Metformin: A Dual-Role Player in Cancer Treatment and Prevention
Abstract
:1. Background
2. Metformin as an Anticancer Drug: In Vivo and In Vitro Studies
| Authors [Ref.] | Year | Study Design | Outcome | Relative Risk (95% CI) a |
|---|---|---|---|---|
| Bowker et al. [11] | 2006 | Cohort | Cancer mortality | 0.8 (0.6–0.9) |
| Currie et al. [19] | 2009 | Cohort | Any cancer | 0.54 (0.43–0.66) |
| Wright et al. [20] | 2009 | Case–control | Prostate cancer | 0.56 (0.32–1.00) |
| Libby et al. [21] | 2009 | Cohort | Any cancer | 0.63 (0.53–0.75) |
| Bowker et al. [22] | 2010 | Cohort | Cancer mortality | 0.8 (0.65–0.98) |
| Dandon et al. [23] | 2010 | Case–control | Liver cancer | 0.15 (0.04–0.5) |
| Bodmer et al. [24] | 2010 | Nested case–control | Breast cancer | 0.44 (0.24–0.82) |
| Lee et al. [25] | 2011 | Cohort | Any cancer | 0.12 (0.08–0.19) |
| Chen et al. [26] | 2011 | Cohort | Liver cancer | 0.24 (0.07–0.8) |
| He et al. [27] | 2011 | Cohort | Prostate: all-cause mortality | 0.55 (0.32–0.94) |
| Monami et al. [28] | 2011 | Nested case–control | Any cancer | 0.28 (0.13–0.57) |
| Bosco et al. [29] | 2011 | Nested case–control | Breast cancer | 0.81 (0.63–0.96) |
| Bodmer et al. [30] | 2011 | Nested case–control | Ovarian cancer | 0.61 (0.3–1.25) |
| Geraldine et al. [31] | 2012 | Cohort | Any cancer | 0.2 (0.03–0.82) |
| Lai et al. [32] | 2012 | Cohort | Lung cancer | 0.55 (0.32–0.94) |
| Lee et al. [33] | 2012 | Cohort | Colorectal: all-cause mortality/cancer mortality | 0.66 (0.45–0.98)/0.66 (0.48–0.92) |
| He et al. [34] | 2012 | Cohort | Breast: all-cause mortality/cancer mortality | 0.52 (0.28–0.97)/0.47 (0.24–0.9) |
| Romero et al. [35] | 2012 | Cohort | Ovarian cancer progression/all-cause mortality | 0.38 (0.16–0.90)/0.43 (0.16–1.19) |
| Bodmer et al. [36] | 2012 | Nested case–control | Pancreatic cancer | 0.43 (0.23–0.8) |
| Bodmer et al. [36] | 2012 | Nested case–control | Colorectal cancer | 1.43 (1.08–1.9) |
| Franciosi et al. [37] | 2013 | Systematic review | Any cancer: all-cause mortality | 0.65 (0.53–0.80) |
| Preston et al. [38] | 2014 | Nested case–control | Prostate cancer | 0.84 (0.74–0.96) |
| Kim et al. [39] | 2014 | Cohort | Gastric cancer | 0.57 (0.37–0.87) b |
| Chen et al. [40] | 2015 | Cohort | Any cancer | 1.36 (1.11–1.67) |
| Calip et al. [41] | 2016 | Cohort | Breast cancer | 0.95 (0.51–1.77) |
| Häggström et al. [42] | 2016 | Cohort | Prostate cancer | 0.96 (0.77–1.19) |
| Franchi et al. [43] | 2017 | Nested case–control | Endometrial cancer | 0.99 (0.80–1.23) |
| Kim et al. [44] | 2018 | Cohort | Any cancer | 0.513 (0.318–0.826) |
| Chang et al. [45] | 2018 | Cohort | Colorectal cancer | 0.36 (0.29–0.44) c, 0.6 (0.49–0.74) d |
| Tang et al. [46] | 2018 | Meta-analysis | Breast cancer/BC all-cause mortality | 0.964 (0.761–1.221)/ 0.652 (0.488–0.873) |
| Kuo et al. [47] | 2019 | Cohort | Prostate cancer | 0.69 (0.49–0.96) |
| Hoiso et al. [48] | 2019 | Cohort | Breast cancer | 0.97 (0.89–1.05) |
| Xiao et al. [49] | 2020 | Meta-analysis | Lung cancer/survival | 0.78 (0.70–0.86)/0.65 (0.55–0.77) |
| Zhang et al. [50] | 2021 | Meta-analysis | Any cancer | 0.70 (0.65–0.76) |
| Kim et al. [51] | 2022 | Cohort | Pancreatic cancer | 1.116 (0.648–1.923) e & 2.769 (1.003–7.642) f |
| Hu et al. [52] | 2023 | Nested case–control | Pancreatic cancer | 0.82 (0.69–0.98) |
| Orchard et al. [53] | 2023 | Cohort | Any cancer/cancer mortality | 0.68 (0.51–0.90)/ 0.72 (0.43–1.19) |
