Alzheimer’s Amyloid Hypothesis and Antibody Therapy: Melting Glaciers?
Abstract
1. Introduction
2. Disease Understanding and Diagnosis
3. Treatment
3.1. Clinical Efficacy

3.2. Approved Amyloid-PET Imaging Probes
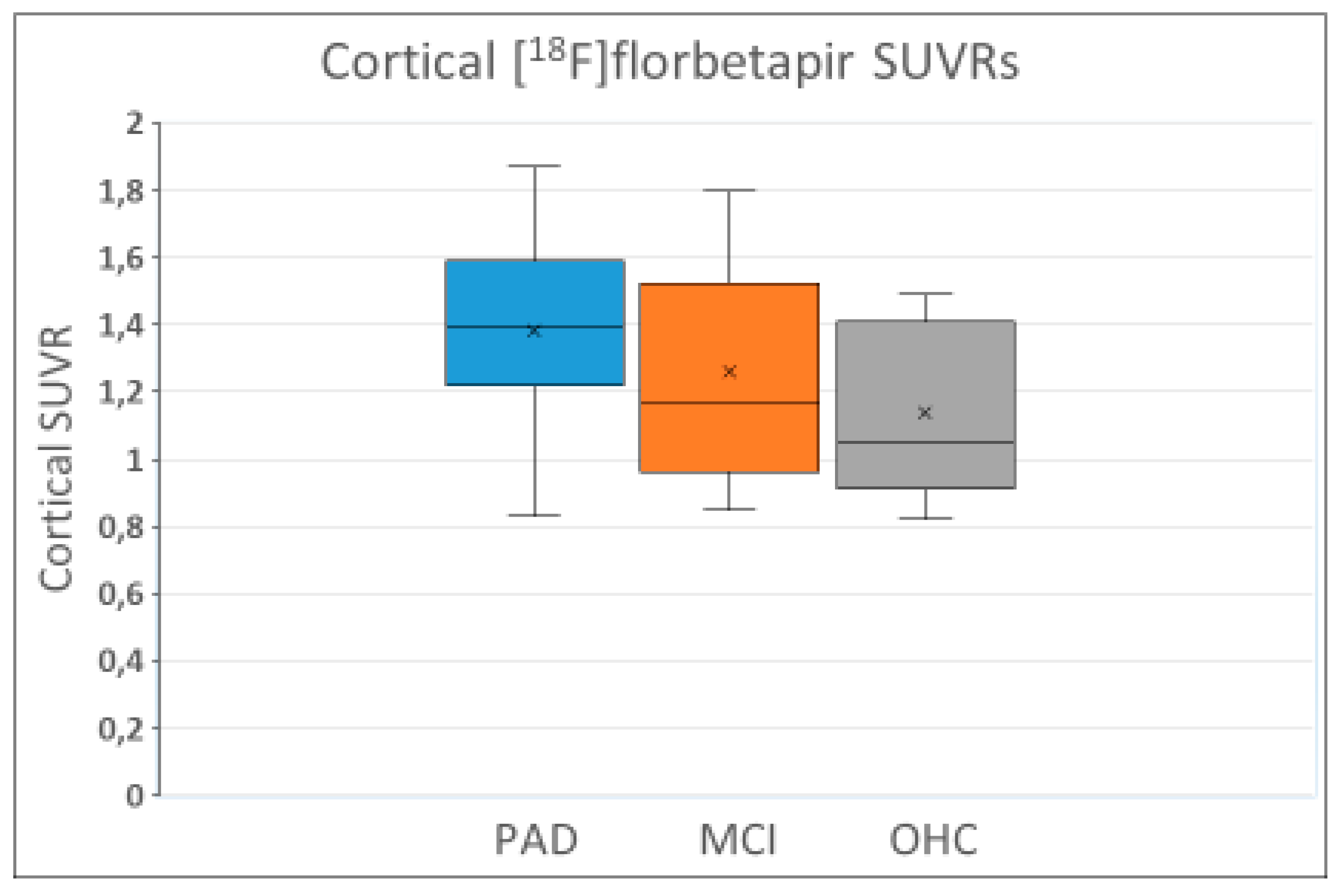
3.3. Amyloid Positivity
3.4. Amyloid Removal

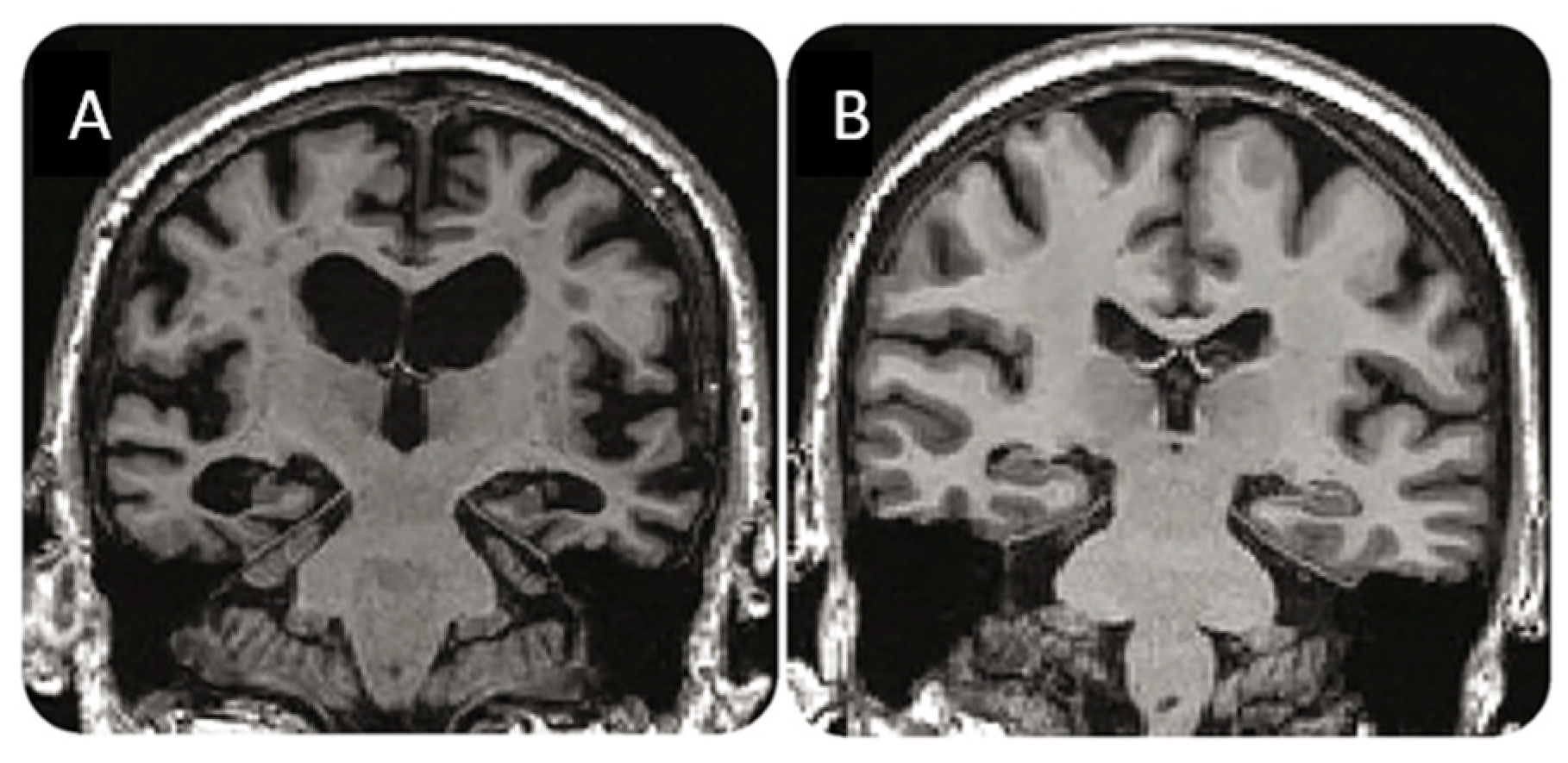
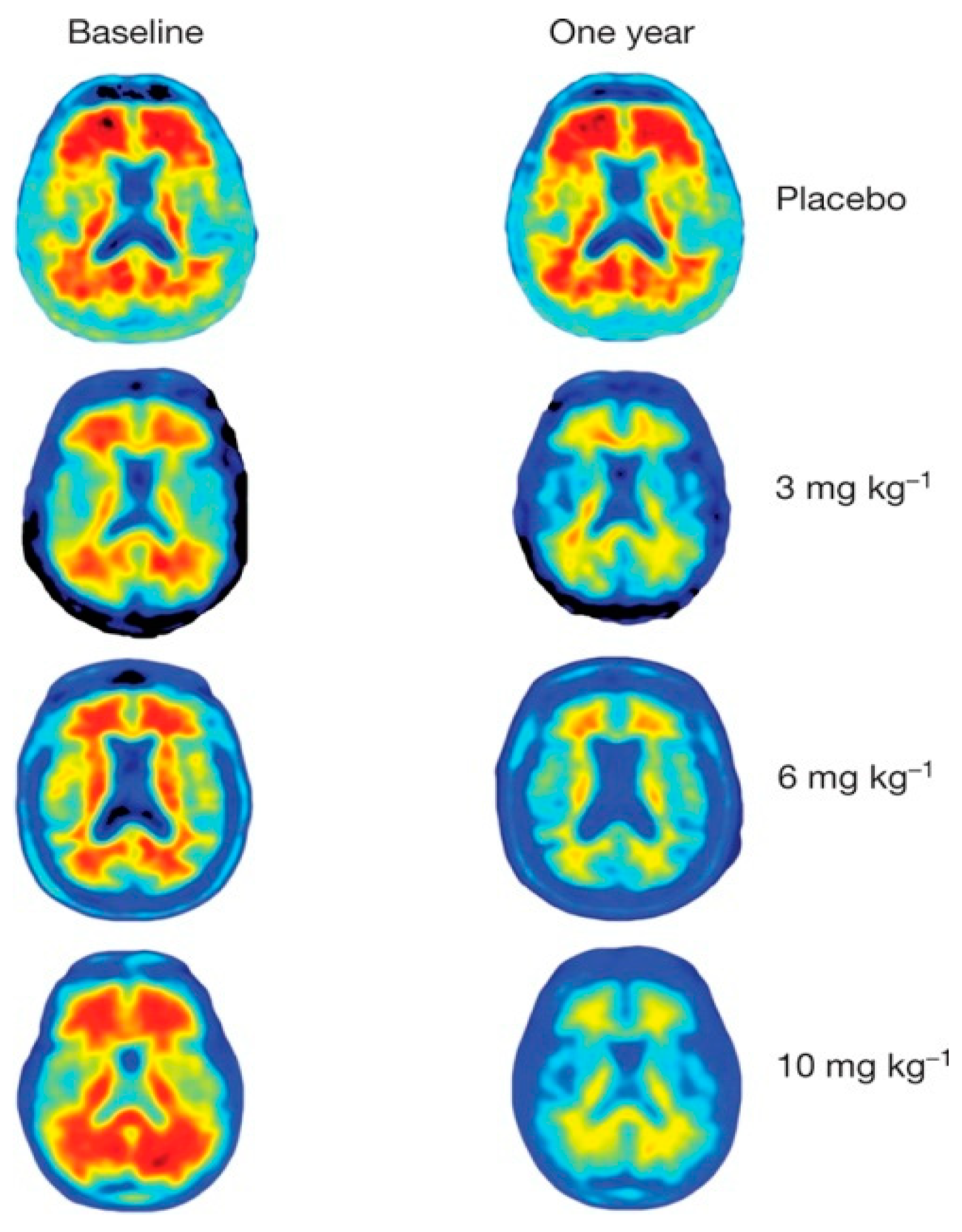
3.5. Summing Up
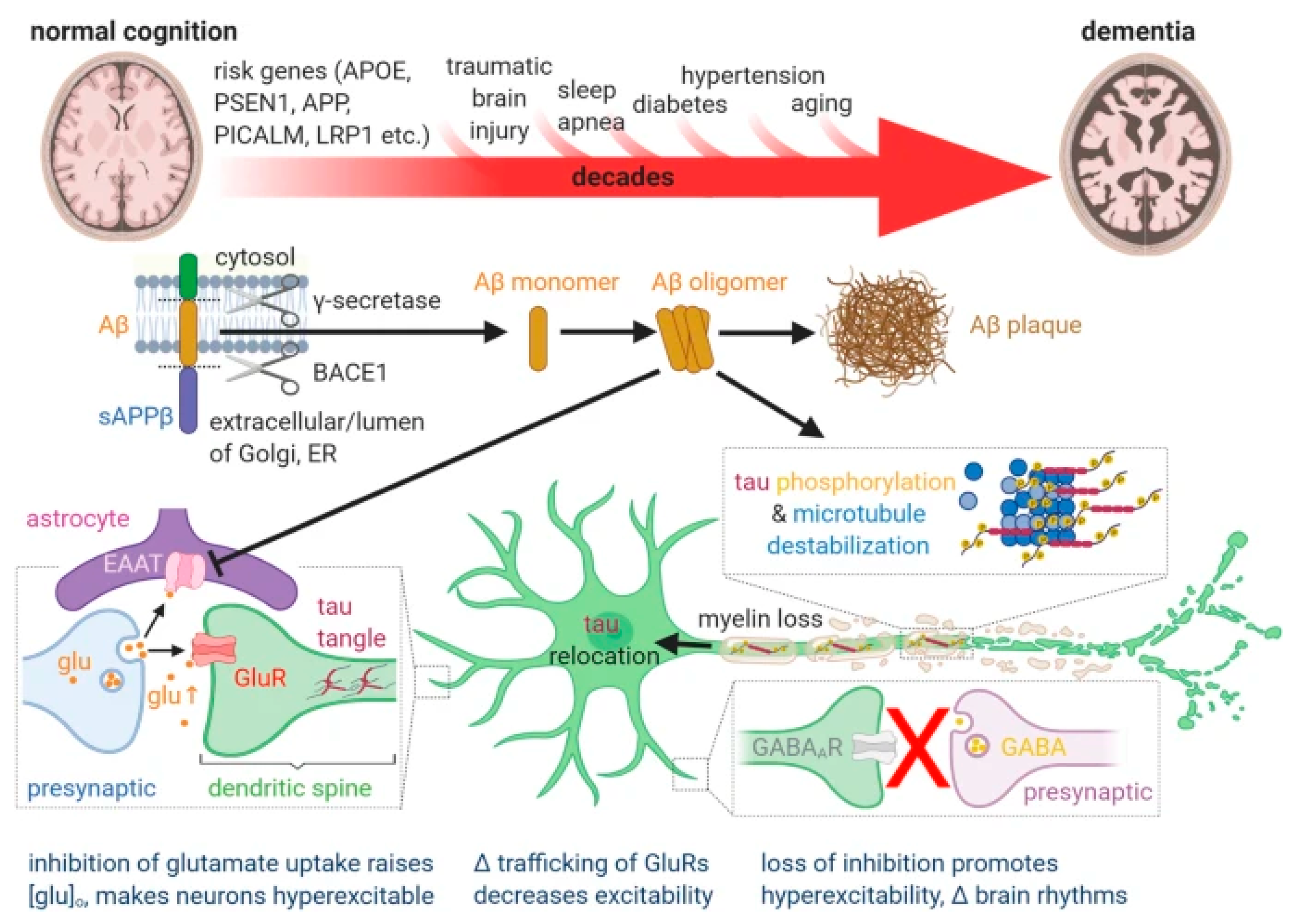
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s disease |
| ADAS-Cog | Alzheimer’s Disease Assessment Scale-Cognitive |
| APOE ε4 | Apolipoprotein E gene |
| ARIA | Amyloid-related imaging abnormality |
| ATN | Amyloid/tau/neuropathology |
| BBB | Blood–brain barrier |
| CDR-SB | Clinical Dementia Rating-Sum of Boxes |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CMS | Centers for Medicare & Medicaid Services |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| FDA | Food and Drug Administration |
| FDG | Fluorodeoxyglucose |
| iADRS | Integrated Alzheimer Disease Rating Scale |
| MCI | Mild cognitive impairment |
| MICD | Minimally important clinical difference |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| PiB | Pittsburgh compound-B |
| RCT | Randomized clinical trial |
| SUVR | Standardized uptake value ratio |
| ThT | Thioflavin-T |
References
