Agroindustrial By-Products as a Source of Biostimulants Enhancing Responses to Abiotic Stress of Horticultural Crops
Abstract
1. Introduction
2. Biostimulants
2.1. Definition and Classification
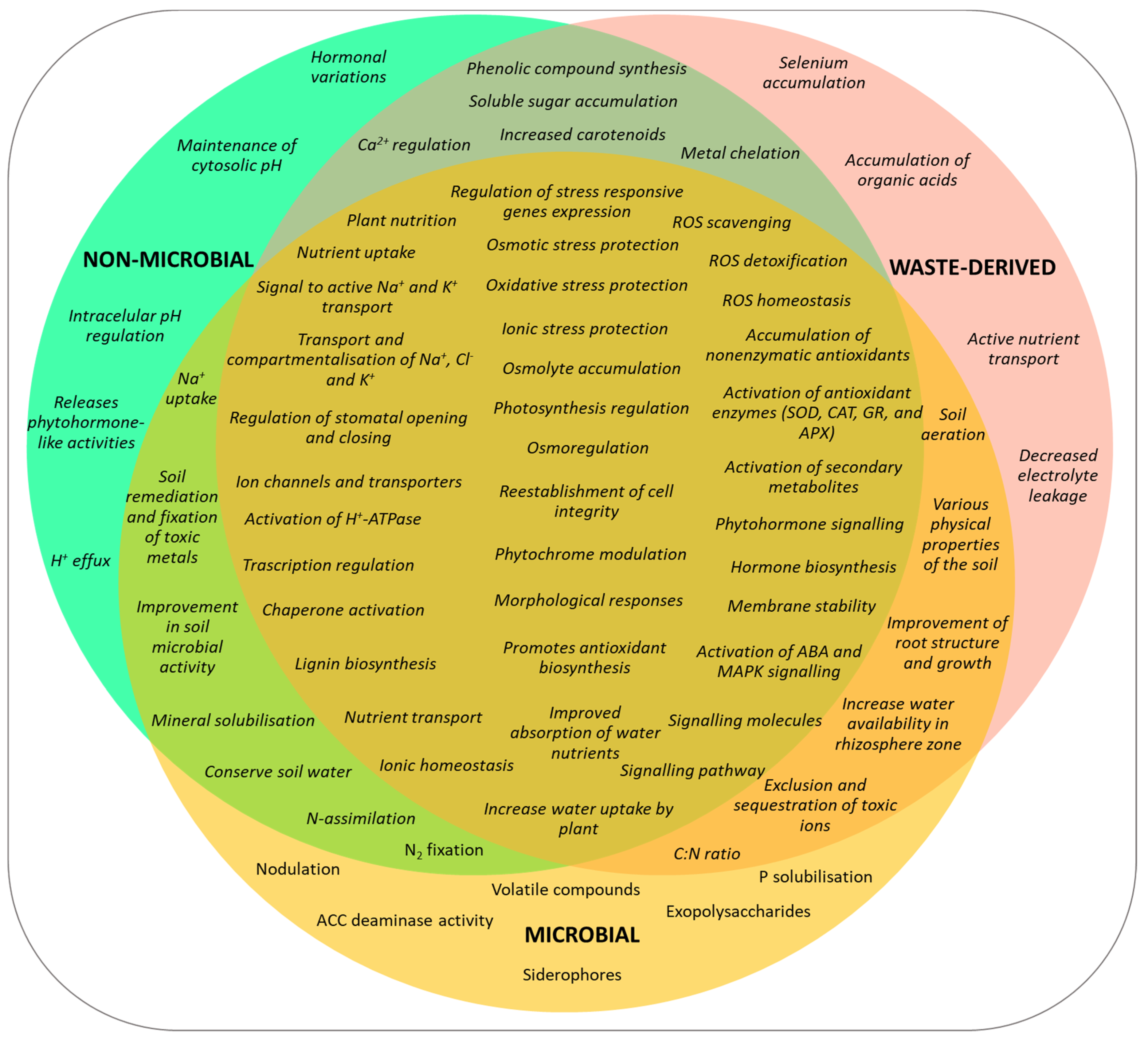
2.2. Mechanisms of Action: Function and Defence against Abiotic Stress
3. Protein Hydrolysates
3.1. Definition and Agronomic Importance
3.2. Effects and Mechanisms on Plant Abiotic Stress Tolerance
4. Agroindustrial By-Products as a Source of Biostimulant

Effect of Protein Hydrolysates on Plant Abiotic Stress Responses
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2020, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of crop response to soil salinity stress: Possible approaches from leaching to nano-management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J.M. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Peña, A.; Delgado-Moreno, L.; Rodríguez-Liébana, J.A. A review of the impact of wastewater on the fate of pesticides in soils: Effect of some soil and solution properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef] [PubMed]
- Manasa, M.R.K.; Katukuri, N.R.; Darveekaran Nair, S.S.; Haojie, Y.; Yang, Z.; bo Guo, R. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737. [Google Scholar] [CrossRef]
- Shahid, S.A. Potential threats to soil functions and mitigation options for sustainable uses. In Terrestrial Environment and Ecosystems of Kuwait: Assessment and Restoration; Springer Nature: Cham, Switzerland, 2024; pp. 3–20. [Google Scholar] [CrossRef]
- Ramos, T.B.; Castanheira, N.; Oliveira, A.R.; Paz, A.M.; Darouich, H.; Simionesei, L.; Farzamian, M.; Gonçalves, M.C. Soil salinity assessment using vegetation indices derived from Sentinel-2 multispectral data. Application to Lezíria Grande, Portugal. Agric. Water Manag. 2020, 241, 106387. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, M.I. Physiological role of gamma-aminobutyric acid in salt stress tolerance. In Salt and Drought Stress Tolerance in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Singam, K.; Juntawong, N.; Cha-Um, S.; Kirdmanee, C. Salt stress induced ion accumulation, ion homeostasis, membrane injury and sugar contents in salt-sensitive rice (Oryza sativa L. spp. indica) roots under isoosmotic conditions. Afr. J. Biotechnol. 2011, 10, 1340–1346. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar] [CrossRef]