| Chemotherapy | Cancer Type | Cancer Cell Line | IC50 of Drug Alone | IC50 of the Drug + Metformin | Metformin Dose | Reference |
|---|---|---|---|---|---|---|
| Cisplatin | Oral squamous cell carcinoma | HSC3 | 17.44 ± 1.10 µM | 8.32 ± 0.92 µM | 10 μM | [54] |
| SCC3 | 9.86 ± 1.55 µM | 3.59 ± 1.02 µM | 10 μM | |||
| TCA8113 | 9.83 ± 1.30 µM | 5.73 ± 0.77 µM | 10 μM | |||
| Ovarian cancer | SKOV3 | 14.35 µg/mL | 11.20 µg/mL | 10 mmol/L | [55] | |
| SKOV3/DPP | 70.26 µg/mL | 6.21 µg/mL | ||||
| Lung cancer | A549 | 20.4 µM | 15.4 µM | 10 mM | [56] | |
| Methotrexate | Ovarian cancer | SKOV3 | 4.21 µg/mL | 2.80 µg/mL | 10 mmol/L | [55] |
| SKOV3/DPP | 15.27 µg/mL | 2.74 µg/mL | 10 mmol/L | |||
| Hepatocellular carcinoma | HepG2 | 29.8 ± 0.6 nM | 14.6 ± 0.8 nM | 2.5 mM | [57] | |
| HepG2/MTX | 219 ± 8 nM | 17 ± 1 nM | ||||
| Doxorubicin | Breast cancer | MCF7 | 0.283 ± 0.036 µM | 0.253 ± 0.031 µM | 10 mM | [58] |
| MCF7/Dox | 3.23 ± 0.14 µM | 1.182 ± 0.1 µM | 10 mM | |||
| Paclitaxel | Prostate cancer | PC-3 cells | 13.170 ± 1.12 nM | 5.423 ± 0.734 nM | 5 mM | [59] |
| Breast cancer | T47D cells | 0.2 mg/mL | 0.048 mg/mL | 40 mg | [60] |
| Metformin Monotherapy | |||||||
|---|---|---|---|---|---|---|---|
| NCT No. | Year ᶴ | Cancer Type | No. of Patients Included | Diabetes Status | Study Design | Results | |
| NCT00930579 [61] | 2014 | BC (DCIS) | 35 | Non-diabetic | Phase II | No proliferation changes though reduction in relevant biomarkers was observed | |
| NCT01447927 [62] | 2015 | Barret’s Esophagus | 74 | Non-diabetic | Phase II | No significant change in pS6K levels | |
| NCT02376166 [63] | 2017 | Prostate cancer | 14 | Non-diabetic | _ | Metformin was well tolerated and exhibited minimal anti-PCa activity | |
| NCT01266486 [64] | 2018 | BC | 41 | Non-diabetic | Phase II | There are two distinct metabolic responses to metformin: the OXPHOS transcriptional response (OTR) and FDG response. The OTR was resistant to metformin, manifested by increased proliferation. Mitochondrial response to metformin in primary breast cancer may define the anti-tumor effect. | |
| NCT03118128 [65] | 2018 | ALL | 102 | Non-diabetic | Phase II | Metformin + chemotherapy is effective in patients with high ABCB1 gene expression | |
| NCT01312467 | 2019 | CRC | 32 | Non-diabetic | Phase II | Non-significant change in pS6K Ser235 | |
| NCT01101438 [66] | 2022 | Early BC | 3649 | Non-diabetic | Phase III | In high-risk operable BC, metformin did not improve the DFS. | |
| Metformin Added to Conventional Chemotherapy | |||||||
| NCT No. | Year ᶴ | Cancer Type | No. of Patients Included | Diabetes Status | Study Design | Chemo-Drug | Results |
| NCT01941953 [67] | 2014 | Refractory metastatic CRC | 22 | Non-diabetic | Phase II | Fluorouracil, leucovorin | Anticancer activity and better response to treatments |
| NCT01971034 [68] | 2015 | Metastatic pancreatic cancer | 41 | Non-diabetic | Phase II | Paclitaxel | Poor tolerance and no prognostic value in patients |
| NCT01210911 [69] | 2015 | Pancreatic cancer | 121 | Non-diabetic | Phase II | Gemcitabine erlotinib | No additional outcome improvement |
| NCT00490139 [70] | 2017 | Breast cancer | 8381 | Diabetic and non-diabetic | Phase III | Trastuzumab, lapatinib, or their combination | Improved the bad prognosis, mainly in primary HER2- and HR-positive breast cancer. |
