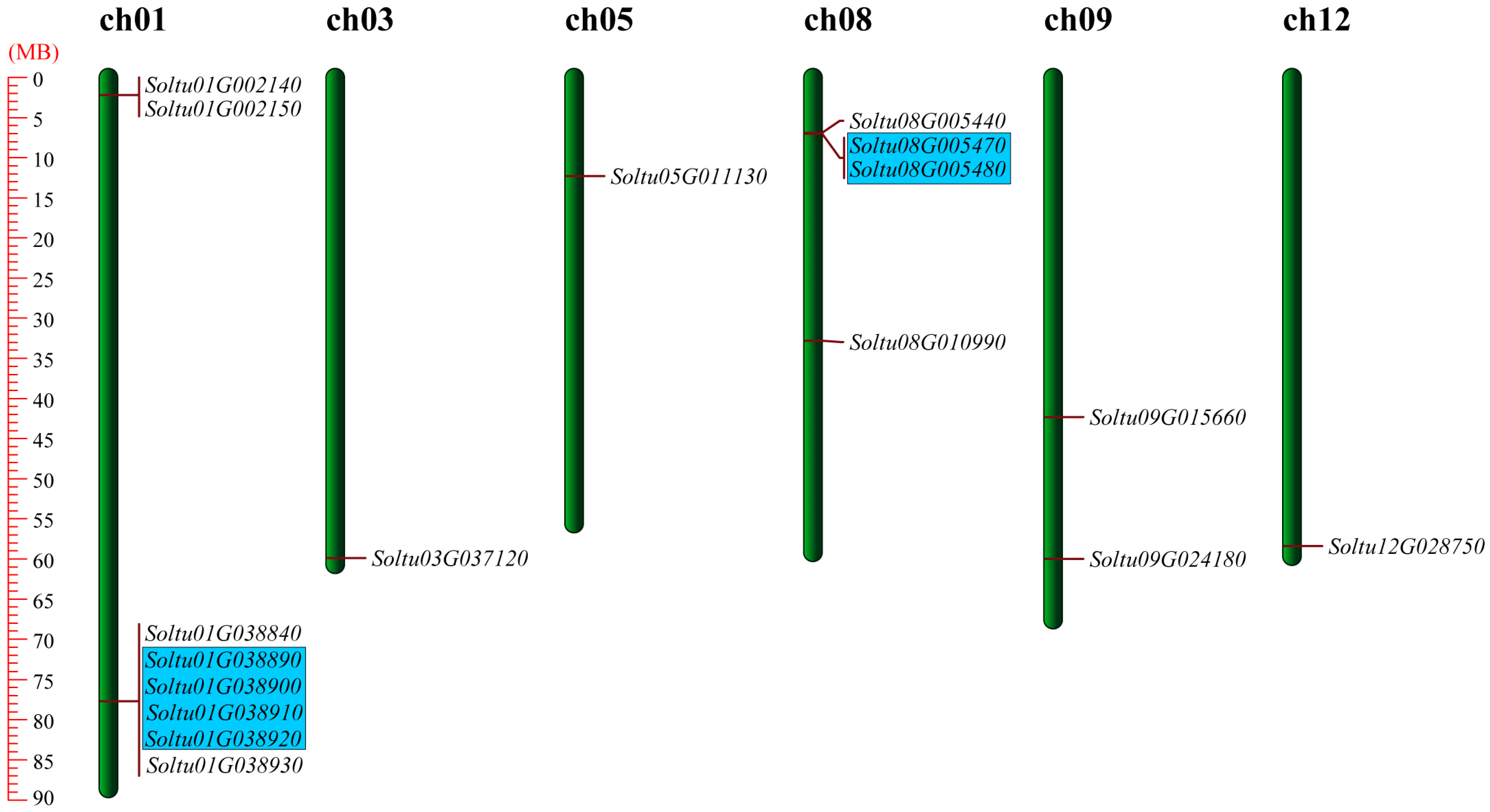
2.1. Identification of StLOXs
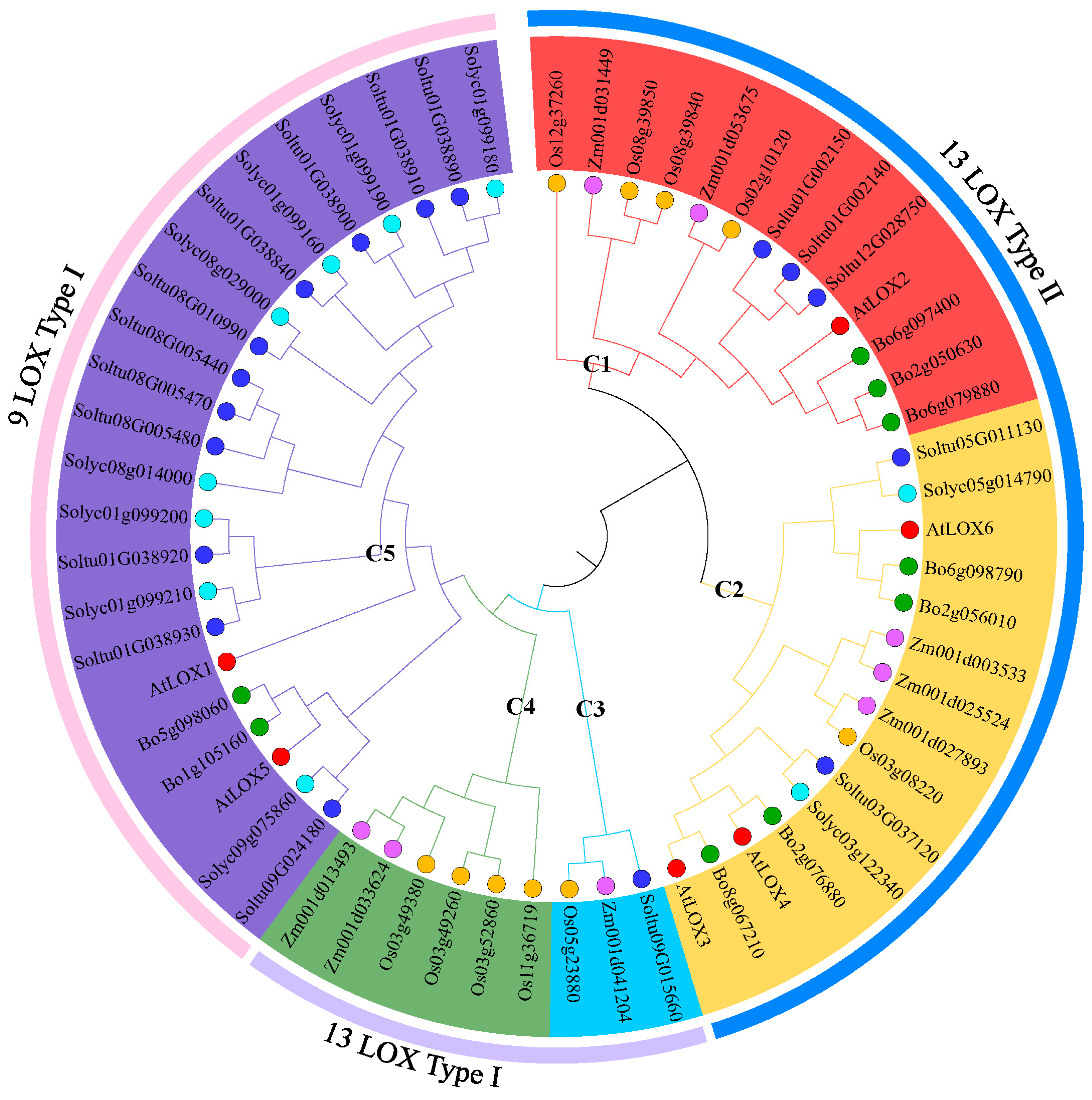
2.2. Phylogenetic Analysis and Classification of StLOXs
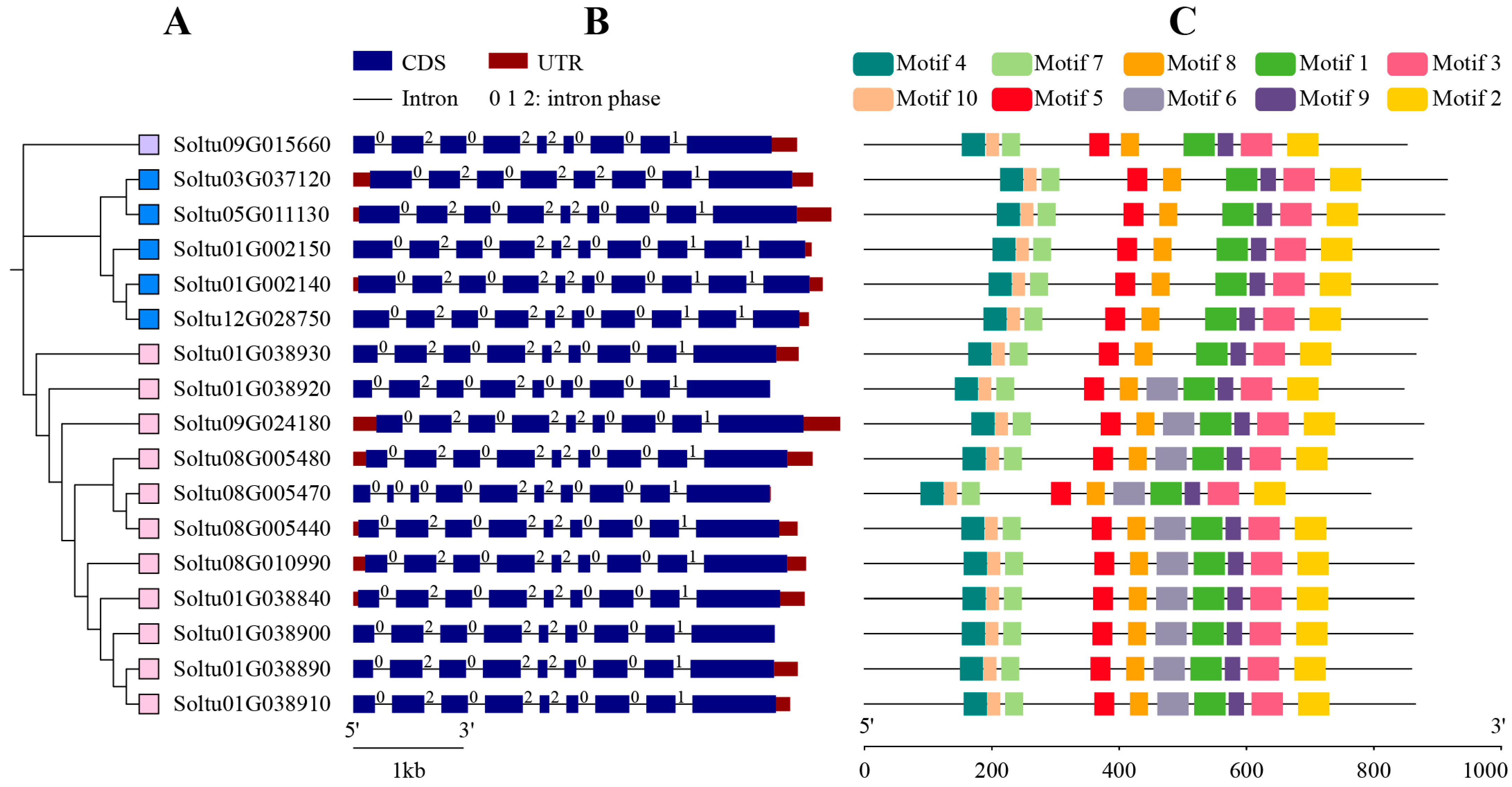
2.3. Gene Structure and Conserved Motifs of StLOXs
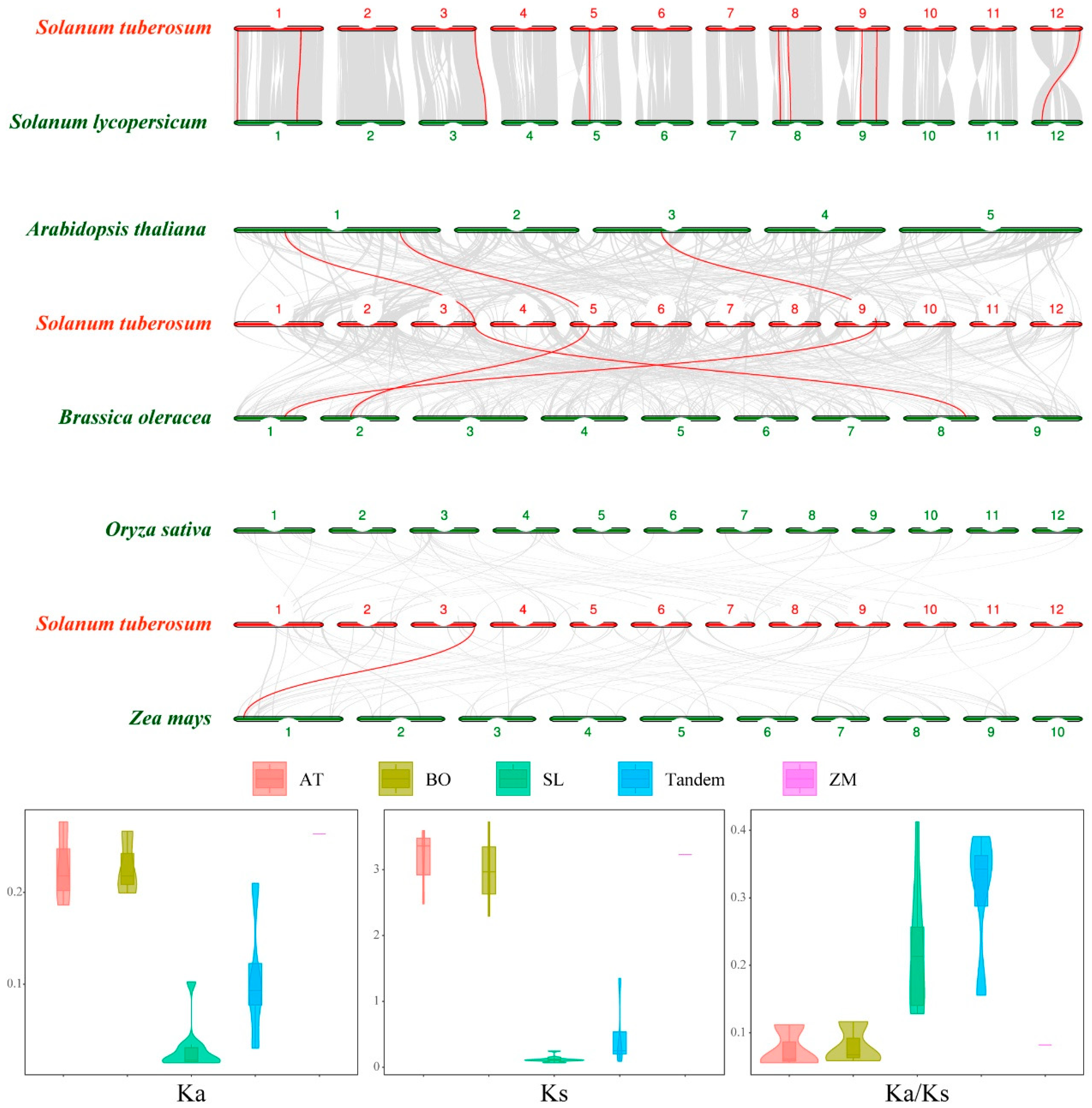
2.4. Gene Duplication Events in the StLOX and Analysis of Synteny with Other Species
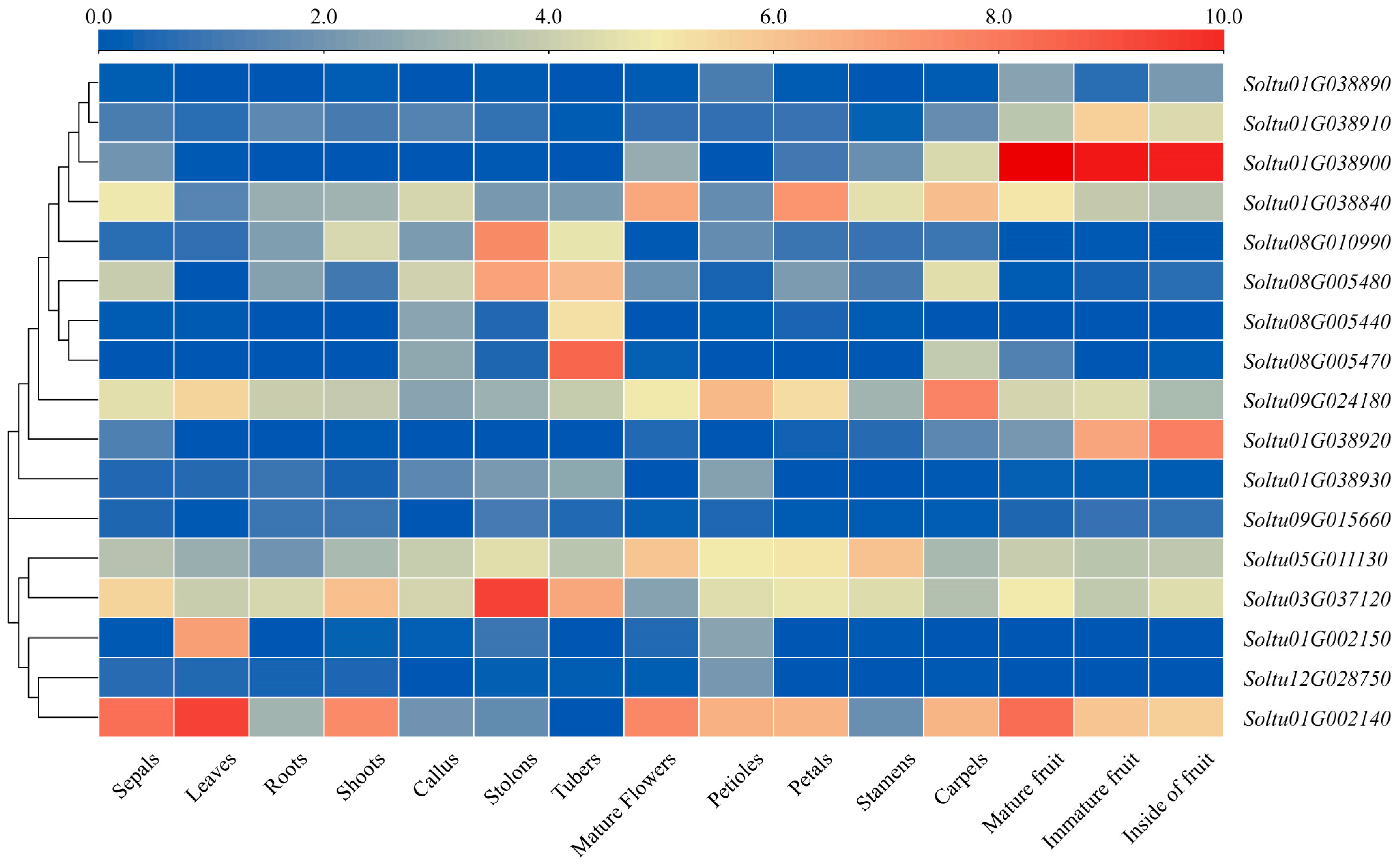
2.5. Patterns of StLOX Gene Expression across Different Tissues in DM Potato
2.6. Patterns of StLOX Gene Expression in Response to Stress Conditions and Hormonal Treatments
2.7. Expression of Six StLOX Genes in Potato Plantlets under NaCl and PEG Treatments
2.8. Cloning and Functional Analysis of StLOX Gene
3. Discussion
4. Materials and Methods
4.1. Identification of LOX Gene Family Members and Analysis of Protein Physicochemical Properties in Potato
4.2. Phylogenetic Tree Construction of LOXs
4.3. Conserved Motif and Gene Structural Characterization of StLOXs
4.4. Duplication Events and Syntenic Patterns of LOX Genes
4.5. Transcriptional Profile Analysis of StLOXs in Potato
4.6. Plant Materials and Treatments
4.7. Extraction of RNA and qPCR