- Shahid, M.J.; AL-Surhanee, A.A.; Kouadri, F.; Ali, S.; Nawaz, N.; Afzal, M.; Rizwan, M.; Ali, B.; Soliman, M.H. Role of microorganisms in the remediation of wastewater in floating treatment wetlands: A review. Sustainability 2020, 12, 5559. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P.J. Salinity, Osmolytes and Compatible Solutes; Springer: Dordrecht, The Netherlands, 2002; pp. 181–204. [Google Scholar] [CrossRef]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. S. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Sade, N.; Del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef]
- Shah, L.R.; Sharma, A.; Nabi, J.; Prasad, J. Breeding approaches for abiotic stress management in vegetable crops. J. Pharmacogn. Phytochem. 2018, 7, 1023–1028. [Google Scholar]
- Lim, M.Y.; Jeong, B.R.; Jung, M.; Harn, C.H. Transgenic tomato plants expressing strawberry d-galacturonic acid reductase gene display enhanced tolerance to abiotic stresses. Plant Biotechnol. Rep. 2016, 10, 105–116. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Sharma, P.; Nagpal, S.; Gupta, R.K.; Sirari, A.; Nair, R.M.; Bindumadhava, H.; Singh, S. Dual microbial inoculation, a game changer?—Bacterial biostimulants with multifunctional growth promoting traits to mitigate salinity stress in spring mungbean. Front. Microbiol. 2021, 11, 600576. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: The importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Research and Markets. Agriculture Biostimulant-Global Market Outlook (2019–2027); Research and Markets; European Union: Maastricht, The Netherlands, 2020. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-based protein hydrolysate improves salinity tolerance in hemp: Agronomical and physiological aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, W.; Lv, H.; Liang, B.; Jin, S.; Li, J.; Zhou, W. Animal-derived plant biostimulant alleviates drought stress by regulating photosynthesis, osmotic adjustment, and antioxidant systems in tomato plants. Sci. Hortic. 2022, 305, 111365. [Google Scholar] [CrossRef]
- European Commission. Circular Economy Research and Innovation—Connecting Economic and Environmental Gains; European Commission: Brussels, Belgium, 2017; Available online: https://ec.europa.eu/programmes/horizon2020/sites/horizon2020/files/ce_booklet.pdf (accessed on 15 January 2024).