- Neve, R.L.; Robakis, N.K. Alzheimer’s disease: A re-examination of the amyloid hypothesis. Trends Neurosci. 1998, 21, 15–19. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Shumeyko, S.A.; Vikhlyantsev, I.M.; Bobylev, A.G. Amyloids: The History of Toxicity and Functionality. Biology 2021, 10, 394. [Google Scholar] [CrossRef]
- Espay, A.J.; Sturchio, A.; Schneider, L.S.; Ezzat, K. Soluble Amyloid-beta Consumption in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 1403–1415. [Google Scholar] [CrossRef]
- Rischel, E.B.; Gejl, M.; Brock, B.; Rungby, J.; Gjedde, A. In Alzheimer’s disease, amyloid beta accumulation is a protective mechanism that ultimately fails. Alzheimers Dement. 2022. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Cavazzoni, P. FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. U.S. Food and Drug Administration. 7 June 2021. Available online: https://www.fda.gov/drugs/our-perspective/fdas-decision-approve-new-treatment-alzheimers-disease (accessed on 21 February 2024).
- U.S. Food and Drug Administration. FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. 6 July 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (accessed on 25 February 2024).
- Alzheimer’s Association. Donanemab for Treatment of Early Alzheimer’s Disease—News Pending FDA Review. 2024. Available online: https://www.alz.org/alzheimers-dementia/treatments/donanemab (accessed on 25 February 2024).