4.8. Cloning of the 3 StLOX Genes
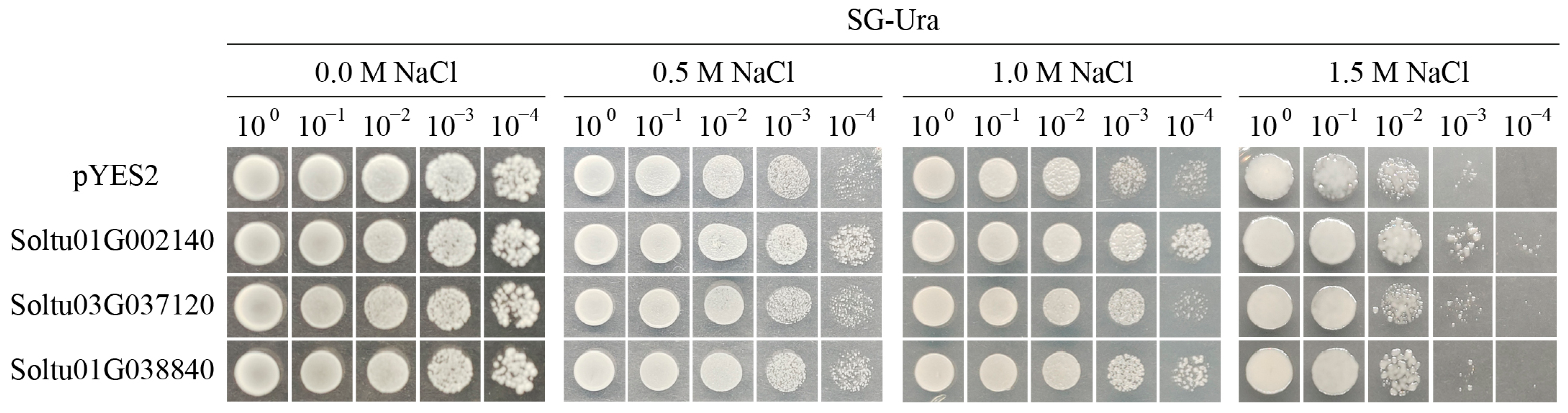
4.9. Salt Stress Tolerance Verification of Candidate Genes in Yeast
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Terp, N.; Göbel, C.; Brandt, A.; Feussner, I. Lipoxygenases during Brassica napus seed germination. Phytochemistry 2006, 67, 2030–2040. [Google Scholar] [CrossRef]
- Walper, E.; Weiste, C.; Mueller, M.J.; Hamberg, M.; Dröge-Laser, W. Screen Identifying Arabidopsis Transcription Factors Involved in the Response to 9-Lipoxygenase-Derived Oxylipins. PLoS ONE 2016, 11, e0153216. [Google Scholar] [CrossRef]
- Huang, D.; Ma, F.; Wu, B.; Lv, W.; Xu, Y.; Xing, W.; Chen, D.; Xu, B.; Song, S. Genome-Wide Association and Expression Analysis of the Lipoxygenase Gene Family in Passiflora edulis Revealing PeLOX4 Might Be Involved in Fruit Ripeness and Ester Formation. Int. J. Mol. Sci. 2022, 23, 12496. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Gardner, H.W. Lipoxygenase-derived aldehydes inhibit fungi pathogenic on soybean. J. Chem. Ecol. 1993, 19, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Zhang, B.; Li, H. The Responses of the Lipoxygenase Gene Family to Salt and Drought Stress in Foxtail Millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant Lipoxygenase: Structure and Function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Bezáková, L.; Holková, I.; Balazová, A.; Obložinský, M.; Mikuš, P. Animal lipoxygenases—Structure, positional specificity of lipoxygenase isoenzymes and their importance. Chem. Listy 2017, 111, 567–574. [Google Scholar]
- Chen, H.M.; Zhu, Z.J.; Chen, J.J.; Yang, R.; Luo, Q.J.; Xu, J.L.; Shan, H.; Yan, X.J. A multifunctional lipoxygenase from Pyropia haitanensis—The cloned and functioned complex eukaryotic algae oxylipin pathway enzyme. Algal Res. 2015, 12, 316–327. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Rybakov, Y.A.; Afanasieva, T.P.; Bachurina, G.P.; Lukina, G.P.; Ezhova, I.E.; Nosova, A.V.; Artjushkina, T.V.; Sineokii, S.P.; Kritskii, M.S. Characterization of Lipoxygenase from Fungi of the Genus Mortierella. Appl. Biochem. Microbiol. 2001, 37, 473–479. [Google Scholar] [CrossRef]
- Teng, L.; Han, W.; Fan, X.; Xu, D.; Zhang, X.; Dittami, S.M.; Ye, N. Evolution and Expansion of the Prokaryote-Like Lipoxygenase Family in the Brown Alga Saccharina japonica. Front. Plant Sci. 2017, 8, 295968. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Steczko, J.; Stec, B.; Otwinowski, Z.; Bolin, J.T.; Walter, R.; Axelrod, B. Crystal Structure of Soybean Lipoxygenase L-1 at 1.4 Å Resolution. Biochemistry 1996, 35, 10687–10701. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef]
- Bannenberg, G.; Martínez, M.; Hamberg, M.; Castresana, C. Diversity of the Enzymatic Activity in the Lipoxygenase Gene Family of Arabidopsis thaliana. Lipids 2009, 44, 85–95. [Google Scholar] [CrossRef]
- Chauvin, A.; Caldelari, D.; Wolfender, J.-L.; Farmer, E.E. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: A role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013, 197, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins Produced by the 9-Lipoxygenase Pathway in Arabidopsis Regulate Lateral Root Development and Defense Responses through a Specific Signaling Cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef]
- Hou, Y.; Ban, Q.; Meng, K.; He, Y.; Han, S.; Jin, M.; Rao, J. Overexpression of persimmon 9-lipoxygenase DkLOX3 confers resistance to Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis. Plant Growth Regul. 2018, 84, 179–189. [Google Scholar] [CrossRef]
- Lim, C.W.; Han, S.-W.; Hwang, I.S.; Kim, D.S.; Hwang, B.K.; Lee, S.C. The Pepper Lipoxygenase CaLOX1 Plays a Role in Osmotic, Drought and High Salinity Stress Response. Plant Cell Physiol. 2015, 56, 930–942. [Google Scholar] [CrossRef]
- Hu, T.; Zeng, H.; Hu, Z.; Qv, X.; Chen, G. Overexpression of the Tomato 13-Lipoxygenase Gene TomloxD Increases Generation of Endogenous Jasmonic Acid and Resistance to Cladosporium fulvum and High Temperature. Plant Mol. Biol. Report. 2013, 31, 1141–1149. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Mattoo, A.K. Genome-wide identification of tomato (Solanum lycopersicum L.) lipoxygenases coupled with expression profiles during plant development and in response to methyl-jasmonate and wounding. J. Plant Physiol. 2018, 231, 318–328. [Google Scholar] [CrossRef]
- Vogt, J.; Schiller, D.; Ulrich, D.; Schwab, W.; Dunemann, F. Identification of lipoxygenase (LOX) genes putatively involved in fruit flavour formation in apple (Malus × domestica). Tree Genet. Genomes 2013, 9, 1493–1511. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Guo, L.; Xu, Q.; Zhao, S.; Li, F.; Yan, X.; Liu, S.; Wei, C. Characterization and Alternative Splicing Profiles of the Lipoxygenase Gene Family in Tea Plant (Camellia sinensis). Plant Cell Physiol. 2018, 59, 1765–1781. [Google Scholar] [CrossRef]
