Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient
Abstract
1. Background
2. Results
2.1. Study Population Characteristics
2.2. Performance of Neuropsychological Symptoms
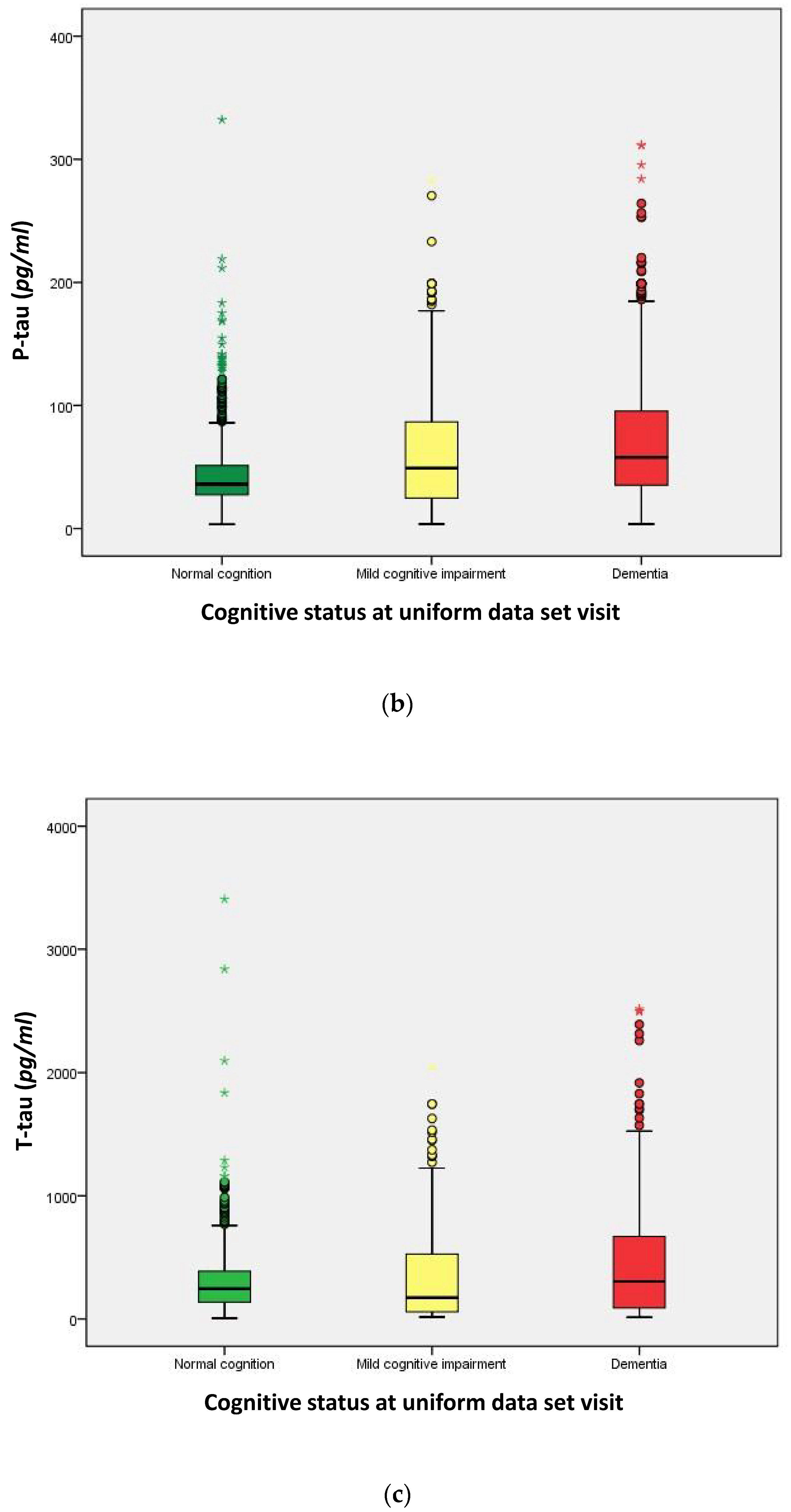
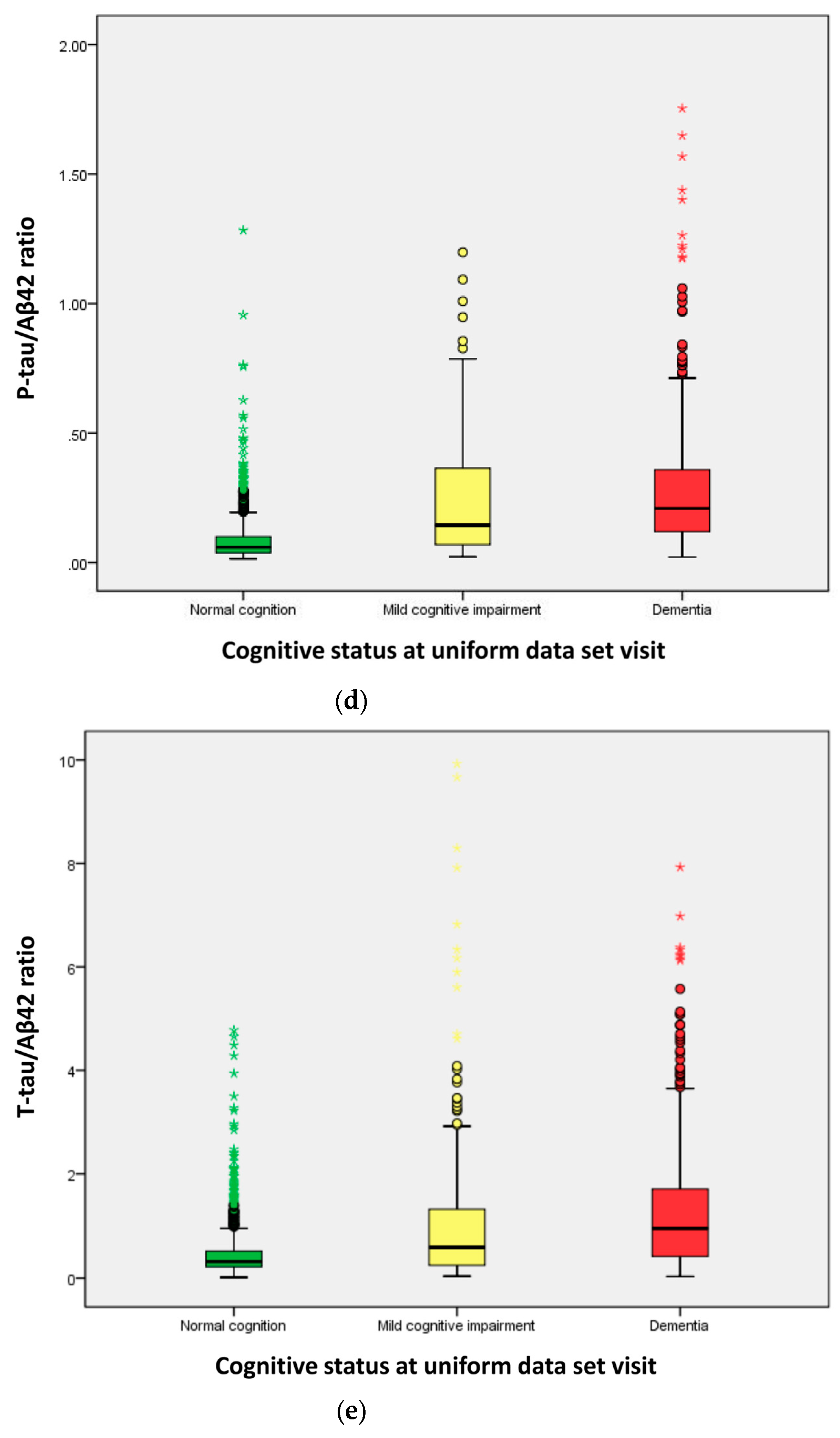
2.3. Distribution of Cerebrospinal Fluid (CSF) Biomarkers
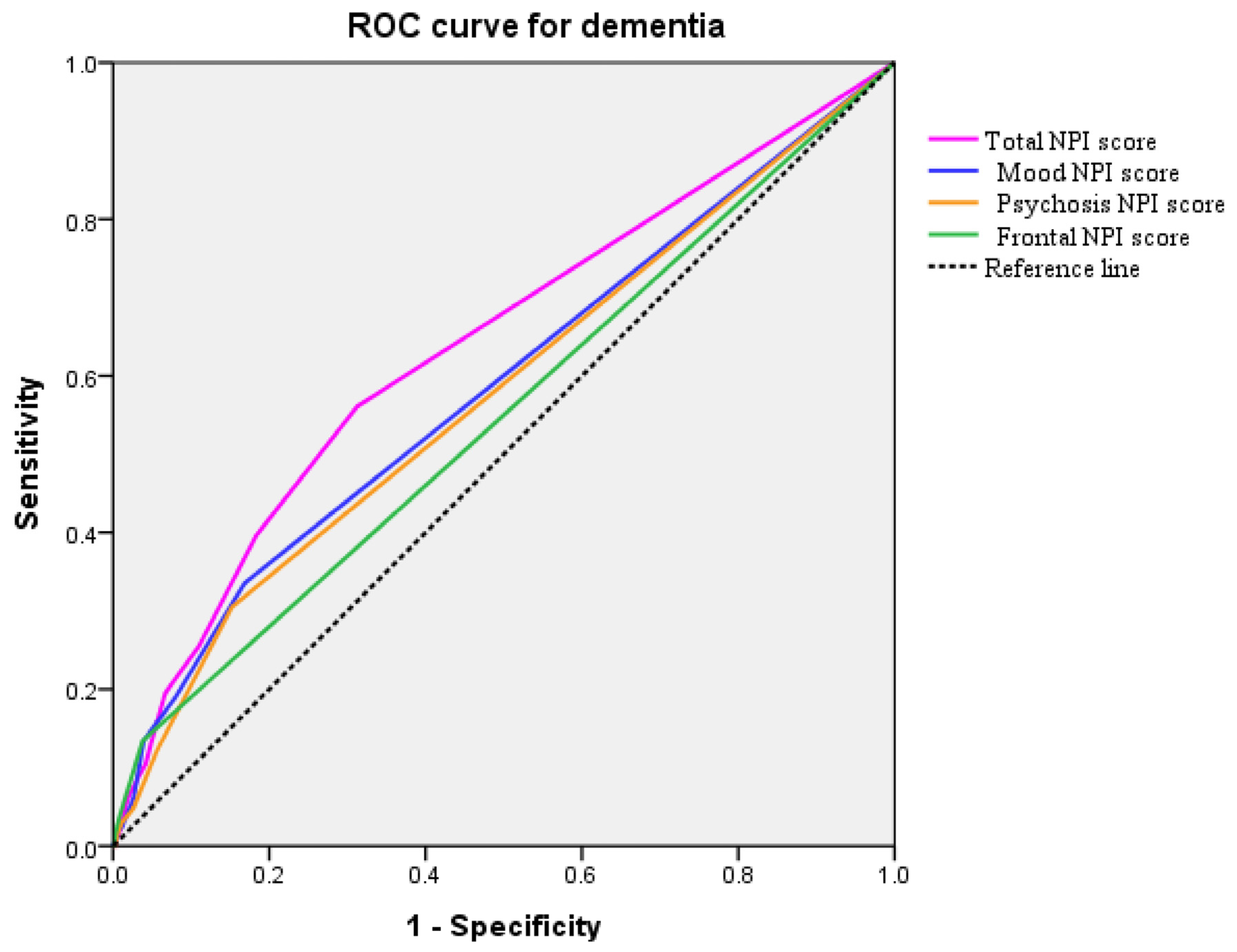
2.4. Prediction of Dementia
3. Methods
3.1. Data Source
3.2. Ethics Statement
3.3. Study Subjects
3.4. Study Variables
3.4.1. Neuropsychiatry Inventory (NPI)
3.4.2. Cerebrospinal Fluid (CSF) Biomarker
- Amyloid-β42 (Aβ42): Aβ42 plays a pivotal role in the pathogenesis of AD, and its levels in the CSF have been closely linked with the disease’s progression and diagnosis. The aggregation of Aβ42 into amyloid plaques in the brain is a hallmark of AD. These plaques disrupt cell-to-cell communication and activate immune responses, which can lead to inflammation and the destruction of neurons. The measurement of Aβ42 levels in the CSF provides an indirect marker of plaque burden within the brain. Importantly, reductions in CSF Aβ42 levels can occur years before the onset of clinical symptoms, making it a potential biomarker for early detection and intervention in AD. Additionally, Aβ42 levels are being explored in the context of clinical trials for amyloid-targeting therapies, serving as a biological endpoint for disease-modifying treatments.