| NCT01666730 | 2018 | Metastatic pancreatic cancer | 31 | Diabetic and non-diabetic | Phase II | Oxaliplatin, fluorouracil, leucovorin calcium | According to RECIST, ≈50% of patients benefited clinically from metformin use |
| NCT01589367 [71] | 2019 | ER-positive breast cancer | 153 | Non-diabetic | Phase II | Letrozole | >10% higher response rate and more patients with Ki67 < 10% |
| NCT01310231 [72] | 2019 | Metastatic breast cancer (MBC) | 40 | Non-diabetic | Phase II | Anthracycline, platinum, taxane, capecitabine/vinorelbine | No significant effect on RR, PFS, or OS |
| NCT02325401 | 2020 | Locally advanced HNSCC | 20 | Non-diabetic | Phase I | Cisplatin and chemoradiation | High OS (≈83.33% for 2 g metformin and ≈100% for 2.5 and 3 g) and 100% PFS with all metformin doses |
| NCT02048384 [73] | 2020 | Pancreatic adenocarcinoma | 22 | N/R | Phase Ib | Rapamycin | Well tolerated, and stable disease associated with exceptionally long survival was achieved |
| NCT01796028 [74] | 2021 | Prostate neoplasms | 100 | Non-diabetic | Phase II | Docetaxel | Failed to improve the outcome |
| NCT04143282 [75] | 2021 | MBC | 50 | Non-diabetic | Phase II | Gemcitabine + carboplatin/paclitaxel; FAC; AC; vinorelbine; capecitabine; paclitaxel | Improved radiologic RR and, better yet, insignificant OS and PFS |
| NCT02115464 [76] | 2021 | LA-NSCLC | 54 | Non-diabetics | RCT: Phase II | Cisplatin ± radiotherapy | Worse treatment efficacy and more toxic effects |
| NCT02755844 | 2022 | Recurrent endometrial cancer | 35 | Diabetic and non-diabetic | Phase I/II | Cyclophosphamide and olaparib | Significant non-progression rate in recurrent advanced or metastatic endometrial cancer |
| NCT05351021 [77] | 2023 | Breast cancer | 73 | Non-diabetic | Phase II | Paclitaxel | Remarkable protection against paclitaxel-induced PN |
| NCT02949700 [78] | 2023 | Head and neck cancer | 16 | Non-diabetic | Phase I/II | Cisplatin-based chemoradiation | 2-year PFS = 90% and OS = 85%. Yet, the small sample size renders effectiveness of metformin as chemo-radiosensitizer unclear |
| NCT04170465 [79] | 2023 | Primary breast cancer | 70 | Non-diabetic | RCT: Phase II | AC-T | Better control of chemotherapy-induced toxicities |
| NCT05840068 [80] | 2023 | MBC | 107 | Non-diabetic | Phase II | N/R | No significant IGF-I reduction in MBC patients on metformin |
| NCT03243851 [81] | 2023 | Glioblastoma | 81 | Non-diabetic | Phase II | Temozolomide | Well tolerated, but no clinical benefit in recurrent/refractory GBM |
3. Mechanism of Action of Anti-Tumorigenic Effect of Metformin
3.1. Metformin Direct Effect (Insulin-Independent)
3.2. Metformin Indirect Effect (Insulin-Dependent)
4. Metformin and Cancer Prevention
4.1. Diabetes
4.2. Aging
4.3. Hyperlipidemia and Dyslipidemia
4.4. Obesity
5. Metformin Use in Different Cancer Types
5.1. Breast Cancer
5.2. Colorectal Cancer (CRC)
5.3. Gastric Cancer (GC)
5.4. Liver Cancer
5.5. Lung Cancer
5.6. Ovarian Cancer
5.7. Pancreatic Cancer
5.8. Prostate Cancer (PCa)
6. Challenges of Metformin Repurposing as an Anticancer Drug
7. Conclusions
Funding
Conflicts of Interest
References
- Galal, M.A.; Alouch, S.S.; Alsultan, B.S.; Dahman, H.; Alyabis, N.A.; Alammar, S.A.; Aljada, A. Insulin Receptor Isoforms and Insulin Growth Factor-like Receptors: Implications in Cell Signaling, Carcinogenesis, and Chemoresistance. Int. J. Mol. Sci. 2023, 24, 15006. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Global Cancer Observatory—Cancer Fact Sheets. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 1 April 2024).