- Rai, A.N.; Mandliya, T.; Kulkarni, P.; Rao, M.; Suprasanna, P. Evolution and Transcriptional Modulation of Lipoxygenase Genes Under Heat, Drought, and Combined Stress in Brassica rapa. Plant Mol. Biol. Report. 2021, 39, 60–71. [Google Scholar] [CrossRef]
- Sarde, S.J.; Kumar, A.; Remme, R.N.; Dicke, M. Genome-wide identification, classification and expression of lipoxygenase gene family in pepper. Plant Mol. Biol. 2018, 98, 375–387. [Google Scholar] [CrossRef]
- Wang, J.; Hu, T.; Wang, W.; Hu, H.; Wei, Q.; Wei, X.; Bao, C. Bioinformatics Analysis of the Lipoxygenase Gene Family in Radish (Raphanus sativus) and Functional Characterization in Response to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2019, 20, 6095. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Yan, H.; Li, W.; Li, Y.; Cai, R.; Xiang, Y. The Lipoxygenase Gene Family in Poplar: Identification, Classification, and Expression in Response to MeJA Treatment. PLoS ONE 2015, 10, e0125526. [Google Scholar] [CrossRef]
- Stokstad, E. The new potato. Science 2019, 363, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 15003. [Google Scholar] [CrossRef] [PubMed]
- RoyChowdhury, M.; Li, X.; Qi, H.; Li, W.; Sun, J.; Huang, C.; Wu, D. Functional Characterization of 9-/13-LOXs in Rice and Silencing Their Expressions to Improve Grain Qualities. BioMed Res. Int. 2016, 2016, 4275904. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Shi, L.-N.; Lu, L.-X.; Ye, J.-R.; Shi, H.-M. The Endophytic Strain ZS-3 Enhances Salt Tolerance in Arabidopsis thaliana by Regulating Photosynthesis, Osmotic Stress, and Ion Homeostasis and Inducing Systemic Tolerance. Front. Plant Sci. 2022, 13, 820837. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37 (Suppl. S2), W202–W208. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, N.; Si, H.; Calderón-Urrea, A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods 2017, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yao, P.; Zhao, J.; Wu, H.; Wang, S.; Chen, Y.; Hu, M.; Wang, T.; Li, C.; Wu, Q. A Novel R2R3-MYB Transcription Factor FtMYB22 Negatively Regulates Salt and Drought Stress through ABA-Dependent Pathway. Int. J. Mol. Sci. 2022, 23, 14549. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Chen, L.; Li, Z.; Wang, W.; Qi, Z.; Li, Y.; Liu, Y.; Liu, Z. Genome-Wide Identification of LOX Gene Family and Its Expression Analysis under Abiotic Stress in Potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2024, 25, 3487. https://doi.org/10.3390/ijms25063487
Zhu J, Chen L, Li Z, Wang W, Qi Z, Li Y, Liu Y, Liu Z. Genome-Wide Identification of LOX Gene Family and Its Expression Analysis under Abiotic Stress in Potato (Solanum tuberosum L.). International Journal of Molecular Sciences. 2024; 25(6):3487. https://doi.org/10.3390/ijms25063487
Chicago/Turabian StyleZhu, Jinyong, Limin Chen, Zhitao Li, Weilu Wang, Zheying Qi, Yuanming Li, Yuhui Liu, and Zhen Liu. 2024. "Genome-Wide Identification of LOX Gene Family and Its Expression Analysis under Abiotic Stress in Potato (Solanum tuberosum L.)" International Journal of Molecular Sciences 25, no. 6: 3487. https://doi.org/10.3390/ijms25063487
APA StyleZhu, J., Chen, L., Li, Z., Wang, W., Qi, Z., Li, Y., Liu, Y., & Liu, Z. (2024). Genome-Wide Identification of LOX Gene Family and Its Expression Analysis under Abiotic Stress in Potato (Solanum tuberosum L.). International Journal of Molecular Sciences, 25(6), 3487. https://doi.org/10.3390/ijms25063487