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta. Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Dante, G.; Scarpelli, D.G.; Burrows, W. Disease. Brittanica. Last Updated 15 February 2024. Available online: https://www.britannica.com/science/disease (accessed on 24 February 2024).
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Fur Psychiatr. Psych. Gerichtl. Medizin. 1907, 64, 146–148. [Google Scholar]
- Tagarelli, A.; Piro, A.; Tagarelli, G.; Lagonia, P.; Quattrone, A. Alois Alzheimer: A hundred years after the discovery of the eponymous disorder. Int. J. Biomed. Sci. 2006, 2, 196–204. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Alavi, A.; Revheim, M.E. Re: Aducanumab-Related ARIA: Paean or Lament? Clin. Nucl. Med. 2023, 48, 505–506. [Google Scholar] [CrossRef]
- Müller, E.G.; Edwin, T.H.; Stokke, C.; Navelsaker, S.S.; Babovic, A.; Bogdanovic, N.; Knapskog, A.B.; Revheim, M.E. Amyloid-β PET-Correlation with cerebrospinal fluid biomarkers and prediction of Alzheimer’s disease diagnosis in a memory clinic. PLoS ONE 2019, 14, e0221365. [Google Scholar] [CrossRef]
- Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. DRAFT Body Text as of 9 October 2023. Available online: https://aaic.alz.org/diagnostic-criteria.asp (accessed on 22 February 2024).