- Phosphorylated tau (P-tau): P-tau is a specific marker for AD pathology. The hyperphosphorylation of tau protein leads to its aggregation into neurofibrillary tangles, another pathological hallmark of AD. These tangles accumulate inside neurons, disrupting their function and eventually leading to cell death. The levels of P-tau in the CSF are correlated with the presence and progression of tau pathology in the brain. Elevated P-tau levels are considered a sign of ongoing neurodegenerative processes and have been associated with cognitive decline and the severity of AD. P-tau levels in the CSF are also being studied as a biomarker for tracking disease progression and response to tau-targeted therapies.
- Total tau (T-tau): T-tau in the CSF is a marker of neuronal damage and neurodegeneration. Unlike Aβ42 and P-tau, which are more specific to AD pathology, elevated T-tau levels can be seen in various conditions that cause neuronal damage, such as traumatic brain injury, stroke, and other neurodegenerative diseases. This makes T-tau a less specific biomarker for AD but valuable in the broader context of neurological damage assessment. In AD, the combination of high T-tau and P-tau levels with low Aβ42 levels in the CSF can significantly enhance diagnostic accuracy, differentiating AD from other forms of dementia and neurodegenerative disorders.
3.5. Covariates
3.6. Statistical Analyses
4. Discussion
5. Future Directions
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Rae-Grant, A. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Chen, P.; Cai, H.; Zhang, Q.; Su, Z.; Cheung, T.; Xiang, Y.T. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: A meta-analysis and systematic review of epidemiology studies. Age Ageing 2022, 51, afac173. [Google Scholar] [PubMed]
- Chen, P.; Cai, H.; Bai, W.; Su, Z.; Tang, Y.L.; Ungvari, G.S.; Ng, C.H.; Zhang, Q.; Xiang, Y.T. Global prevalence of mild cognitive impairment among older adults living in nursing homes: A meta-analysis and systematic review of epidemiological surveys. Transl. Psychiatry 2023, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public. Health 2020, 5, e661–e671. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Jutkowitz, E.; Bynum, J.P.; Mitchell, S.L.; Cocoros, N.M.; Shapira, O.; Haynes, K.; McCarthy, E.P. Diagnosed prevalence of Alzheimer’s disease and related dementias in Medicare Advantage plans. Alzheimers Dement. 2020, 12, e12048. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hou, N.N.; Wu, H.M.; Zuo, X.; Lian, Y.Z.; Zhang, C.N.; Zhu, J.H. Prevalence of Alzheimer’s Disease and Parkinson’s Disease in China: An Updated Systematical Analysis. Front. Aging Neurosci. 