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence#heading-One (accessed on 2 December 2023).
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Donnelly, L.; Emslie-Smith, A.; Alessi, D.; Morris, A. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ahmed, K.B.; Garcia-Prieto, C.; Huang, P. Metabolic alterations in cancer cells and therapeutic implications. Chin. J. Cancer 2011, 30, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Iwamoto, T.; Jordan, L.; Purdie, C.; Bray, S.; Baker, L.; Jellema, G.; Deharo, S.; Hardie, D.G.; Pusztai, L.; et al. Evidence for biological effects of metformin in operable breast cancer: A pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res. Treat. 2011, 128, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Algire, C.; Moiseeva, O.; Deschênes-Simard, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Viollet, B.; Ferbeyre, G.; Pollak, M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res. 2012, 5, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef]
- Kurelac, I.; Iommarini, L.; Vatrinet, R.; Amato, L.B.; De Luise, M.; Leone, G.; Girolimetti, G.; Umesh Ganesh, N.; Bridgeman, V.L.; Ombrato, L. Inducing cancer indolence by targeting mitochondrial Complex I is potentiated by blocking macrophage-mediated adaptive responses. Nat. Commun. 2019, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Bi, Y.; Li, S.; Zhang, Q.; Zhao, G.; Guo, Y.; Song, Q. Reduced Risk of Lung Cancer with Metformin Therapy in Diabetic Patients: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2014, 180, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Zheng, Z.-J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced Risk of Colorectal Cancer with Metformin Therapy in Patients with Type 2 Diabetes: A meta-analysis. Diabetes Care 2011, 34, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Zheng, Z.-J.; Shi, R.; Su, Q.; Jiang, Q.; Kip, K.E. Metformin for Liver Cancer Prevention in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Li, S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2014, 16, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Farmer, R.E.; Ford, D.; Forbes, H.J.; Chaturvedi, N.; Kaplan, R.; Smeeth, L.; Bhaskaran, K. Metformin and cancer in type 2 diabetes: A systematic review and comprehensive bias evaluation. Int. J. Epidemiol. 2017, 46, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.J.; Poole, C.D.; Gale, E. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Stanford, J.L. Metformin use and prostate cancer in Caucasian men: Results from a population-based case–control study. Cancer Causes Control 2009, 20, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Bowker, S.; Yasui, Y.; Veugelers, P.; Johnson, J. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: Assessing effects of time-varying exposure. Diabetologia 2010, 53, 1631–1637. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Mas, M.D.; Casarin, P.; Zanette, G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010, 30, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Meier, C.; Krähenbühl, S.; Jick, S.S.; Meier, C.R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 2010, 33, 1304–1308. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Chen, T.M.; Lin, C.C.; Huang, P.T.; Wen, C.F. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J. Gastroenterol. Hepatol. 2011, 26, 858–865. [Google Scholar] [CrossRef]
- He, X.-X.; Tu, S.; Lee, M.-H.; Yeung, S.-C. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann. Oncol. 2011, 22, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Colombi, C.; Balzi, D.; Dicembrini, I.; Giannini, S.; Melani, C.; Vitale, V.; Romano, D.; Barchielli, A.; Marchionni, N. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2011, 34, 129–131. [Google Scholar] [CrossRef]
- Bosco, J.L.F.; Antonsen, S.; Sørensen, H.T.; Pedersen, L.; Lash, T.L. Metformin and incident breast cancer among diabetic women: A population-based case–control study in Denmark. Cancer Epidemiol. Biomark. Prev. 2011, 20, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.S.; Meier, C.R. Use of metformin and the risk of ovarian cancer: A case–control analysis. Gynecol. Oncol. 2011, 123, 200–204. [Google Scholar] [CrossRef]