- Okafor, M.; Nye, J.A.; Shokouhi, M.; Shaw, L.M.; Goldstein, F.; Hajjar, I. 18F-Flortaucipir PET Associations with Cerebrospinal Fluid, Cognition, and Neuroimaging in Mild Cognitive Impairment due to Alzheimer’s Disease. J. Alzheimers Dis. 2020, 74, 589–601. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Amyloid Biomarker Study Group; Aalten, P.; Aarsland, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Roberts, R.O.; Aakre, J.A.; Kremers, W.K.; Vassilaki, M.; Knopman, D.S.; Mielke, M.M.; Alhurani, R.; Geda, Y.E.; Machulda, M.M.; Coloma, P.; et al. Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol. 2018, 75, 970–979. [Google Scholar] [CrossRef]
- Michalowska, M.M.; Herholz, K.; Hinz, R.; Amadi, C.; McInnes, L.; Anton-Rodriguez, J.M.; Karikari, T.K.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; et al. Evaluation of in vivo staging of amyloid deposition in cognitively unimpaired elderly aged 78–94. Mol. Psychiatry 2022, 27, 4335–4342. [Google Scholar] [CrossRef]
- Baik, K.; Kim, H.R.; Park, M.; Lee, Y.; Na, H.K.; Sohn, Y.H.; Seong, J.K.; Lee, P.H. Effect of Amyloid on Cognitive Performance in Parkinson’s Disease and Dementia with Lewy Bodies. Mov. Disord. 2023, 38, 278–285. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Jansen, W.J.; Rabinovici, G.D.; Knol, D.L.; van der Flier, W.M.; van Berckel, B.N.; Scheltens, P.; Visser, P.J.; Amyloid PET Study Group; Verfaillie, S.C.; et al. Prevalence of amyloid PET positivity in dementia syndromes: A meta-analysis. JAMA 2015, 313, 1939–1949. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Chen, K.; Liu, X.; Roontiva, A.; Thiyyagura, P.; Ayutyanont, N.; Joshi, A.D.; Clark, C.M.; Mintun, M.A.; Pontecorvo, M.J.; et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch. Neurol. 2011, 68, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Kepe, V.; Moghbel, M.C.; Långström, B.; Zaidi, H.; Vinters, H.V.; Huang, S.C.; Satyamurthy, N.; Doudet, D.; Mishani, E.; Cohen, R.M.; et al. Amyloid-β positron emission tomography imaging probes: A critical review. J. Alzheimers Dis. 2013, 36, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Scarioni, M.; Carandini, T.; Basilico, P.; Cadioli, M.; Giulietti, G.; Arighi, A.; Caprioli, M.; Serra, L.; Sina, C.; et al. CSF β-amyloid and white matter damage: A new perspective on Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 352–357. [Google Scholar] [CrossRef]
- Pietroboni, A.M.; Colombi, A.; Carandini, T.; Sacchi, L.; Fenoglio, C.; Marotta, G.; Arighi, A.; De Riz, M.A.; Fumagalli, G.G.; Castellani, M.; et al. Amyloid PET imaging and dementias: Potential applications in detecting and quantifying early white matter damage. Alzheimers Res. Ther. 2022, 14, 33. [Google Scholar] [CrossRef]
- Surmak, A.J.; Wong, K.P.; Cole, G.B.; Hirata, K.; Aabedi, A.A.; Mirfendereski, O.; Mirfendereski, P.; Yu, A.S.; Huang, S.C.; Ringman, J.M.; et al. Probing Estrogen Sulfotransferase-Mediated Inflammation with [11C]-PiB in the Living Human Brain. J. Alzheimers Dis. 2020, 73, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Rogne, A.G.; Müller, E.G.; Udnaes, E.; Sigurdardottir, S.; Raudeberg, R.; Connelly, J.P.; Revheim, M.E.; Hassel, B.; Dahlberg, D. β-Amyloid may accumulate in the human brain after focal bacterial infection: An 18 F-flutemetamol positron emission tomography study. Eur. J. Neurol. 2021, 28, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; Wang, Y.; Zeng, F. Case of Autosomal Dominant Alzheimer Disease With Negative Findings From PiB-PET Examination. Neurol. Genet. 2023, 10, e200119. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, N.S.; Chung, C.H.; Lin, F.H.; Chiang, C.P.; Yeh, C.B.; Huang, S.Y.; Lu, R.B.; Chang, H.A.; Kao, Y.C.; Yeh, H.W.; et al. Anti-herpetic Medications and Reduced Risk of Dementia in Patients with Herpes Simplex Virus Infections-a Nationwide, Population-Based Cohort Study in Taiwan. Neurotherapeutics 2018, 15, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, R.F. Overwhelming Evidence for a Major Role for Herpes Simplex Virus Type 1 (HSV1) in Alzheimer’s Disease (AD); Underwhelming Evidence against. Vaccines 2021, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Ecarnot, F.; Boccardi, V.; Calcagno, A.; Franceschi, C.; Fülop, T.; Itzhaki, R.F.; Michel, J.P.; Panza, F.; Rainero, I.; Solfrizzi, V.; et al. Dementia, infections and vaccines: 30 years of controversy. Aging Clin Exp. Res. 2023, 35, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Malignant Brain Aging: The Formidable Link Between Dysregulated Signaling Through Mechanistic Target of Rapamycin Pathways and Alzheimer’s Disease (Type 3 Diabetes). J. Alzheimers Dis. 2023, 95, 1301–1337. [Google Scholar] [CrossRef] [PubMed]
- Celsis, P.; Agniel, A.; Puel, M.; Rascol, A.; Marc-Vergnes, J.P. Focal cerebral hypoperfusion and selective cognitive deficit in dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry. 1987, 50, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. Deciphering Alzheimer’s Disease Pathogenic Pathway: Role of Chronic Brain Hypoperfusion on p-Tau and mTOR. J. Alzheimers Dis. 2021, 79, 1381–1396. [Google Scholar] [CrossRef]
- Hattori, Y.; Kakino, Y.; Hattori, Y.; Iwashita, M.; Uchiyama, H.; Noda, K.; Yoshimoto, T.; Iida, H.; Ihara, M. Long-Term Resveratrol Intake for Cognitive and Cerebral Blood Flow Impairment in Carotid Artery Stenosis/Occlusion. J. Stroke. 2024, 26, 64–74. [Google Scholar] [CrossRef]
- Hase, Y.; Jobson, D.; Cheong, J.; Gotama, K.; Maffei, L.; Hase, M.; Hamdan, A.; Ding, R.; Polivkoski, T.; Horsburgh, K.; et al. Hippocampal capillary pericytes in post-stroke and vascular dementias and Alzheimer’s disease and experimental chronic cerebral hypoperfusion. Acta. Neuropathol. Commun. 2024, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.L.; Jiang, H.; Wildburger, N.; Esparza, T.J. Non-canonical soluble amyloid-beta aggregates and plaque buffering: Controversies and future directions for target discovery in Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 62. [Google Scholar] [CrossRef]
- Zhang, W.B.; Huang, Y.; Guo, X.R.; Zhang, M.Q.; Yuan, X.S.; Zu, H.B. DHCR24 reverses Alzheimer’s disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice. Acta Neuropathol. Commun. 2023, 11, 102. [Google Scholar] [CrossRef]
- García-Lluch, G.; Peña-Bautista, C.; Royo, L.M.; Baquero, M.; Cañada-Martínez, A.J.; Cháfer-Pericás, C. Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study. Pharmaceutics 2023, 15, 924. [Google Scholar] [CrossRef] [PubMed]
- Mullane, K.; Williams, M. Alzheimer’s disease beyond amyloid: Can the repetitive failures of amyloid-targeted therapeutics inform future approaches to dementia drug discovery? Biochem. Pharmacol. 2020, 177, 113945. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022, 8, e12295. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics 2023, 13, 4138–4165. [Google Scholar] [CrossRef]
- Kelly, L.; Sharp, M.M.; Thomas, I.; Brown, C.; Schrag, M.; Antunes, L.V.; Solopova, E.; Martinez-Gonzalez, J.; Rodríguez, C.; Carare, R.O. Targeting lysyl-oxidase (LOX) may facilitate intramural periarterial drainage for the treatment of Alzheimer’s disease. Cereb. Circ.-Cogn. Behav. 2023, 5, 100171. [Google Scholar] [CrossRef] [PubMed]
- Terao, I.; Kodama, W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 2024, 94, 102203. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Orgogozo, J.-M.; Gilman, S.; Dartigues, J.-F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bullock, R.; Love, S.; Neal, J.W.; et al. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Buckland, G.R.; Harrison, C.H.; Page, A.; Harris, S.; Love, S.; Neal, J.W.; Holmes, C.; Boche, D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain 2019, 142, 2113–2126. [Google Scholar] [CrossRef]
- Biogen. Biogen to Realign Resources for Alzheimer’s Disease Franchise. 31 January 2024. NEWS RELEASE. Available online: https://investors.biogen.com/news-releases/news-release-details/biogen-realign-resources-alzheimers-disease-franchise (accessed on 26 February 2024).