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- European Commission. Regulation of the European Parliament and of the Council laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114. [Google Scholar]
- Huygens, D.; Saveyn, H.G.M.; Tonini, D.; Eder, P.; Delgado Sancho, L. Technical Proposals for Selected New Fertilising Materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009)—Process and Quality Criteria, and Assessment of Environmental and Market Impacts for Precipitated Phosphate Salts & Derivates, Thermal Oxidation Materials & Derivates and Pyrolysis & Gasification Materials, EUR 29841 EN.; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-09888-1. [Google Scholar]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The futuristic sustainable approach for alleviating crop productivity and abiotic stress tolerance. J. Plant Growth Regul. 2023, 43, 659–674. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno-Ramón, H.; Ibáñez-Asensio, S.; Vicente, O. Effect of a biostimulant based on polyphenols and glycine betaine on tomato plants’ responses to salt stress. Agronomy 2022, 12, 2142. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Frąc, M.; Pieczywek, P.; Pertile, G.; Chibrikov, V.; Nosalewicz, A.; Feledyn-Szewczyk, B.; Sas-Paszt, L.; Zdunek, A. Microbial biostimulants affect the development of pathogenic microorganisms and the quality of fresh strawberries (Fragaria ananassa Duch.). Sci. Hortic. 2024, 327, 112793. [Google Scholar] [CrossRef]
- Kałuzewicz, A.; Krzesiński, W.; Spizewski, T.; Zaworska, A. Effect of biostimulants on several physiological characteristics and chlorophyll content in broccoli under drought stress and re-watering. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 197–202. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.S.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Tavarini, S.; Passera, B.; Martini, A.; Avio, L.; Sbrana, C.; Giovannetti, M.; Angelini, L.G. Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind. Crops Prod. 2018, 111, 899–907. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Arroussi, H.E.L.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.L.; Aafsar, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Villalobos-Escobedo, J.M.; Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Ramírez, F.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, A. The fungal NADPH oxidase is an essential element for the molecular dialog between Trichoderma and Arabidopsis. Plant J. 2020, 103, 2178–2192. [Google Scholar] [CrossRef]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant mode of action: Impact of biostimulant on whole-plant level. In The Chemical Biology of Plant Biostimulants; Geelan, D., Xu, L., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 205–227. [Google Scholar] [CrossRef]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome engineering and plant Biostimulants for sustainable crop improvement and mitigation of biotic and abiotic Stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Calone, R.; Rodríguez-Heredia, R.; Asaff-Torres, A.; Boscaiu, M.; Ibáñez-Asensio, S.; Moreno-Ramón, H.; Vicente, O. Use of a biostimulant to mitigate the effects of excess salinity in soil and irrigation water in tomato plants. Plants 2023, 12, 1190. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits: A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef]
- Aktsoglou, D.-C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Protein hydrolysates supplement in the nutrient solution of soilless grown fresh peppermint and spearmint as a tool for improving product quality. Agronomy 2021, 11, 317. [Google Scholar] [CrossRef]
- Martín, M.-H.J.; Ángel, M.-M.M.; Aarón, S.-L.J.; Israel, B.-G. Protein hydrolysates as biostimulants of plant growth and development. In Biostimulants: Exploring Sources and Applications; Springer: Singapore, 2022; pp. 141–175. [Google Scholar] [CrossRef]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arriogoni, G.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Monterumici, C.M.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Hortechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Liu, N.; Qu, P.; Huang, H.; Wei, Z. Soybean protein hydrolysate-formaldehyde-urea block copolymer for controlled release fertilizer. Environ. Pollut. Bioavailab. 2019, 31, 94–102. [Google Scholar] [CrossRef]
- Ambrosini, S.; Prinsi, B.; Zamboni, A.; Espen, L.; Zanzoni, S.; Santi, C.; Varanini, Z.; Pandolfini, T. Chemical characterization of a collagen-derived protein hydrolysate and biostimulant activity assessment of its peptidic components. J. Agric. Food Chem. 2020, 70, 11201–11211. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; Placa, G.G.L.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; Bella, S.L.; Mauro, R.P.; Anna, F.D.; et al. Celery (Apium graveolens L.) performances as subjected to different sources of protein hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Garcia-Santiago, J.C.; Cavazos, C.J.; Gonzalez-Fuentes, J.; Zermeno-Gonzalez, A.; Alvarado, E.R.; Duarte, A.R.; Preciado-Rangel, P.; Troyo-Dieguez, E.; Ramos, F.M.P.; Valdez-Aguilar, L.A.; et al. Effect of fish-derived protein hydrolysate, animal-based organic fertilisers and irrigation method on the growth and quality of grape tomatoes. Biol. Agric. Hortic. 2021, 37, 107–124. [Google Scholar] [CrossRef]
- Liatile, P.C.; Potgieter, G.; Moloi, M.J. A natural biostimulant consisting of a mixture of fish protein hydrolysates and kelp extract enhances the physiological, biochemical, and growth responses of spinach under different water levels. Plants 2022, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Tsopmo, A.; Thimmanagari, M.; Dutta, A. Corn distillers solubles by two-step proteolytic hydrolysis as a new source of plant-based protein hydrolysates with ACE and DPP4 inhibition activities. Food Chem. 2023, 401, 134120. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Cristofano, F.; Colla, G.; Pii, Y.; Lucini, L.; Rouphael, Y. A Graminaceae-derived protein hydrolysate and its fractions provide differential growth and modulate qualitative traits of lettuce grown under non-saline and mild salinity conditions. Sci. Hortic. 2023, 319, 112130. [Google Scholar] [CrossRef]
- Testani, E.; Campanelli, G.; Leteo, F.; Ciaccia, C.; Canali, S.; Tittarelli, F. Sweet pepper (Capsicum annuum L.) organic seedling production: The role of compost, cultivar, and protein hydrolyzate. Compost Sci. Util. 2017, 25, 112–119. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of plant-derived protein hydrolysates on yield, quality and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; Silva-Valdiviezo, D.; Calone, R.; Lupuţ, I.; Ibáñez-Asensio, S.; Boscaiu, M.; Moreno-Ramón, H.; Vicente, O. Biochemical responses to salt stress and biostimulant action in tomato plants grown in two different soil types. Horticulturae 2023, 9, 1209. [Google Scholar] [CrossRef]
- Zhang, L.; Freschi, G.; Rouphael, Y.; De Pascale, S.; Lucini, L. The differential modulation of secondary metabolism induced by a protein hydrolysate and a seaweed extract in tomato plants under salinity. Front Plant Sci. 2023, 13, 1072782. [Google Scholar] [CrossRef]
- Tallarita, A.V.; Vecchietti, L.; Golubkina, N.A.; Sekara, A.; Cozzolino, E.; Mirabella, M.; Cuciniello, A.; Maiello, R.; Cenvinzo, V.; Lombardi, P.; et al. Effects of plant biostimulation time span and soil electrical conductivity on greenhouse tomato Miniplum yield and quality in diverse crop seasons. Plants 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Cholakova-Bimbalova, R.; Petrov, V.; Vassilev, A. Photosynthetic performance of young maize (Zea mays L.) plants exposed to chilling stress can be improved by the application of protein hydrolysates. Acta Agrobot. 2019, 72, 1769. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 25, 108784. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant. Sci. 2019, 10, 47. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Colla, G.; Cardarelli, M.; Pii, Y.; Lucini, L.; Rouphael, Y. Modulation of morpho-physiological and metabolic profiles of lettuce subjected to salt stress and treated with two vegetal-derived biostimulants. Plants 2023, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Zuzunaga-Rosas, J.; Calone, R.; Mircea, D.M.; Shakya, R.; Ibáñez-Asensio, S.; Boscaiu, M.; Fita, A.; Moreno-Ramón, H.; Vicente, O. Mitigation of salt stress in lettuce by a biostimulant that protects the root absorption zone and improves biochemical responses. Front. Plant Sci. 2024, 15, 1341714. [Google Scholar] [CrossRef]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.-O.; Barone, A.; Rigano, M.M. Phenotyping to dissect the biostimulant action of a protein hydrolysate in tomato plants under combined abiotic stress. Plant Physiol. Biochem. 2022, 179, 32–43. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous pig blood-derived protein hydrolysates as a promising method for alleviation of salt stress in tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Bhuimbar, M.V.; Dandge, P.B. Production of organic liquid biofertilizer from fish waste and study of its plant growth promoting effect. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 235–243. [Google Scholar] [CrossRef]
- Liya, S.M.; Umesh, M. Bioconversion of chicken feather waste into feather hydrolysate by multifaceted keratinolytic Bacillus tropicus LS27 and new insights into its antioxidant and plant growth-promoting properties. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Guyomard, H.; Bureau, J.C.; Chatellier, V.; Détang-Dessendre, C.; Dupraz, P.; Jacquet, F.; Reboud, X.; Réquillart, V.; Soler, L.G.; Tysebaert, M. The Green Deal and the CAP: Policy Implications to Adapt Farming Practices and to Preserve the EU’s Natural Resources; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium; Available online: http://www.europarl.europa.eu/RegData/etudes/STUD/2020/629214/IPOL_STU(2020)629214_EN.pdf (accessed on 20 January 2024).