- Geraldine, N.; Marc, A.; Carla, T.; Chantal, M.; Stefaan, B.; Welcome, W.; Frank, B. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res. Clin. Pract. 2012, 97, 331–336. [Google Scholar] [CrossRef]
- Lai, S.-W.; Liao, K.-F.; Chen, P.-C.; Tsai, P.-Y.; Hsieh, D.P.H.; Chen, C.-C. Antidiabetes drugs correlate with decreased risk of lung cancer: A population-based observation in Taiwan. Clin. Lung Cancer 2012, 13, 143–148. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.I.; Jeon, S.M.; Hong, S.P.; Cheon, J.H.; Kim, W.H. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int. J. Cancer 2012, 131, 752–759. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Esteva, F.; Ensor, J.; Hortobagyi, G.; Lee, M.-H.; Yeung, S.-C. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann. Oncol. 2012, 23, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.L.; McCormick, A.; McEwen, K.A.; Park, S.; Karrison, T.; Yamada, S.D.; Pannain, S.; Lengyel, E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012, 119, 61. [Google Scholar] [CrossRef]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.S.; Meier, C.R. Use of metformin is not associated with a decreased risk of colorectal cancer: A case–control analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.A.; Riis, A.H.; Ehrenstein, V.; Breau, R.H.; Batista, J.L.; Olumi, A.F.; Mucci, L.A.; Adami, H.O.; Sørensen, H.T. Metformin use and prostate cancer risk. Eur. Urol. 2014, 66, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, S.; Cho, S.J.; Park, J.H.; Choi, I.J.; Lee, Y.J.; Lee, E.; Kook, M.C.; Kim, C.; Ryu, K. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: A nationwide cohort study. Aliment. Pharmacol. Ther. 2014, 39, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Kok, V.C.; Chien, C.-H.; Horng, J.-T.; Tsai, J.J. Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: A cohort study using metformin as the comparator. Ther. Clin. Risk Manag. 2015, ume 11, 1315–1323. [Google Scholar]
- Calip, G.S.; Yu, O.; Elmore, J.G.; Boudreau, D.M. Comparative safety of diabetes medications and risk of incident invasive breast cancer: A population-based cohort study. Cancer Causes Control 2016, 27, 709–720. [Google Scholar] [CrossRef]
- Häggström, C.; Van Hemelrijck, M.; Zethelius, B.; Robinson, D.; Grundmark, B.; Holmberg, L.; Gudbjörnsdottir, S.; Garmo, H.; Stattin, P. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int. J. Cancer 2017, 140, 611–617. [Google Scholar] [CrossRef]
- Franchi, M.; Asciutto, R.; Nicotra, F.; Merlino, L.; La Vecchia, C.; Corrao, G.; Bosetti, C. Metformin, other antidiabetic drugs, and endometrial cancer risk: A nested case–control study within Italian healthcare utilization databases. Eur. J. Cancer Prev. 2017, 26, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.; Chun, K.H.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kim, Y.S.; Woo, J.-T.; Nam, M.-S.; Baik, S.H. Metformin reduces the risk of cancer in patients with type 2 diabetes: An analysis based on the Korean National Diabetes Program Cohort. Medicine 2018, 97, e0036. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Tsai, H.-L.; Kung, Y.-T.; Yeh, Y.-S.; Huang, C.-W.; Ma, C.-J.; Chiu, H.-C.; Wang, J.-Y. Dose-dependent relationship between metformin and colorectal cancer occurrence among patients with Type 2 Diabetes—A nationwide cohort study. Transl. Oncol. 2018, 11, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Satkunam, M.; Pond, G.R.; Steinberg, G.R.; Blandino, G.; Schünemann, H.J.; Muti, P. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2018, 27, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Sung, F.C.; Hsieh, P.F.; Chang, H.P.; Wu, K.L.; Wu, H.C. Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: A nationwide population-based cohort study. Cancer Med. 2019, 8, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Hosio, M.; Urpilainen, E.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Puistola, U.; Läärä, E.; Jukkola, A.; Karihtala, P. Association of antidiabetic medication and statins with breast cancer incidence in women with type 2 diabetes. Breast Cancer Res. Treat. 2019, 175, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, F.; Liu, J.; Xu, J.; Wu, Q.; Li, X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, Y.-J.; Kang, H.-T. Metformin Use May Increase Risk of Pancreatic Cancer in Diabetic Women: An Analysis of the Korean National Health Insurance Service-National Health Screening Cohort Database. Korean J. Fam. Med. 2022, 43, 327. [Google Scholar] [CrossRef]