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Eisai Global. Lecanemab Confirmatory Phase 3 CLARITY AD Study Met Primary Endpoint, Showing Highly Statistically Significant Reduction of Clinical Decline in Large Global Clinical Study of 1795 Participants with Early Alzheimer’s Disease. 28 September 2022. Available online: https://www.eisai.com/news/2022/news202271.html (accessed on 21 February 2024).
- PR Newswire. Lilly’s Donanemab Significantly Slowed Cognitive and Functional De-cline in Phase 3 Study of Early Alzheimer’s Disease. 3 May 2023. Available online: https://www.prnewswire.com/news-releases/lillys-donanemab-significantly-slowed-cognitive-and-functional-decline-in-phase-3-study-of-early-alzheimers-disease-301814001.html (accessed on 21 February 2024).
- Rountree, S.D.; Chan, W.; Pavlik, V.N.; Darby, E.J.; Siddiqui, S.; Doody, R.S. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009, 1, 7. [Google Scholar] [CrossRef]
- Tricco, A.C.; Ashoor, H.M.; Soobiah, C.; Rios, P.; Veroniki, A.A.; Hamid, J.S.; Ivory, J.D.; Khan, P.A.; Yazdi, F.; Ghassemi, M.; et al. Comparative Effectiveness and Safety of Cognitive Enhancers for Treating Alzheimer’s Disease: Systematic Review and Network Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Ashoor, H.M.; Rios, P.; Seitidis, G.; Stewart, L.; Clarke, M.; Tudur-Smith, C.; Mavridis, D.; Hemmelgarn, B.R.; Holroyd-Leduc, J.; et al. Comparative safety and efficacy of cognitive enhancers for Alzheimer’s dementia: A systematic review with individual patient data network meta-analysis. BMJ. Open 2022, 12, e053012. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Xu, H.; Garcia-Ptacek, S.; Jönsson, L.; Wimo, A.; Nordström, P.; Eriksdotter, M. Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality. Neurology 2021, 96, e2220–e2230. [Google Scholar] [CrossRef]
- Andrews, J.S.; Desai, U.; Kirson, N.Y.; Zichlin, M.L.; Ball, D.E.; Matthews, B. Disease sever-ity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement. 2019, 5, 354–363. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; O’Dell, R.S.; Mecca, A.P. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimeir’s disease clinical trial. Alzheimers Dement. 2023, 9, e12388. [Google Scholar] [CrossRef]
- Andrews, J.S.; Desai, U.; Kirson, N.Y.; Matthews, B.R. Response to van Dyck, O’Dell, & Mecca letter to the editor regarding Andrews et al. (2019). Alzheimers Dement. 2023, 9, e12387. [Google Scholar]
- Lansdall, C.J.; McDougall, F.; Butler, L.M.; Delmar, P.; Pross, N.; Qin, S.; McLeod, L.; Zhou, X.; Kerchner, G.A.; Doody, R.S. Establishing clinically meaningful change on outcome assessments frequently used in trials of mild cognitive impairment due to Alzheimer’s disease. J. Prev. Alzheimers Dis. 2023, 10, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ebell, H.E.; Barry, H.C.; Baduni, I.; Grasso, G. Clinically Important Benefits and Harms of Monoclonal Antibodies Targeting Amyloid for the Treatment of Alzheimer Disease: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2024, 22, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis From Cohort Studies. Front. Aging. Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Thambisetty, M.; Howard, R. Lecanemab trial in AD brings hope but requires greater clarity. Nat. Rev. Neurol. 2023, 19, 132–133. [Google Scholar] [CrossRef]
- Alves, F.; Kalinowski, P.; Ayton, S. Accelerated Brain Volume Loss Caused by Anti-β-Amyloid Drugs: A Systematic Review and Meta-analysis. Neurology 2023, 100, e2114–e2124. [Google Scholar] [CrossRef]
- Centers for Medicare & Medicaid Services. Beta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease. CAG-00431R. 13 October 2023. Available online: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=308 (accessed on 28 February 2024).
- Høilund-Carlsen, P.F.; Alavi, A.; Barrio, J.R. Better Access to Amyloid-PET Scans Won’t Reduce Racial Inequities in Alzheimer’s. STAT+. 14 November 2022. Available online: https://www.statnews.com/2022/11/14/better-access-amyloid-pet-scans-alzheimers-inequities (accessed on 28 February 2024).
- Villemagne, V.L.; Klunk, W.E.; Mathis, C.A.; Rowe, C.C.; Brooks, D.J.; Hyman, B.T.; Ikonomovic, M.D.; Ishii, K.; Jack, C.R.; Jagust, W.J.; et al. Aβ Imaging: Feasible, pertinent, and vital to progress in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2012, 39, 209–219. [Google Scholar] [CrossRef]
- Alavi, A.; Barrio, J.R.; Werner, T.J.; Khosravi, M.; Newberg, A.; Høilund-Carlsen, P.F. Suboptimal validity of amyloid imaging-based diagnosis and management of Alzheimer’s disease: Why it is time to abandon the approach. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 225–230. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Revheim, M.E.; Costa, T.; Alavi, A.; Kepp, K.P.; Sensi, S.L.; Perry, G.; Barrio, J.R.; Vissel, B. Passive Alzheimer’s Immunotherapy: A promising or uncertain option? Ageing Res. Rev. 2023, 90, 101996. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.B.; Keum, G.; Liu, J.; Small, G.W.; Satyamurthy, N.; Kepe, V.; Barrio, J.R. Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates. Proc. Natl. Acad. Sci. USA 2010, 107, 6222–6227. [Google Scholar] [CrossRef]
- Cselényi, Z.; Jönhagen, M.E.; Forsberg, A.; Halldin, C.; Julin, P.; Schou, M.; Johnström, P.; Varnäs, K.; Svensson, S.; Farde, L. Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J. Nucl. Med. 2012, 53, 415–424. [Google Scholar] [CrossRef]
- Choi, S.R.; Golding, G.; Zhuang, Z.; Zhang, W.; Lim, N.; Hefti, F.; Benedum, T.E.; Kilbourn, M.R.; Skovronsky, D.; Kung, H.F. Preclinical properties of 18F-AV-45: A PET agent for Abeta plaques in the brain. J. Nucl. Med. 2009, 50, 1887–1894. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, X.; Qiao, J.; Zhu, L. Study the pharmacokinetics of AV-45 in rat plasma and metabolism in liver microsomes by ultra-performance liquid chromatography with mass spectrometry. Biomed. Chromatogr. 2012, 26, 666–671. [Google Scholar] [CrossRef]