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Casadesus, A.; Perez-Llorca, M.; Munne-Bosch, S.; Polo, J. An enzymatically hydrolyzed animal protein-based biostimulant (Pepton) increases salicylic acid and promotes growth of tomato roots under temperature and nutrient stress. Front. Plant Sci. 2020, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, Y.; Meenakshisundaram, S.; Raja, K.; Balaiah, A. Sustainable and efficient-recycling approach of chicken feather waste into liquid protein hydrolysate with biostimulant efficacy on plant, soil fertility and soil microbial consortium: A perspective to promote the circular economy. Process Saf. Environ. Prot. 2023, 170, 573–583. [Google Scholar] [CrossRef]
- Kumar, S.; Chinnannan, K.; Thamilarasan, S.K.; Seralathan, M.; Shanmuganathan, R.; Padikasan, I.A. Enzymatically hydrolysed sago bagasse improves physiological, biochemical and molecular attributes of Solanum lycopersicum. Biocatal. Agric. Biotechnol. 2019, 17, 499–506. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein hydrolysates modulate leaf proteome and metabolome in water-stressed grapevines. Sci. Hortic. 2020, 270, 109413. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A novel protein hydrolysate-based biostimulant improves tomato performances under drought stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Harizanova, A.; Koleva-Valkova, L.; Vassilev, A. Effects of the protein hydrolysate pretreatment on cucumber plants exposed to chilling stress. Acta Agrobot. 2022, 75, 1–8. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Simova-Stoilova, L.; Kostadinova, A.; Yuperlieva-Mateeva, B.; Karakicheva, T.; Vassileva, V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in Protease, DHN, and HSP gene expression in maize. Agronomy 2022, 12, 1127. [Google Scholar] [CrossRef]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef] [PubMed]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jorda, M.; Quinones, A.; Intrigliolo, D.S. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. Rojo Brillante grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]

| Source Material | Type of Stress | Crop | Effect on the Plants | Reference |
|---|---|---|---|---|
| Agroindustrial by-products of plant origin | ||||
| Rice and oat husks | Salt stress | Tomato (Solanum lycopersicum L.) | Promotes plant hydration and improves photosynthesis and primary metabolism. | [45,57,82] |
| Lettuce (Lactuca sativa L.) | Promotes growth and protects and stimulates the absorption zone of the roots. | [89] | ||
| Graminaceae | Salt stress | Lettuce (Lactuca sativa L.) | Promotes growth, increasing the fresh weight of shoots. | [78] |
| Pumpkin seeds | Salt stress | Common bean (Phaseolus vulgaris L.) | Enhances the nutritional status. | [73] |
| Fluid agri-food vinaigrette of fruits | Salt stress | Tomato (Solanum lycopersicum L.) | Increased photosynthesis activity and recovery of membrane integrity. | [83] |
| Fabaceae or Leguminosae | Salt stress | Hemp (Cannabis sativa L.) | Protection of the photosynthetic system, increase in seed yield and residual biomass. | [34] |
| Tomato (Solanum lycopersicum L.) | Improved yield and quality of fruits. | [84] | ||
| Legume seeds | Salt stress | Lettuce (Lactuca sativa L.) | Favours an increase in fresh yield, dry biomass and root dry weight. | [51] |
| Water stress | Tomato (Solanum lycopersicum L.) | Favours an increase in biomass. | [53] | |
| Legume biomass | Water stress | Grapevine (Vitis vinifera L.) | Promotes photosynthesis, vegetative growth and nutrient absorption. | [100] |