- Hu, J.; Fan, H.-D.; Gong, J.-P.; Mao, Q.-S. The relationship between the use of metformin and the risk of pancreatic cancer in patients with diabetes: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 1–21. [Google Scholar] [CrossRef]
- Orchard, S.G.; Lockery, J.E.; Broder, J.C.; Ernst, M.E.; Espinoza, S.; Gibbs, P.; Wolfe, R.; Polekhina, G.; Zoungas, S.; Loomans-Kropp, H.A. Association of metformin, aspirin, and cancer incidence with mortality risk in adults with diabetes. JNCI Cancer Spectr. 2023, 7, pkad017. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, W.; Xie, J.; Wang, Y.; Han, S.; Wei, Z.; Ni, Y.; Dong, Y.; Han, W. Metformin sensitizes the response of oral squamous cell carcinoma to cisplatin treatment through inhibition of NF-κB/HIF-1α signal axis. Sci. Rep. 2016, 6, 35788. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, N.; Li, D.; Zou, G.; Chen, Y. Metformin improves the sensitivity of ovarian cancer cells to chemotherapeutic agents. Oncol. Lett. 2019, 18, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.P.; Tortelli, T.C., Jr.; Pavan, I.C.B.; Silva, F.R.; Granato, D.C.; Peruca, G.F.; Pauletti, B.A.; Domingues, R.R.; Bezerra, R.M.N.; De Moura, L.P.; et al. Metformin impairs cisplatin resistance effects in A549 lung cancer cells through mTOR signaling and other metabolic pathways. Int. J. Oncol. 2021, 58, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Sun, L.; Chen, X.; Wei, H.; Suo, C.; Feng, J.; Yuan, M.; Shen, S.; Jia, W.; et al. Metformin sensitises hepatocarcinoma cells to methotrexate by targeting dihydrofolate reductase. Cell Death Dis. 2021, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-Irannejad, V.; Samadi, N.; Yousefi, B.; Salehi, R.; Velaei, K.; Zarghami, N. Metformin enhances doxorubicin sensitivity via inhibition of doxorubicin efflux in P-gp-overexpressing MCF-7 cells. Chem. Biol. Drug Des. 2018, 91, 269–276. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, X.; Tang, H.; Ye, D.; Liu, J. Combination of metformin and paclitaxel suppresses proliferation and induces apoptosis of human prostate cancer cells via oxidative stress and targeting the mitochondria-dependent pathway. Oncol. Lett. 2019, 17, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Al-Kofahi, T.; Altrad, B.; Amawi, H.; Aljabali, A.A.; Abul-Haija, Y.M.; Obeid, M.A. Paclitaxel-loaded niosomes in combination with metformin: Development, characterization and anticancer potentials. Ther. Deliv. 2024, 15, 109–118. [Google Scholar] [CrossRef]
- Kalinsky, K.; Crew, K.D.; Refice, S.; Xiao, T.; Wang, A.; Feldman, S.M.; Taback, B.; Ahmad, A.; Cremers, S.; Hibshoosh, H.; et al. Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Investig. 2014, 32, 150–157. [Google Scholar] [CrossRef]
- Chak, A.; Buttar, N.S.; Foster, N.R.; Seisler, D.K.; Marcon, N.; Schoen, R.E.; Cruz-Correa, M.; Falk, G.W.; Sharma, P.; Hur, C.; et al. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 665–672.e4. [Google Scholar] [CrossRef]
- Galsky, M.D.; Shahin, M.; Olson, A.; Shaffer, D.R.; Gimpel-Tetra, K.; Tsao, C.-K.; Baker, C.; Leiter, A.; Holland, J.; Sablinski, T.; et al. Telemedicine-enabled clinical trial of metformin in patients (pts) with biochemically-recurrent prostate cancer (PCa). J. Clin. Oncol. 2017, 35 (Suppl. 6), 243. [Google Scholar] [CrossRef]