- Silverman, D.H.S.; Melega, W.P. Molecular Imaging of Biological Processes with PET: Evaluating Biologic Bases of Cerebral Function. In PET Molecular Imaging and Its Biological Applications; Phelps, M.E., Ed.; Springer: New York, NY, USA, 2003; Chapter 7; pp. 509–583. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Klunk, W.E.; Abrahamson, E.E.; Mathis, C.A.; Price, J.C.; Tsopelas, N.D.; Lopresti, B.J.; Ziolko, S.; Bi, W.; Paljug, W.R.; et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 2008, 131, 1630–1645. [Google Scholar] [CrossRef]
- Kadir, A.; Marutle, A.; Gonzalez, D.; Schöll, M.; Almkvist, O.; Mousavi, M.; Mustafiz, T.; Darreh-Shori, T.; Nennesmo, I.; Nordberg, A. Positron emission tomography imaging and clinical progression in relation to molecular pathology in the first Pittsburgh Compound B positron emission tomography patient with Alzheimer’s disease. Brain 2011, 134, 301–317. [Google Scholar] [CrossRef]
- Sojkova, J.; Driscoll, I.; Iacono, D.; Zhou, Y.; Codispoti, K.E.; Kraut, M.A.; Ferrucci, L.; Pletnikova, O.; Mathis, C.A.; Klunk, W.E.; et al. In vivo fibrillar beta-amyloid detected using [11C]PiB positron emission tomography and neuropathologic assessment in older adults. Arch. Neurol. 2011, 68, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Trudler, D.; Nazor, K.L.; Eisele, Y.S.; Grabauskas, T.; Dolatabadi, N.; Parker, J.; Sultan, A.; Zhong, Z.; Goodwin, M.S.; Levites, Y.; et al. Soluble α-synuclein-antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc. Natl. Acad. Sci. USA 2021, 118, e2025847118. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Kaneta, T.; Okamura, N.; Tashiro, M.; Iwata, R.; Takanami, K.; Fukuda, H.; Takahashi, S.; Yanai, K.; Kudo, Y.; et al. Pitfalls of voxel-based amyloid PET analyses for diagnosis of Alzheimer’s disease: Artifacts due to non-specific uptake in the white matter and the skull. Tohoku J. Exp. Med. 2014, 234, 175–181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collins-Praino, L.E.; Francis, Y.I.; Griffith, E.Y.; Wiegman, A.F.; Urbach, J.; Lawton, A.; Ho-nig, L.S.; Cortes, E.; Vonsattel, J.P.; Canoll, P.D.; et al. Soluble amyloid beta levels are elevated in the white matter of Alzheimer’s patients, independent of cortical plaque severity. Acta. Neuropathol. Commun. 2014, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, A.; Silva-Rodríguez, J.; Aldrey, J.M.; Cortés, J.; Pías-Peleteiro, J.M.; Ruibal, Á.; Aguiar, P.; Alzheimer’s Disease Neuroimaging Initiative. 18F-florbetapir PET as a marker of myelin integrity across the Alzheimer’s disease spectrum. Eur. J. Nucl. Med. Mol. Imaging. 2022, 49, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Recent update on the heterogeneity of the Alzheimer’s disease spec-trum. J. Neural. Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Yu, L.; Leurgans, S.E.; Wilson, R.S.; Brookmeyer, R.; Schneider, J.A.; Bennett, D.A. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann. Neurol. 2019, 85, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Janssen, O.; Tijms, B.M.; Vos, S.J.B.; Ossenkoppele, R.; Visser, P.J.; Amyloid Biomarker Study Group. Prevalence Estimates of Amyloid Abnormality Across the Alzheimer Disease Clinical Spectrum. JAMA Neurol. 2022, 79, 228–243. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019, 76, 1174–1183. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Alavi, A. Aducanumab (Marketed as Aduhelm) Approval Is Likely Based on Misinterpretation of PET Imaging Data. J. Alzheimers Dis. 2021, 84, 1457–1460. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Revheim, M.E.; Costa, T.; Kepp, K.P.; Castellani, R.J.; Perry, G.; Alavi, A.; Barrio, J.R. FDG-PET versus Amyloid-PET Imaging for Diagnosis and Response Evaluation in Alzheimer’s Disease: Benefits and Pitfalls. Diagnostics 2023, 13, 2254. [Google Scholar] [CrossRef] [PubMed]
- López-González, F.J.; Moscoso, A.; Efthimiou, N.; Fernández-Ferreiro, A.; Piñeiro-Fiel, M.; Archibald, S.J.; Aguiar, P.; Silva-Rodríguez, J.; Alzheimer’s Disease Neuroimaging Initiative. Spill-in counts in the quantification of 18F-florbetapir on Aβ-negative subjects: The effect of including white matter in the reference region. EJNMMI Phys. 2019, 6, 27. [Google Scholar] [CrossRef]
- Flores, S.; Chen, C.D.; Su, Y.; Dincer, A.; Keefe, S.J.; McKay, N.S.; Paulick, A.M.; Perez-Carrillo, G.G.; Wang, L.; Hornbeck, R.C.; et al. Investigating Tau and Amyloid Tracer Skull Binding in Studies of Alzheimer Disease. J. Nucl. Med. 2023, 64, 287–293. [Google Scholar] [CrossRef]
- Alavi, A.; Werner, T.J.; Høilund-Carlsen, P.F.; Zaidi, H. Correction for Partial Volume Effect Is a Must, Not a Luxury, to Fully Exploit the Potential of Quantitative PET Imaging in Clinical Oncology. Mol. Imaging Biol. 2018, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Reish, N.J.; Jamshidi, P.; Stamm, B.; Flanagan, M.E.; Sugg, E.; Tang, M.; Donohue, K.L.; McCord, M.; Krumpelman, C.; Mesulam, M.M.; et al. Multiple Cerebral Hemorrhages in a Patient Receiving Lecanemab and Treated with t-PA for Stroke. N. Engl. J. Med. 2023, 388, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Shanes, E.D.; McCord, M.; Reish, N.J.; Flanagan, M.E.; Mesulam, M.M.; Jamshidi, P. Neuropathology of Anti-Amyloid-β Immunotherapy: A Case Report. J. Alzheimers Dis. 2023, 93, 803–813. [Google Scholar] [CrossRef]
- Solopova, E.; Romero-Fernandez, W.; Harmsen, H.; Ventura-Antunes, L.; Wang, E.; Shos-tak, A.; Maldonado, J.; Donahue, M.J.; Schultz, D.; Coyne, T.M.; et al. Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanem-ab for Alzheimer’s disease. Nat. Commun. 2023, 14, 8220. [Google Scholar] [CrossRef]
- Perry, V.H.; Andersson, P.B.; Gordon, S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993, 16, 268–273. [Google Scholar] [CrossRef]
- Lassmann, H.; Zimprich, F.; Rössler, K.; Vass, K. Inflammation in the nervous system. Basic mechanisms and immunological concepts. Rev. Neurol. 1991, 147, 763–781. [Google Scholar]
- Askenase, M.H.; Sansing, L.H. Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage. Semin. Neurol. 2016, 36, 288–297. [Google Scholar] [CrossRef]
- Dunleavy, K. 3 More Deaths among Patients on Biogen’s Aduhelm Fuel Safety Concerns, Though no Link Established: Analyst. Fierce Pharm 16 February 2022. Available online: https://www.fiercepharma.com/pharma/death-3-more-patients-biogen-s-aduhelm-fuel-more-concern-about-drug-s-safety-though-no-link (accessed on 21 February 2024).
- Høilund-Carlsen, P.F.; Revheim, M.E.; Alavi, A. Alzheimer’s Disease at a Crossroad: Time to Part from Amyloid to More Promising Aspects-Atherosclerosis for a Start. J. Alzheimers Dis. 2022, 88, 455–458. [Google Scholar] [CrossRef]

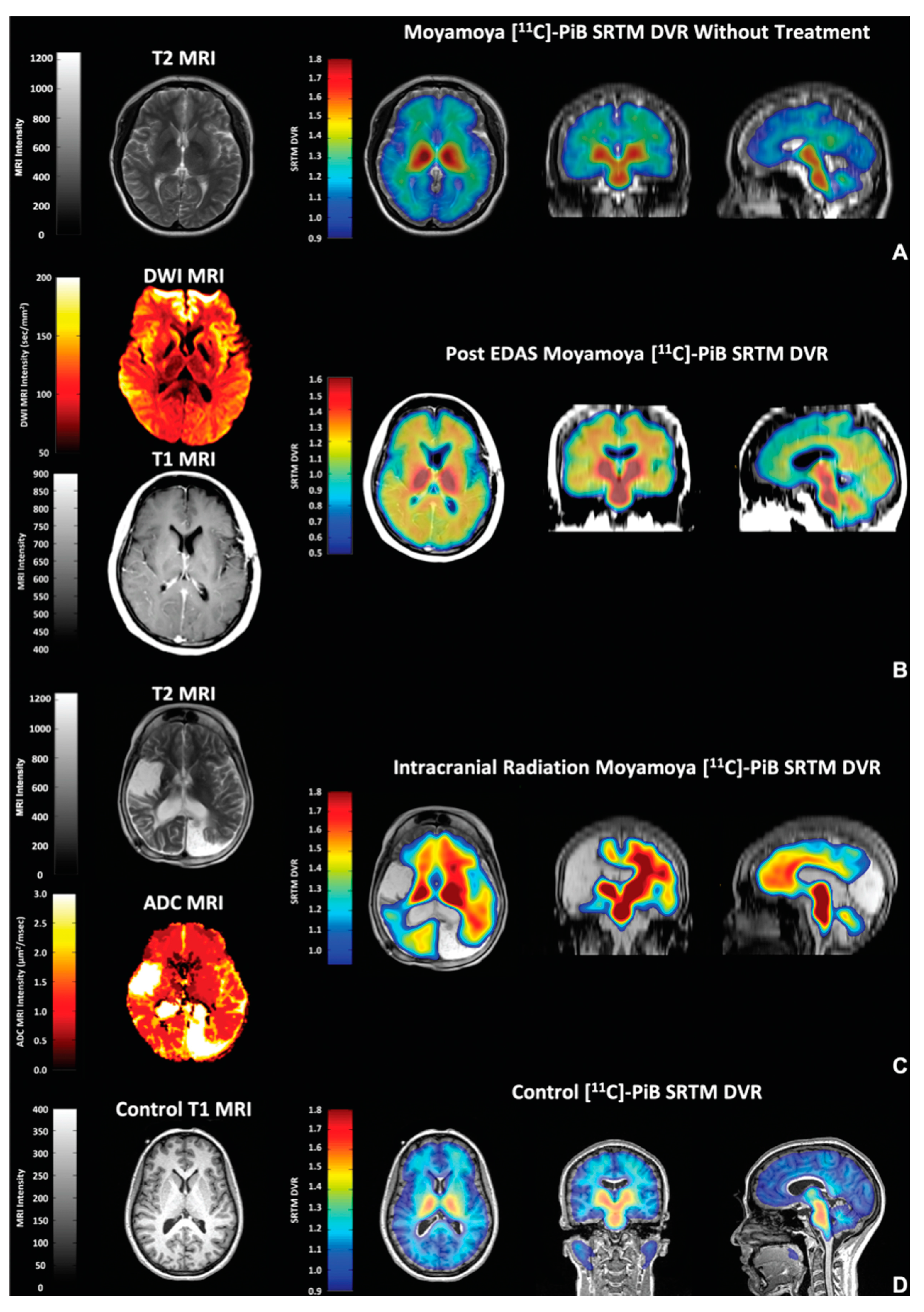
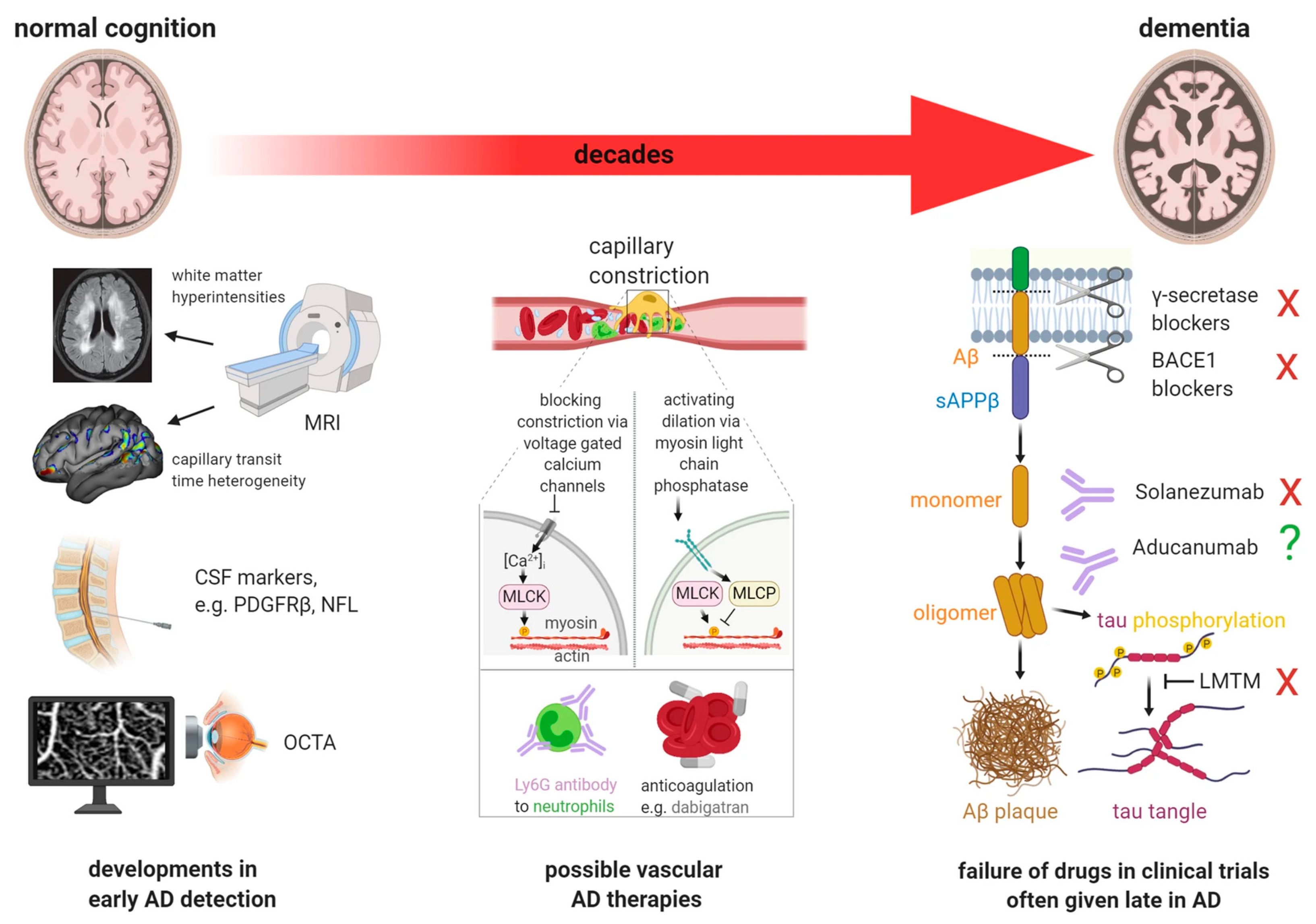
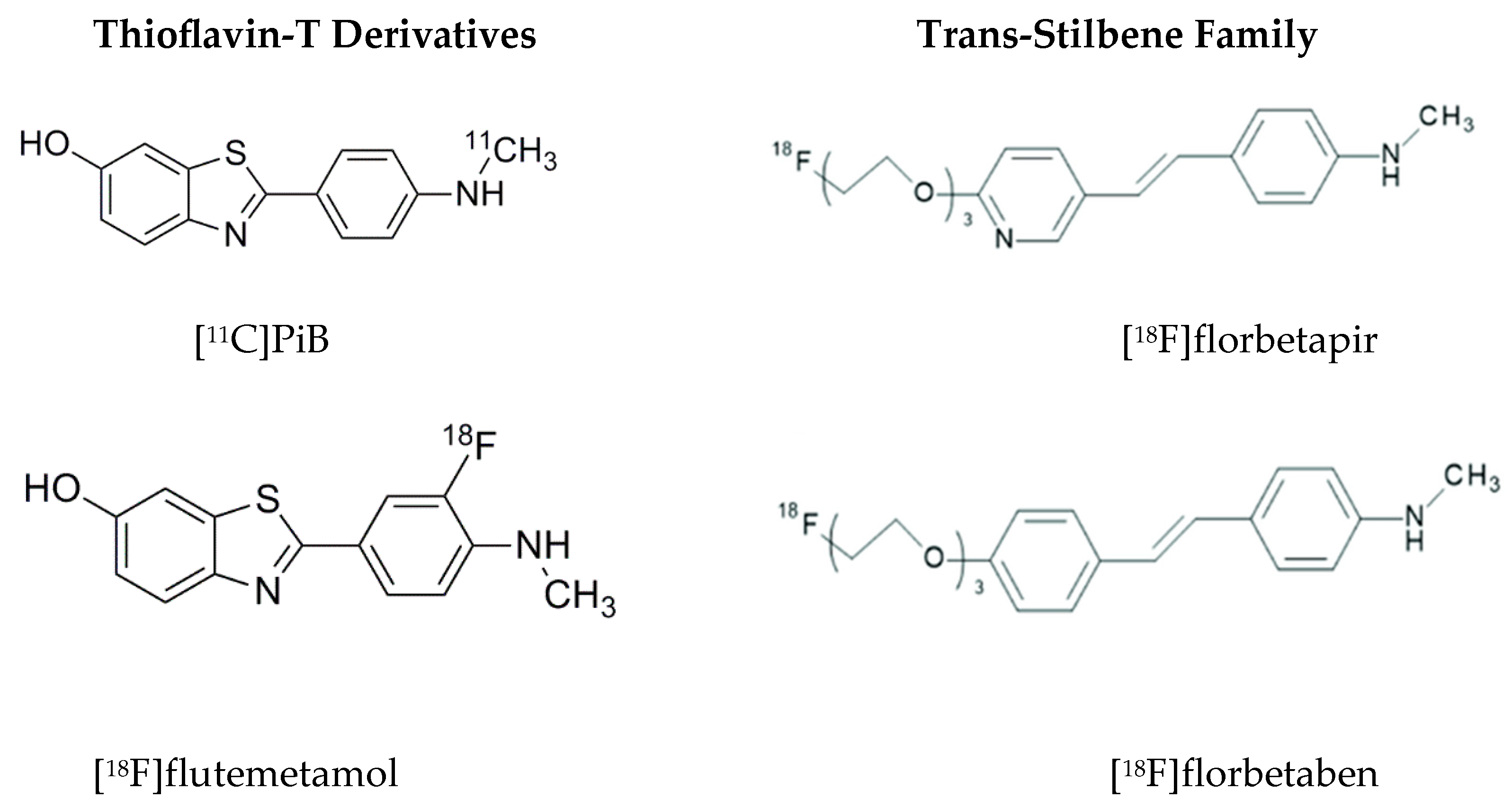
| Topic | Issue | Question | Common Perception | Independent Evidence |
|---|---|---|---|---|
| Cause of AD | Cortical amyloid deposition | Valid? | Yes | No |
| Diagnosis | ATN classification | Valid? | Yes | No |
| Amyloid positivity | By PET | Valid marker of AD? | Yes | No |
| Amyloid positivity | By CSF/plasma tests | Valid marker of AD? | Yes | No |
| Passive immunotherapy | Efficacy | Effective? | Yes | No |
| Passive immunotherapy | Amyloid removal | Therapy-induced? | Yes | No |
| Passive immunotherapy | ARIAs | Therapy-induced? | Yes | Yes |
| Passive immunotherapy | Brain volume loss | Therapy-induced? | Unknown | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Høilund-Carlsen, P.F.; Alavi, A.; Castellani, R.J.; Neve, R.L.; Perry, G.; Revheim, M.