2020, 12, 603854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X. The prevalence of Alzheimer’s disease in the Chinese Han population: A meta-analysis. Neurol. Res. 2020, 42, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dunne, R.A.; Aarsland, D.; O’brien, J.T.; Ballard, C.; Banerjee, S.; Fox, N.C.; Isaacs, J.D.; Underwood, B.R.; Perry, R.J.; Chan, D.; et al. Mild Cognitive Impairment: The Manchester consensus. J. Am. Geriatr. Soc. 2021, 50, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Zetterberg, H. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Pickett, E.K.; Herrmann, A.G.; McQueen, J.; Abt, K.; Dando, O.; Tulloch, J.; Jain, P.; Dunnett, S.; Sohrabi, S.; Fjeldstad, M.P.; et al. Amyloid Beta and Tau Cooperate to Cause Reversible Behavioral and Transcriptional Deficits in a Model of Alzheimer’s Disease. Cell Rep. 2019, 29, 3592–3604.e5. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2020, 16, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Korecka, M.; Figurski, M.J.; Toledo, J.B.; Blennow, K.; Zetterberg, H. The Alzheimer’s Disease Neuroimaging Initiative 2 Biomarker Core: A review of progress and plans. Alzheimers Dement. 2015, 11, 772–791. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H. Review: Tau in biofluids—Relation to pathology, imaging and clinical features. Neuropathol. Appl. Neurobiol. 2017, 43, 194–199. [Google Scholar] [CrossRef]
- Leuzy, A.; Cullen, N.C.; Mattsson-Carlgren, N.; Hansson, O. Current advances in plasma and cerebrospinal fluid biomarkers in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 266–274. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Abeta Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. 2022, 17, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Subjective Cognitive Decline Initiative. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Mangialasche, F.; Camarda, C.; Ercolani, S.; Camarda, R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J. Alzheimers Dis. 2009, 18, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug. Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Pouryamout, L.; Dams, J.; Wasem, J.; Dodel, R.; Neumann, A. Economic evaluation of treatment options in patients with Alzheimer’s disease: A systematic review of cost-effectiveness analyses. Drugs 2012, 72, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Keogh-Brown, M.R.; Jensen, H.T.; Arrighi, H.M.; Smith, R.D. The Impact of Alzheimer’s Disease on the Chinese Economy. EBioMedicine 2016, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.I.; Qin, W.; Gauthier, S. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018, 14, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Dauphinot, V.; Potashman, M.; Levitchi-Benea, M.; Su, R.; Rubino, I.; Krolak-Salmon, P. Economic and caregiver impact of Alzheimer’s disease across the disease spectrum: A cohort study. Alzheimers Res. Ther. 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Krell-Roesch, J.; Rakusa, M.; Syrjanen, J.A.; van Harten, A.C.; Lowe, V.J.; Jack, C.R., Jr.; Geda, Y.E. Association between CSF biomarkers of Alzheimer’s disease and neuropsychiatric symptoms: Mayo Clinic Study of Aging. Alzheimers Dement. 2022, 19, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Cotta Ramusino, M.; Perini, G.; Vaghi, G.; Dal Fabbro, B.; Capelli, M.; Picascia, M.; Costa, A. Correlation of Frontal Atrophy and CSF Tau Levels With Neuropsychiatric Symptoms in Patients With Cognitive Impairment: A Memory Clinic Experience. Front. Aging Neurosci. 2021, 13, 595758. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Kazis, D. A meta-analysis on CSF neurogranin levels for the diagnosis of Alzheimer’s disease and mild cognitive impairment. Aging Clin. Exp. Res. 2020, 32, 1639–1646. [Google Scholar] [CrossRef]
- Cousins, O.; Hodges, A.; Schubert, J.; Veronese, M.; Turkheimer, F.; Miyan, J.; Engelhardt, B.; Roncaroli, F. The blood–CSF–brain route of neurological disease: The indirect pathway into the brain. Neuropathol. Appl. Neurobiol. 2022, 48, e12789. [Google Scholar] [CrossRef] [PubMed]
- Besser, L.; Kukull, W.; Knopman, D.S.; Chui, H.; Galasko, D.; Weintraub, S.; Neuropsychology Work Group. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis. Assoc. Disord. 2018, 32, 351–358. [Google Scholar] [CrossRef]
- Besser, L.M.; Kukull, W.A.; Teylan, M.A.; Bigio, E.H.; Cairns, N.J.; Kofler, J.K.; Nelson, P.T. The Revised National Alzheimer’s Coordinating Center’s Neuropathology Form-Available Data and New Analyses. J. Neuropathol. Exp. Neurol. 2018, 77, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Weintraub, S.; Chui, H.C.; Cummings, J.; DeCarli, C.; Ferris, S.; Kukull, W.A. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 2006, 20, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Boada, M.; Borson, S.; Chilukuri, M.; Dubois, B.; Ingram, J.; Iwata, A.; Porsteinsson, A.P.; Possin, K.L.; Rabinovici, G.D.; et al. Early Detection of Mild Cognitive Impairment (MCI) in Primary Care. J. Prev. Alzheimers Dis. 2020, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Mielke, M.M.; Appleby, B.S.; Oh, E.S.; Geda, Y.E.; Lyketsos, C.G. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am. J. Geriatr. Psychiatry 2013, 21, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Mallo, S.C.; Patten, S.B.; Ismail, Z.; Pereiro, A.X.; Facal, D.; Otero, C.; Juncos-Rabadán, O. Does the neuropsychiatric inventory predict progression from mild cognitive impairment to dementia? A systematic review and meta-analysis. Ageing Res. Rev. 2020, 58, 101004. [Google Scholar] [CrossRef] [PubMed]