- Lord, S.R.; Cheng, W.C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K.; et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018, 28, 679–688.e4. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Penafiel, C.; Olarte-Carrillo, I.; Ceron-Maldonado, R.; Rozen-Fuller, E.; Kassack-Ipina, J.J.; Melendez-Mier, G.; Collazo-Jaloma, J.; Martinez-Tovar, A. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J. Transl. Med. 2018, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Miranda, V.C.; Faria, L.D.; Braghiroli, M.I.F.M.; Jacobs, M.; Sabbaga, J.; Hoff, P.M.; Riechelmann, R.P. A phase II trial of metformin and fluorouracil (MetFU) for patients (pts) with metastatic colorectal cancer (mCRC) refractory to standard treatment. J. Clin. Oncol. 2014, 32 (Suppl. 3), 601. [Google Scholar] [CrossRef]
- Braghiroli, M.I.; de Celis Ferrari, A.C.; Pfiffer, T.E.; Alex, A.K.; Nebuloni, D.; Carneiro, A.S.; Caparelli, F.; Senna, L.; Lobo, J.; Hoff, P.M.; et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience 2015, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathot, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Sonnenblick, A.; Agbor-Tarh, D.; Bradbury, I.; Di Cosimo, S.; Azim, H.A., Jr.; Fumagalli, D.; Sarp, S.; Wolff, A.C.; Andersson, M.; Kroep, J.; et al. Impact of Diabetes, Insulin, and Metformin Use on the Outcome of Patients with Human Epidermal Growth Factor Receptor 2-Positive Primary Breast Cancer: Analysis From the ALTTO Phase III Randomized Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, W.; Kim, E.-K.; Jung, Y.; Kim, H.-A.; Chae, S.M.; Lee, E.S.; Ahn, S.-H.; Kim, T.H.; Jeong, J.; et al. Phase II randomized study of neoadjuvant metformin plus letrozole versus placebo plus letrozole for ER-positive postmenopausal breast cancer [METEOR Study]. J. Clin. Oncol. 2019, 37 (Suppl. 15), 576. [Google Scholar] [CrossRef]
- Pimentel, I.; Lohmann, A.E.; Ennis, M.; Dowling, R.J.O.; Cescon, D.; Elser, C.; Potvin, K.R.; Haq, R.; Hamm, C.; Chang, M.C.; et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 2019, 48, 17–23. [Google Scholar] [CrossRef]
- Bever, K.M.; Borazanci, E.H.; Thompson, E.A.; Durham, J.N.; Pinero, K.; Jameson, G.S.; Vrana, A.; Liu, M.; Wilt, C.; Wu, A.A.; et al. An exploratory study of metformin with or without rapamycin as maintenance therapy after induction chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncotarget 2020, 11, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Pujalte Martin, M.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B.; et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rabea, H.; Hassan, A.; Elberry, A.A. Metformin as an Adjuvant Treatment in Non-Diabetic Metastatic Breast Cancer. Bahrain Med. Bull 2021, 43, 477–481. [Google Scholar]
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in Combination with Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: The OCOG-ALMERA Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1333–1341. [Google Scholar] [CrossRef]
- Bakry, H.M.; Mansour, N.O.; ElKhodary, T.R.; Soliman, M.M. Efficacy of metformin in prevention of paclitaxel-induced peripheral neuropathy in breast cancer patients: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1181312. [Google Scholar] [CrossRef]
- Kemnade, J.O.; Florez, M.; Sabichi, A.; Zhang, J.; Jhaveri, P.; Chen, G.; Chen, A.; Miller-Chism, C.; Shaun, B.; Hilsenbeck, S.G.; et al. Phase I/II trial of metformin as a chemo-radiosensitizer in a head and neck cancer patient population. Oral Oncol. 2023, 145, 106536. [Google Scholar] [CrossRef]
- Serageldin, M.A.; Kassem, A.B.; El-Kerm, Y.; Helmy, M.W.; El-Mas, M.M.; El-Bassiouny, N.A. The Effect of Metformin on Chemotherapy-Induced Toxicities in Non-diabetic Breast Cancer Patients: A Randomised Controlled Study. Drug Saf. 2023, 46, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Essa, N.; Elgendy, M.; Gabr, A.; Alharbi, a.; Tashkandi, H.; Salem, H.; Harakeh, S.; Boshra, M. The efficacy of metformin as adjuvant to chemotherapy on IGF levels in non-diabetic female patients with progressive and non-progressive metastatic breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5200–5210. [Google Scholar]