| Soybean and lupine | Water stress | Grapevine (Vitis vinifera L.) | Increase in soluble solids. | [86] |
| Sugar cane molasses with yeast extract | Drought stress | Tomato (Solanum lycopersicum L.) | Accumulation of antioxidant compounds, favours growth and productivity. | [101] |
| Heat stress | Tomato (Solanum lycopersicum L.) | Favours the net photosynthetic rate and growth in plants. | [90] | |
| Other by-products of plant origin | Chilling stress | Cucumber (Cucumis sativus L.) | Activation of the antioxidant enzyme system. | [102] |
| Heat stress | Maize (Zea mays L.) | Promotes growth and stabilisation of photosynthesis. | [103] | |
| Metal stress | Mehirugi (Kandelia obovate) | Promotes the elimination of free radicals. | [104] | |
| Agroindustrial by-products of animal origin | ||||
| Pig blood | Salt stress | Tomato (Solanum lycopersicum L.) | Promotes plant growth, chlorophyll levels and photosynthetic efficiency. | [92] |
| Drought stress | Tomato (Solanum lycopersicum L.) | Regulates stomatal opening and promotes photosynthetic capacity. | [35] | |
| Casein | Water stress | Grapevine (Vitis vinifera L.) | Increases the total anthocyanin content of the berries. | [86] |
| Trimmings and shavings of bovine hides | Hypoxic stress | Maize (Zea mays L.) | Influences the growth of roots and shoots. | [91] |
| Chicken feathers | Metal stress | Spinach (Spinacia oleracea L.) | Favours plant growth-promoting activity. | [94] |
| - | Maize (Zea mays L.) | Promotes nutrition, yield and grain quality. | [81] | |
| - | Maize (Zea mays L.) | Promotes seed germination and plant growth. | [98] | |
| Fish protein | Drought stress | Spinach (Spinacia oleracea L.) | Favours photosynthetic efficiency and growth parameters. | [76] |
| Fish waste (skin and scales) | - | Chilli (Capsicum annum) | Increases the length of shoots and roots and the number of leaves and pods. | [93] |
| - | Cowpea (Vigna unguiculata) | Favours the formation of root nodules and fruits and increases fresh and dry weight. | [93] | |
| Other by-products of animal origin | Salt stress | Kaki persimmon (Diospyros kaki L.) | Reduces chloride absorption and leaf necrosis. | [105] |
| Chilling stress | Maize (Zea mays L.) | Increases photosynthetic activity. | [85] | |
| Transplant stress | Sweet pepper (Capsicum annuum L.) | Favours transplantation and increased yield. | [79] | |
| Low-temperature stress | Tomato (Solanum lycopersicum L.) | Promotes the growth of primary and lateral roots of plants. | [97] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuzunaga-Rosas, J.; Boscaiu, M.; Vicente, O. Agroindustrial By-Products as a Source of Biostimulants Enhancing Responses to Abiotic Stress of Horticultural Crops. Int. J. Mol. Sci. 2024, 25, 3525. https://doi.org/10.3390/ijms25063525
Zuzunaga-Rosas J, Boscaiu M, Vicente O. Agroindustrial By-Products as a Source of Biostimulants Enhancing Responses to Abiotic Stress of Horticultural Crops. International Journal of Molecular Sciences. 2024; 25(6):3525. https://doi.org/10.3390/ijms25063525
Chicago/Turabian StyleZuzunaga-Rosas, Javier, Monica Boscaiu, and Oscar Vicente. 2024. "Agroindustrial By-Products as a Source of Biostimulants Enhancing Responses to Abiotic Stress of Horticultural Crops" International Journal of Molecular Sciences 25, no. 6: 3525. https://doi.org/10.3390/ijms25063525
APA StyleZuzunaga-Rosas, J., Boscaiu, M., & Vicente, O. (2024). Agroindustrial By-Products as a Source of Biostimulants Enhancing Responses to Abiotic Stress of Horticultural Crops. International Journal of Molecular Sciences, 25(6), 3525. https://doi.org/10.3390/ijms25063525