- Wiels, W.A.; Wittens, M.M.J.; Zeeuws, D.; Baeken, C.; Engelborghs, S. Neuropsychiatric Symptoms in Mild Cognitive Impairment and Dementia Due to AD: Relation With Disease Stage and Cognitive Deficits. Front. Psychiatry 2021, 12, 707580. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, E.; Pereira, J.B.; Xu, H.; Secnik, J.; Winblad, B.; Eriksdotter, M.; Nägga, K.; Religa, D. Behavioral and Psychological Symptoms of Dementia in Different Dementia Disorders: A Large-Scale Study of 10,000 Individuals. J. Alzheimer’s Dis. 2022, 87, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarado, M.; Gago, B.; Navalpotro-Gomez, I.; Jimenez-Urbieta, H.; Rodriguez-Oroz, M.C. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2016, 31, 861–881. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; van den Berg, E.; Singleton, E.H.; Baart, S.J.; Coesmans, M.; Leeuwis, A.E.; Papma, J.M. Neuropsychiatric and Cognitive Symptoms Across the Alzheimer Disease Clinical Spectrum: Cross-sectional and Longitudinal Associations. Neurology 2021, 97, e1276–e1287. [Google Scholar] [CrossRef] [PubMed]

| Variables | Normal Cognition (n = 977) | MCI (n = 270) | Dementia (n = 649) | p Value |
|---|---|---|---|---|
| Demography | ||||
| Age (year, n, %) | 0.045 | |||
| <65 y | 197 (20.2) | 54 (20.0) | 163 (25.1) | |
| ≥65 y | 780 (79.8) | 216 (80.0) | 486 (74.9) | |
| Sex (n, %) | <0.001 | |||
| Male | 418 (42.8) | 156 (57.8) | 342 (52.7) | |
| Female | 559 (57.2) | 114 (42.2) | 307 (47.3) | |
| Race/Ethnicity (n, %) | 0.027 | |||
| White | 860 (88.0) | 241 (89.3) | 576 (88.8) | |
| Black/African American | 71 (0.73) | 8 (0.30) | 35 (0.54) | |
| Others | 46 (0.47) | 21 (0.78) | 38 (0.59) | |
| Education (n, %) | <0.001 | |||
| High school or less | 134 (13.7) | 62 (23.0) | 202 (31.3) | |
| Bachelor degree | 412 (42.3) | 103 (38.3) | 265 (41.1) | |
| Master’s or doctoral degree | 429 (44.0) | 104 (38.7) | 178 (27.6) | |
| Marital status (n, %) | <0.001 | |||
| Married | 679 (69.6) | 200 (74.1) | 516 (79.5) | |
| Widowed/divorced/separated | 241 (24.7) | 63 (23.3) | 112 (17.3) | |
| Never married | 55 (0.56) | 7 (0.26) | 21 (0.32) | |
| Living situation (n, %) | <0.001 | |||
| Living alone | 242 (24.8) | 51 (18.9) | 85 (13.1) | |
| Living with others | 735 (75.2) | 219 (81.1) | 564 (86.9) | |
| Family history (with dementia) (n, %) | <0.001 | |||
| No | 335 (34.3) | 89 (33.0) | 213 (32.8) | |
| Yes | 612 (62.6) | 155 (57.4) | 382 (58.9) | |
| Unknown | 30 (0.31) | 26 (0.96) | 54 (0.83) | |
| Lifestyles/Physical status | ||||
| Smoking | 0.912 | |||
| Yes | 451 (46.7) | 128 (47.9) | 306 (47.5) | |
| Alcohol abuse | 0.407 | |||
| Yes | 43 (0.44) | 13 (0.48) | 38 (0.59) | |
| Other substances abuse | 0.412 | |||
| Yes | 9 (0.09) | 2 (0.07) | 10 (0.15) | |
| Level of independence | <0.001 | |||
| Independent | 969 (99.2) | 185 (68.5) | 209 (32.2) | |
| Dependent | 8 (0.08) | 85 (31.5) | 440 (67.8) | |
| Body mass index (kg/m2, n, %) | 0.820 | |||
| <18.5 | 13 (0.13) | 6 (0.22) | 8 (0.12) | |
| 18.5~29.9 | 722 (73.9) | 198 (73.3) | 484 (74.6) | |
| ≧30 | 242 (24.8) | 66 (24.4) | 157 (24.2) | |
| Vision | <0.001 | |||
| Functionally normal | 939 (96.1) | 247 (91.5) | 586 (90.3) | |
| Hearing | 0.461 | |||
| Functionally normal | 901 (92.2) | 244 (90.4) | 589 (90.8) | |
| Comorbidities (Yes, n, %) | ||||
| Cardiovascular disease | 259 (26.5) | 74 (27.4) | 172 (26.5) | 0.953 |
| Stroke/Transient ischemic attack (TIA) | 40 (0.41) | 19 (0.70) | 44 (0.68) | 0.029 |
| Parkinson’s disease (PD) | 17 (0.17) | 15 (0.56) | 29 (0.45) | 0.001 |
| Seizures/Other neurological condition | 23 (0.24) | 8 (0.30) | 26 (0.40) | 0.161 |
| Traumatic brain injury (TBI) | 91 (0.93) | 35 (13.0) | 58 (0.89) | 0.144 |
| DM | 99 (10.1) | 49 (18.1) | 69 (10.6) | 0.001 |
| Hypertension | 443 (45.3) | 146 (54.1) | 311 (47.9) | 0.038 |
| Hypercholesterolemia | 543 (55.6) | 153 (56.7) | 348 (53.6) | 0.628 |
| B12 deficiency | 65 (0.67) | 16 (0.59) | 42 (0.65) | 0.912 |
| Thyroid disease | 188 (19.2) | 52 (19.3) | 98 (15.1) | 0.082 |
| Arthritis | 461 (47.2) | 73 (27.0) | 113 (17.4) | <0.001 |
| Sleep disorders | 268 (27.4) | 66 (24.4) | 100 (15.4) | <0.001 |
| Psychological disease | 35 (0.36) | 20 (0.74) | 38 (0.59) | 0.014 |
| Cancer | 102 (10.4) | 26 (0.96) | 30 (0.46) | <0.001 |
| Medication usage | ||||
| NSAIDs | 410 (42.0) | 113 (41.9) | 213 (32.8) | 0.001 |
| Anticoagulant or antiplatelet agent | 322 (33.0) | 92 (34.1) | 190 (29.3) | 0.207 |
| Antipsychotic agent | 122 (12.5) | 48 (17.8) | 110 (16.9) | 0.015 |
| Antiparkinson agent | 43 (0.44) | 19 (0.70) | 23 (0.35) | 0.065 |
| Hormone therapy | 50 (0.51) | 6 (0.22) | 11 (0.17) | 0.001 |
| Antihypertensive or blood pressure | 434 (44.4) | 149 (55.2) | 328 (50.5) | 0.002 |
| Lipid-lowering medication | 384 (39.3) | 125 (46.3) | 294 (45.3) | 0.021 |
| Diabetes medication | 72 (0.74) | 36 (13.3) | 49 (0.76) | 0.005 |
| Antidepressant | 218 (22.3) | 93 (34.4) | 261 (40.2) | <0.001 |
| Variables | Normal Cognition (n = 977) | MCI (n = 270) | Dementia (n = 649) | p Value |
|---|---|---|---|---|
| NPI (Yes, n, %) | ||||
| Delusions | 5 (00.5) | 14 (05.2) | 97 (14.9) | <0.001 |
| Hallucinations | 2 (00.2) | 7 (02.6) | 43 (06.6) | <0.001 |
| Agitation | 49 (05.0) | 56 (20.7) | 227 (35.0) | <0.001 |
| Depression | 93 (09.5) | 86 (31.9) | 284 (43.8) | <0.001 |
| Anxiety | 44 (04.5) | 70 (25.9) | 247 (38.1) | <0.001 |
| Elation | 8 (00.8) | 10 (03.7) | 31 (04.8) | <0.001 |
| Apathy | 26 (02.7) | 62 (23.0) | 294 (45.3) | <0.001 |
| Disinhibition | 24 (02.5) | 41 (15.2) | 151 (23.3) | <0.001 |
| Irritability | 96 (09.8) | 81 (30.0) | 257 (39.6) | <0.001 |
| Aberrant motor behavior | 6 (00.6) | 17 (06.3) | 117 (18.0) | <0.001 |
| Night-time behavior | 80 (08.2) | 58 (21.5) | 173 (26.7) | <0.001 |
| Appetite | 48 (04.9) | 36 (13.3) | 161 (24.8) | <0.001 |
| NPI-group (Yes, n, %) | ||||
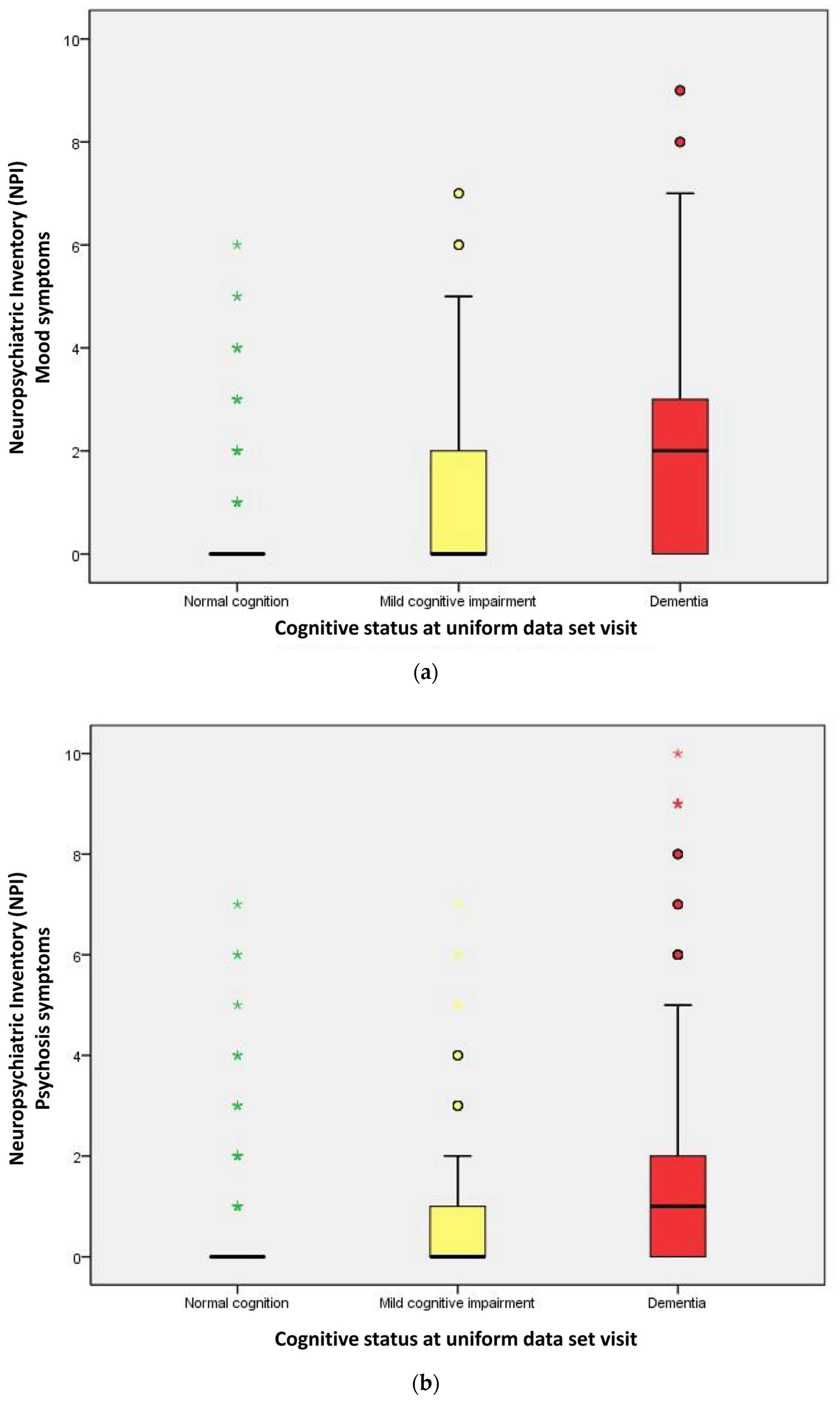
| Mood symptoms | 124 (12.7) | 131 (48.5) | 459 (70.7) | <0.001 |
| Psychosis symptoms | 123 (12.6) | 112 (41.5) | 359 (55.3) | <0.001 |
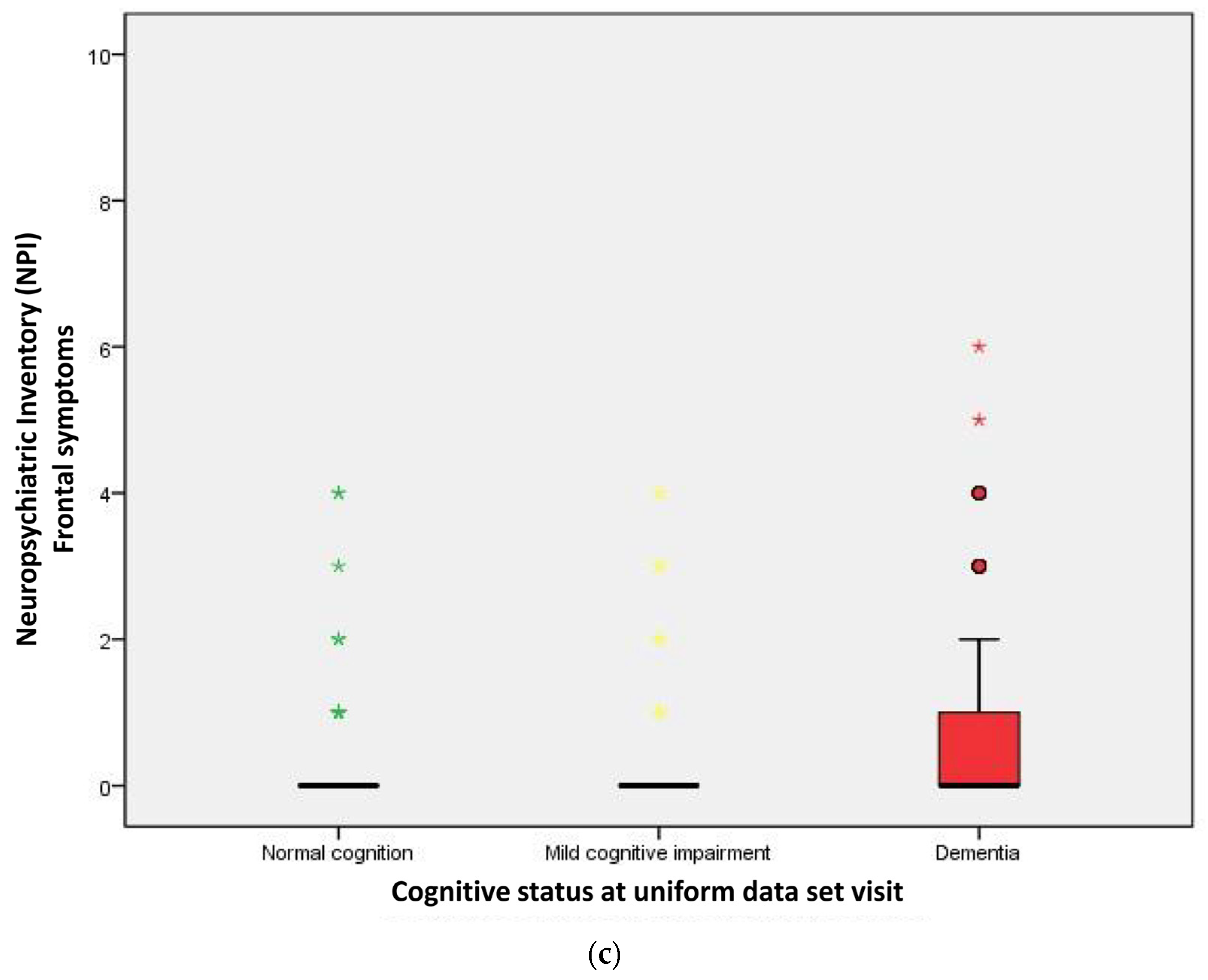
| Frontal symptoms | 29 (03.0) | 45 (16.7) | 166 (25.6) | <0.001 |
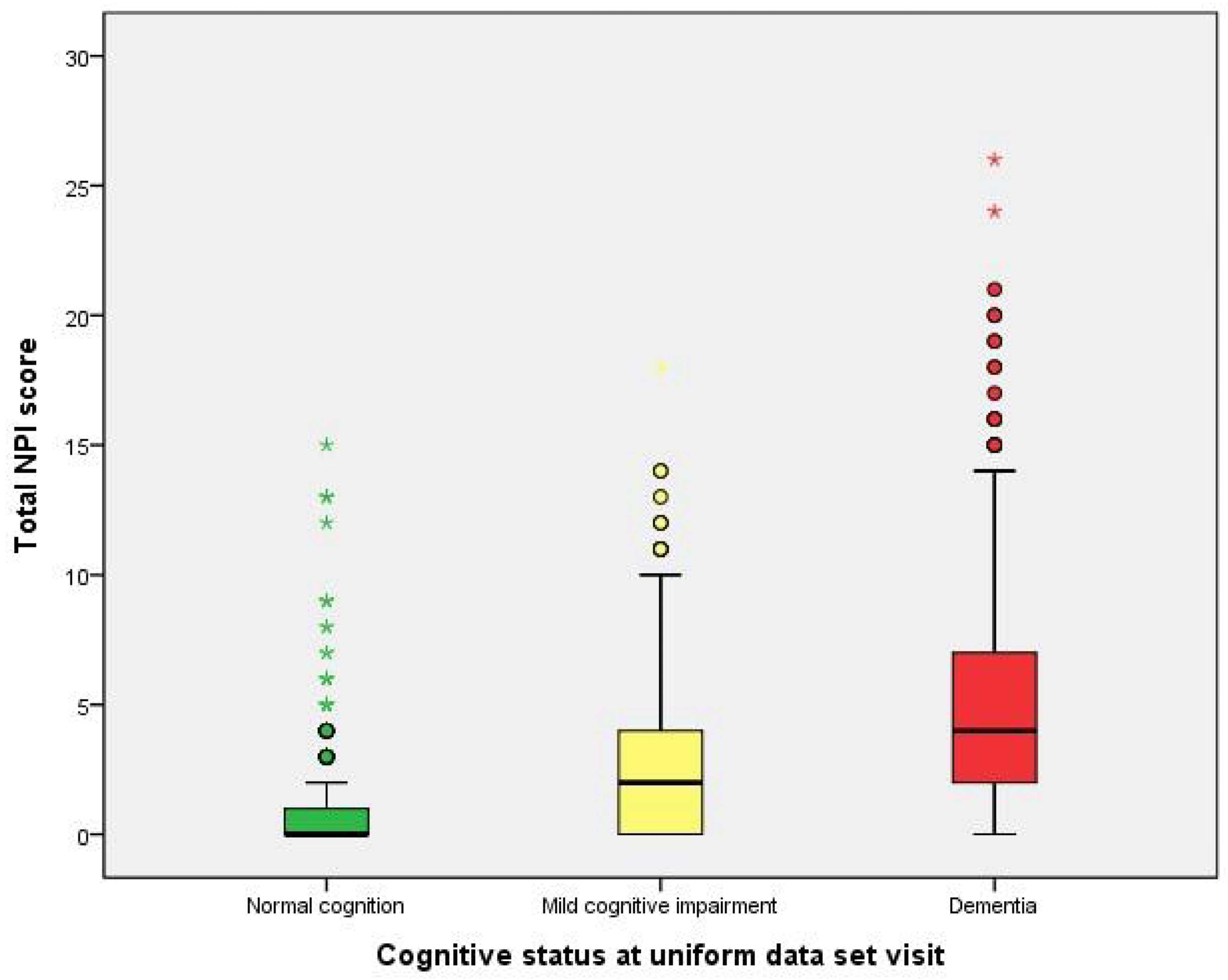
| Total NPI score (median) | 0.00 | 2.00 | 4.00 | <0.001 a |
| NPI-group score (median) | ||||
| Mood symptoms score | 0.00 | 0.00 | 2.00 | <0.001 a |
| Psychosis symptoms score | 0.00 | 0.00 | 1.00 | <0.001 a |
| Frontal symptoms score | 0.00 | 0.00 | 0.00 | <0.001 a |
| CSF biomarkers (median) | ||||
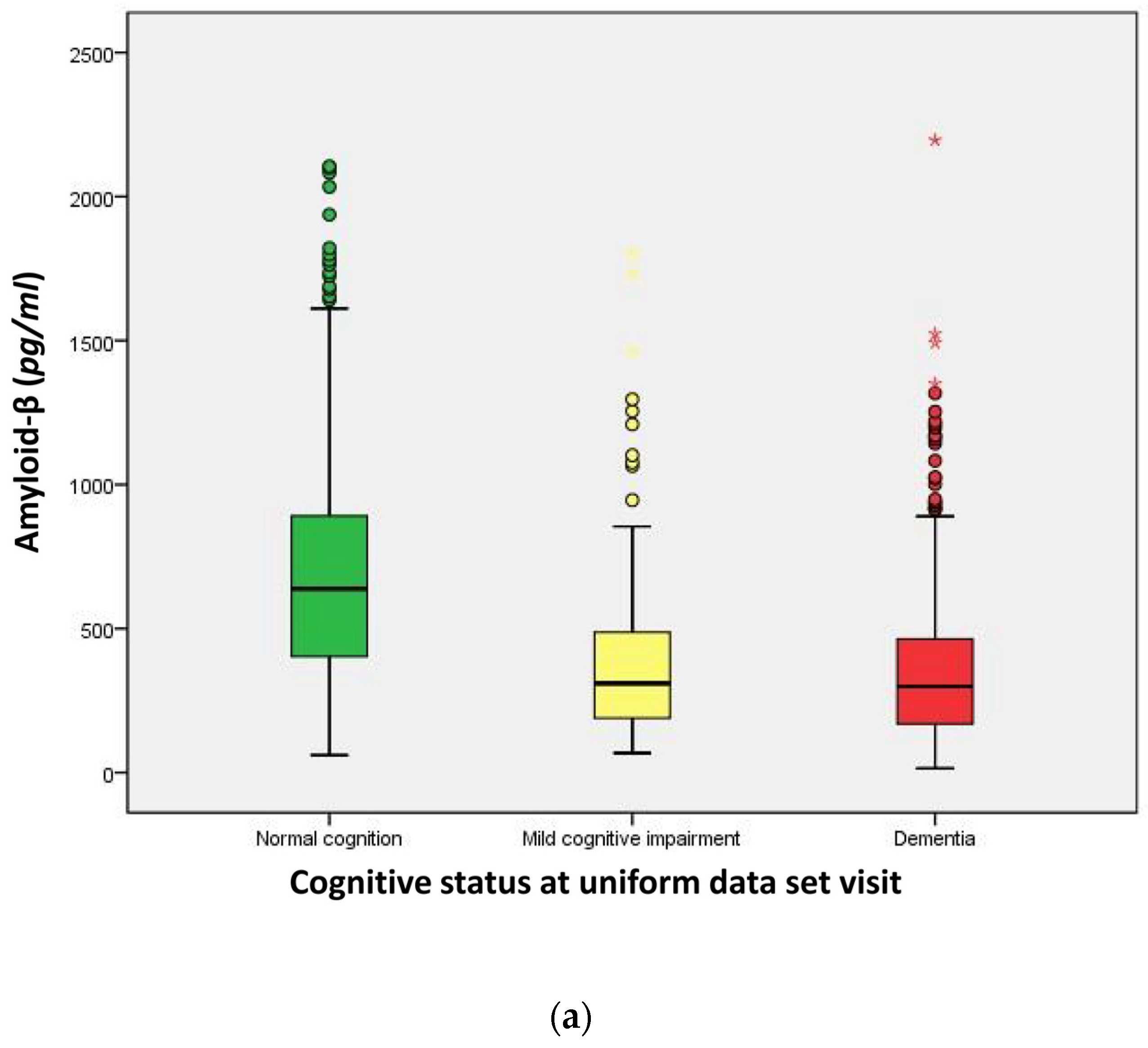
| Amyloid-β (Aβ42, (pg/mL)) | 656.0 | 300.6 | 298.8 | <0.001 a |
| P-tau181P (pg/mL) | 36.00 | 49.12 | 58.29 | <0.001 a |
| T-tau (pg/mL) | 241.0 | 140.6 | 298.3 | <0.001 a |
| P-tau/Aβ42 ratio | 0.058 | 0.144 | 0.209 | <0.001 a |
| T-tau/Aβ42 ratio | 0.305 | 0.560 | 0.941 | <0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Wang, S.-I.; Lin, H.-C.; Lin, E.S.; Yang, F.-P.; Chang, C.-M.; Wei, J.C.-C. Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient. Int. J. Mol. Sci. 2024, 25, 3919. https://doi.org/10.3390/ijms25073919
Hsu C-C, Wang S-I, Lin H-C, Lin ES, Yang F-P, Chang C-M, Wei JC-C. Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient. International Journal of Molecular Sciences. 2024; 25(7):3919. https://doi.org/10.3390/ijms25073919
Chicago/Turabian StyleHsu, Ching-Chi, Shiow-Ing Wang, Hong-Chun Lin, Eric S. Lin, Fan-Pei Yang, Ching-Mao Chang, and James Cheng-Chung Wei. 2024. "Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient" International Journal of Molecular Sciences 25, no. 7: 3919. https://doi.org/10.3390/ijms25073919
APA StyleHsu, C.-C., Wang, S.-I., Lin, H.-C., Lin, E. S., Yang, F.-P., Chang, C.-M., & Wei, J. C.-C. (2024). Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient. International Journal of Molecular Sciences, 25(7), 3919. https://doi.org/10.3390/ijms25073919