- Yoon, W.S.; Chang, J.H.; Kim, J.H.; Kim, Y.J.; Jung, T.Y.; Yoo, H.; Kim, S.H.; Ko, Y.C.; Nam, D.H.; Kim, T.M.; et al. Efficacy and safety of metformin plus low-dose temozolomide in patients with recurrent or refractory glioblastoma: A randomized, prospective, multicenter, double-blind, controlled, phase 2 trial (KNOG-1501 study). Discover. Oncol. 2023, 14, 90. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Janzer, A.; German, N.J.; Gonzalez-Herrera, K.N.; Asara, J.M.; Haigis, M.C.; Struhl, K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 10574–10579. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, C.; Kiyici, S.; Budak, F.; Oral, B.; Guclu, M.; Duran, C.; Selimoglu, H.; Erturk, E.; Tuncel, E.; Imamoglu, S. The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res. Clin. Pract. 2008, 81, 56–60. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.; Nogueira, V.; Fontaine, E.; Averet, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhausl, W. Thiazolidinediones, like metformin, inhibit respiratory complex I: A common mechanism contributing to their antidiabetic actions? Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.; Alquier, T.; Carling, D.; Hardie, D. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zierath, J. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. CB 2003, 13, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gowans, G.J.; Hardie, D.G. AMPK: A cellular energy sensor primarily regulated by AMP. Biochem. Soc. Trans. 2014, 42, 71–75. [Google Scholar] [CrossRef]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.-I.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Gwinn, D.; Shackelford, D.; Egan, D.; Mihaylova, M.; Mery, A.; Vasquez, D.; Turk, B.; Shaw, R. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Jung, C.H.; Seo, M.; Otto, N.M.; Kim, D.H. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy 2011, 7, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, M.A.; Berrino, F.; Gariboldi, M.; Melani, C.; Mogavero, A.; Negri, T.; Pasanisi, P.; Pilotti, S. Targeting metabolism for cancer treatment and prevention: Metformin, an old drug with multi-faceted effects. Oncogene 2013, 32, 1475–1487. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Clemmons, D.R. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol. Endocrinol. 2010, 24, 1218–1229. [Google Scholar] [CrossRef]
- Vazquez-Martin, A.; Oliveras-Ferraros, C.; Del Barco, S.; Martin-Castillo, B.; Menendez, J.A. If mammalian target of metformin indirectly is mammalian target of rapamycin, then the insulin-like growth factor-1 receptor axis will audit the efficacy of metformin in cancer clinical trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, e207–e209. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Karube, Y.; Tanaka, H.; Osada, H.; Tomida, S.; Tatematsu, Y.; Yanagisawa, K.; Yatabe, Y.; Takamizawa, J.; Miyoshi, S.; Mitsudomi, T. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005, 96, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Merritt, W.M.; Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Spannuth, W.A.; Schmandt, R.; Urbauer, D.; Pennacchio, L.A.; Cheng, J.-F.; Nick, A.M. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 2008, 359, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T. A MicroRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Xu, B.; Li, L. A New Role for an Old Drug: Metformin Targets Micro RNA s in Treating Diabetes and Cancer. Drug Dev. Res. 2015, 76, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, N.; Firouzabadi, N.; Fatehi, R. How metformin affects various malignancies by means of microRNAs: A brief review. Cancer Cell Int. 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, J.; Gao, Y.; Ghazal, S.; Lu, L.; Bellone, S.; Yang, Y.; Liu, N.; Zhao, X.; Santin, A.D.; et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene 2015, 34, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Algire, C.; Amrein, L.; Zakikhani, M.; Panasci, L.; Pollak, M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr.-Relat. Cancer 2010, 17, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef]
- Karuman, P.; Gozani, O.; Odze, R.D.; