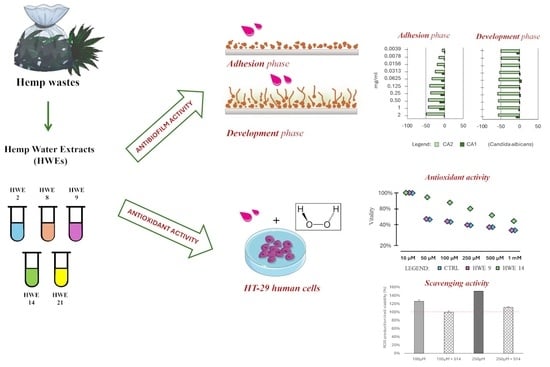
Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. HWEs Chemo-Physical Characterization
2.2. HWEs Chemical NMR Characterization
2.3. Antifungal Susceptibility Testing
2.4. Influence of HWEs on Biofilm Adhesion and Biofilm Development
2.5. Antioxidant Activity of HWEs on HT-29 Colon Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Hemp Waste
4.2. Extraction Procedure
4.2.1. Short-Term Maceration Process
4.2.2. Protein Precipitation
4.2.3. Long-Term Maceration Process
4.3. Hemp Water Extracts (HWEs)
4.4. HWEs Chemo-Physical Characterization
4.4.1. PH, Electrical Conductivity (EC) and Salt Concentration
4.4.2. Dry Weight
4.4.3. Protein Quantification by Bradford Assay
4.4.4. NMR
4.5. Antimicrobial Activity Assay
4.6. Biofilm Formation
4.7. HWEs Activity on HT-29 Colon Rectal Human Tumor Cell Line
4.7.1. HT-29 Cell Culture
4.7.2. Antioxidant Activity and MTT Cell Viability Assay
4.7.3. Evaluation of Intracellular ROS Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berardo, M.E.V.; Mendieta, J.R.; Villamonte, M.D.; Colman, S.L.; Nercessian, D. Antifungal and antibacterial activities of Cannabis sativa L. resins. J. Ethnopharmacol. 2024, 318, 116839. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef]
- Gupte, A.P.; Basaglia, M.; Casella, S.; Favaro, L. Rice waste streams as a promising source of biofuels: Feedstocks, biotechnologies and future perspectives. Renew. Sustain. Energy Rev. 2022, 167, 112673. [Google Scholar] [CrossRef]
- Persia, D.; Mangiavacchi, F.; Marcotullio, M.C.; Rosati, O. Cannabinoids as multifaceted compounds. Phytochemistry 2023, 212, 113718. [Google Scholar] [CrossRef]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; López, V. Evaluation of two different Cannabis sativa L. extracts as antioxidant and neuroprotective agents. Front. Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 19, 174167. [Google Scholar] [CrossRef]
- Baratta, F.; Pignata, I.; Ravetto Enri, L.; Brusa, P. Cannabis for medical use: Analysis of recent clinical trials in view of current legislation. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef]
- Skliamis, K.; Benschop, A.; Korf, D.J. Cannabis users and stigma: A comparison of users from European countries with different cannabis policies. Eur. J. Criminol. 2022, 19, 1483–1500. [Google Scholar] [CrossRef]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and process modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-biofilm activity of cannabidiol against Candida Albicans. Microorg. 2021, 9, 441. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Mazzara, E.; Carletti, R.; Petrelli, R.; Mustafa, A.M.; Caprioli, G.; Fiorini, D.; Scortichini, S.; Dall’Acqua, S.; Sut, S.; Nuñez, S. Green extraction of hemp (Cannabis sativa L.) using microwave method for recovery of three valuable fractions (essential oil, phenolic compounds and cannabinoids): A central composite design optimization study. J. Sci. Food Agric. 2022, 102, 6220–6235. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165771. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.; Chapman, K.E.; Colborn, K.L.; Knupp, K.G. Duration of use of oral cannabis extract in a cohort of pediatric epilepsy patients. Epilepsia 2017, 58, 123–127. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Pratap Singh, A. Hemp (Cannabis sativa L.) extract: Anti-microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684. [Google Scholar] [CrossRef]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds From Cannabis sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Coordination, G.; Alastruey-Izquierdo, A.; World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; 9240060251; Organización Mundial de la Salud (OMS): Ginebra, Suiza, 2022. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R. The antifungal pipeline: Fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.E.; Henriques, M.; Silva, S. Disinfectants to fight oral Candida biofilms. Fungal Biofilms Relat. Infect. Adv. Microbiol. Infect. Dis. Public Health 2016, 3, 83–93. [Google Scholar]
- Ramage, G.; Vande Walle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.; Henriques, M.; Silva, S. In vitro anti-Candida activity of Glycyrrhiza glabra L. Ind. Crops Prod. 2016, 83, 81–85. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.-H.; Lee, J.; Kim, M.-S.; Lee, Y.-G.; Hwang, J.-T.; Choi, S.-Y.; Yoon, H.-G.; Lim, T.-G.; Lee, S.-H. Water Extract of Capsella bursa-pastoris Mitigates Doxorubicin-Induced Cardiotoxicity by Upregulating Antioxidant Enzymes. Int. J. Mol. Sci. 2023, 24, 15912. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. Trends Anal. Chem. 2023, 71, 117201. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M. A multimethodological characterization of Cannabis sativa L. inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef]
- Benzoic Acid, Biological Magnetic Resonance Data Bank. 15 September 2016 ed.. 2016. Available online: https://bmrb.io/metabolomics/mol_summary/show_data.php?id=bmse000300 (accessed on 15 January 2024). [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G. Biological magnetic resonance data bank. Nucleic Acids Res. 2023, 51, D368–D376. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.; Zhang, Y.; Du, H.; Xu, T.; Xu, X.; Zhang, J.; Kuang, T.; Lai, X.; Fan, G. 1H NMR-based metabolomics coupled with molecular docking reveal the anti-diabetic effects and potential active components of Berberis vernae on type 2 diabetic rats. Front. Pharmacol. 2020, 11, 932. [Google Scholar] [CrossRef]
- L-Valine, HMDB0000883. Available online: https://hmdb.ca/spectra/nmr_one_d/1582 (accessed on 15 January 2024).
- Isoleucine, HMDB0000172. Available online: https://hmdb.ca/spectra/nmr_one_d/1136 (accessed on 15 January 2024).
- Leucine, HMDB0000687. Available online: https://hmdb.ca/spectra/nmr_one_d/1477 (accessed on 15 January 2024).
- D-Lactic Acid, HMDB0001311. Available online: https://hmdb.ca/spectra/nmr_one_d/4830 (accessed on 15 January 2024).
- Gamma-Aminobutyric Acid, HMDB0000112. Available online: https://hmdb.ca/spectra/nmr_one_d/1088 (accessed on 15 January 2024).
- De Falco, B.; Incerti, G.; Pepe, R.; Amato, M.; Lanzotti, V. Metabolomic fingerprinting of Romaneschi globe artichokes by NMR spectroscopy and multivariate data analysis. Phytochem. Anal. 2016, 27, 304–314. [Google Scholar] [CrossRef]
- Glycerol, HMDB0000131. Available online: https://hmdb.ca/spectra/nmr_one_d/4829 (accessed on 15 January 2024).
- Corte, L.; Casagrande Pierantoni, D.; Tascini, C.; Roscini, L.; Cardinali, G. Biofilm specific activity: A measure to quantify microbial biofilm. Microorganisms 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Moscariello, C.; Matassa, S.; Esposito, G.; Papirio, S. From residue to resource: The multifaceted environmental and bioeconomy potential of industrial hemp (Cannabis sativa L.). Resour. Conserv. Recycl. 2021, 175, 105864. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Menexis, N.; Iakovidis, D.; Makris, D.P.; Goula, A. A green extraction process to recover polyphenols from byproducts of hemp oil processing. Recycling 2018, 3, 15. [Google Scholar] [CrossRef]
- Kart, D.; Ciftci, S.Y.; Nemutlu, E. Altered metabolomic profile of dual-species biofilm: Interactions between Proteus mirabilis and Candida albicans. Microbiol. Res. 2020, 230, 126346. [Google Scholar] [CrossRef]
- Nedzesky, N.; Matela, A.; Lavelle, T.; Carson, B. The Effects of Glycerol on Biofilm Production. J. Stud. Res. 2021, 10. [Google Scholar] [CrossRef]
- Perez-Castillo, Y.; Lima, T.C.; Ferreira, A.R.; Silva, C.R.; Campos, R.S.; Neto, J.B.; Magalhães, H.I.; Cavalcanti, B.C.; Júnior, H.V.; de Sousa, D.P. Bioactivity and molecular docking studies of derivatives from cinnamic and benzoic acids. BioMed Res. Int. 2020, 2020, 6345429. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic acid as an antimicrobial for poultry production: A review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.E. Sustainable biomass materials for biomedical applications. ACS Biomater. Sci. Eng. 2019, 5, 2079–2092. [Google Scholar] [CrossRef]
- Antonisamy, A.J.; Marimuthu, S.; Malayandi, S.; Rajendran, K.; Lin, Y.-C.; Andaluri, G.; Lee, S.L.; Ponnusamy, V.K. Sustainable approaches on industrial food wastes to value-added products–A review on extraction methods, characterizations, and its biomedical applications. Environ. Res. 2023, 217, 114758. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Abouelwafa, R.; Meddich, A.; Souabi, S.; Winterton, P.; Merlina, G.; Revel, J.-C.; Pinelli, E.; Hafidi, M. PLFAs of the microbial communities in composting mixtures of agro-industry sludge with different proportions of household waste. Int. Biodeterior. Biodegrad. 2010, 64, 614–621. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Renu, S.; Sahu, P.K.; Singh, V. Agrowaste bioconversion and microbial fortification have prospects for soil health, crop productivity, and eco-enterprising. Int. J. Recycl. Org. Waste Agric. 2019, 8, 457–472. [Google Scholar] [CrossRef]
- Lok, B.; Adam, M.A.A.; Kamal, L.Z.M.; Chukwudi, N.A.; Sandai, R.; Sandai, D. The assimilation of different carbon sources in Candida albicans: Fitness and pathogenicity. Med. Mycol. 2021, 59, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Xia, C.; Cheng, Y.; Guo, X.; Li, Y. Effects of malic acid and citric acid on growth performance, antioxidant capacity, haematology and immune response of Carassius auratus gibelio. Aquac. Res. 2020, 51, 2766–2776. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Dallas, D.C. Mass spectral profiling of caseinomacropeptide extracted from feeding material and jejunal fluid using three methods–ethanol precipitation, perchloric acid precipitation, and ultrafiltration. Food Chem. 2023, 398, 133864. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Lee, H.-H. A new SVM method for an indirect matrix converter with common-mode voltage reduction. IEEE Trans. Ind. Inform. 2013, 10, 61–72. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.; Arendrup, M.; Arikan, S.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.; Donnelly, J. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. Def 2008, 9, 1–13. [Google Scholar]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Casagrande Pierantoni, D.; Corte, L.; Casadevall, A.; Robert, V.; Cardinali, G.; Tascini, C. How does temperature trigger biofilm adhesion and growth in Candida albicans and two non-Candida albicans Candida species? Mycoses 2021, 64, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Casagrande Pierantoni, D.; Roscini, L.; Corte, L.; Bernardo, M.; Bassetti, M.; Tascini, C.; Cardinali, G. Qualitative and quantitative change of the tolerance to liposomal amphotericin B triggered by biofilm maturation in C. parapsilosis. Med. Mycol. 2020, 58, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Chiaradia, E.; Tognoloni, A.; Gambelunghe, A.; Meschini, C.; Palmieri, L.; Muzi, G.; Urbanelli, L.; Emiliani, C.; Tancini, B. Effect of curcumin on protein damage induced by rotenone in dopaminergic PC12 cells. Int. J. Mol. Sci. 2020, 21, 2761. [Google Scholar] [CrossRef]
- Cesaretti, A.; Calzoni, E.; Montegiove, N.; Bianconi, T.; Alebardi, M.; La Serra, M.A.; Consiglio, G.; Fortuna, C.G.; Elisei, F.; Spalletti, A. Lighting-Up the Far-Red Fluorescence of RNA-Selective Dyes by Switching from Ortho to Para Position. Int. J. Mol. Sci. 2023, 24, 4812. [Google Scholar] [CrossRef]








| HWE ID | Plant Variety | Plant Material | Extraction Method | Other Treatments |
|---|---|---|---|---|
| S2 | Cannabis sativa L. cv. Strawberry | Waste dry flowers and leaves | 72 h water extraction | Mechanical extraction + MWE-assisted process |
| S8 | Cannabis sativa L. cv. Strawberry | Waste green flowers and leaves | 72 h water extraction | Mechanical extraction + MWE-assisted process |
| S14 | Cannabis sativa L. cv. Strawberry | Waste dry flowers and leaves | 72 h water extraction | Mechanical extraction + protein precipitation |
| S9 | Cannabis sativa L. cv. Strawberry | Waste dry flowers and leaves | 30 days water extraction | Mechanical extraction |
| S21 | Cannabis sativa L. cv. Strawberry | Waste dry flowers and leaves | 60 days water extraction | Mechanical extraction |
| Sample ID | S2 | S8 | S14 | S9 | S21 |
|---|---|---|---|---|---|
| Conductivity (μS cm−1) | 205 | 4870 | 138.8 | 9003 | 10500 |
| pH | 8.37 | 8.11 | 5.74 | 6.37 | 8.16 |
| Fresh weight (g mL−1) | 0.983 | 0.983 | 0.981 | 1.004 | 0.967 |
| Dry weight (g) | 0.030 | 0.003 | 0.011 | 0.013 | 0.008 |
| Dry matter (%) | 1.0 | 0.3 | 1.1 | 1.30 | 0.8 |
| Bradford (mg mL−1) | 0.43 | 0.72 | 2.9 | 0.55 | 5.7 |
| Species | Collection n° | ID |
|---|---|---|
| Candida albicans | CMC2042 | CA1 |
| Candida albicans | CMC1959 | CA2 |
| Candida tropicalis | CMC1827 | CT1 |
| Candida tropicalis | CMC1839 | CT2 |
| Candida tropicalis | CMC2052 | CT3 |
| Candida parapsilosis | CMC1973 | CP1 |
| Candida parapsilosis | CMC2006 | CP2 |
| Candida parapsilosis | CMC1951 | CP3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, L.; Casagrande Pierantoni, D.; Conti, A.; Calzoni, E.; Corte, L.; Santi, C.; Rosati, O.; Cardinali, G.; Emiliani, C. Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells. Int. J. Mol. Sci. 2024, 25, 3979. https://doi.org/10.3390/ijms25073979
Donati L, Casagrande Pierantoni D, Conti A, Calzoni E, Corte L, Santi C, Rosati O, Cardinali G, Emiliani C. Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells. International Journal of Molecular Sciences. 2024; 25(7):3979. https://doi.org/10.3390/ijms25073979
Chicago/Turabian StyleDonati, Leonardo, Debora Casagrande Pierantoni, Angela Conti, Eleonora Calzoni, Laura Corte, Claudio Santi, Ornelio Rosati, Gianluigi Cardinali, and Carla Emiliani. 2024. "Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells" International Journal of Molecular Sciences 25, no. 7: 3979. https://doi.org/10.3390/ijms25073979
APA StyleDonati, L., Casagrande Pierantoni, D., Conti, A., Calzoni, E., Corte, L., Santi, C., Rosati, O., Cardinali, G., & Emiliani, C. (2024). Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells. International Journal of Molecular Sciences, 25(7), 3979. https://doi.org/10.3390/ijms25073979