-E.; Barrio, J.R. Alzheimer’s Amyloid Hypothesis and Antibody Therapy: Melting Glaciers? Int. J. Mol. Sci. 2024, 25, 3892. https://doi.org/10.3390/ijms25073892
Høilund-Carlsen PF, Alavi A, Castellani RJ, Neve RL, Perry G, Revheim M-E, Barrio JR. Alzheimer’s Amyloid Hypothesis and Antibody Therapy: Melting Glaciers? International Journal of Molecular Sciences. 2024; 25(7):3892. https://doi.org/10.3390/ijms25073892
Chicago/Turabian StyleHøilund-Carlsen, Poul F., Abass Alavi, Rudolph J. Castellani, Rachael L. Neve, George Perry, Mona-Elisabeth Revheim, and Jorge R. Barrio. 2024. "Alzheimer’s Amyloid Hypothesis and Antibody Therapy: Melting Glaciers?" International Journal of Molecular Sciences 25, no. 7: 3892. https://doi.org/10.3390/ijms25073892
APA StyleHøilund-Carlsen, P. F., Alavi, A., Castellani, R. J., Neve, R. L., Perry, G., Revheim, M.-E., & Barrio, J. R. (2024). Alzheimer’s Amyloid Hypothesis and Antibody Therapy: Melting Glaciers? International Journal of Molecular Sciences, 25(7), 3892. https://doi.org/10.3390/ijms25073892










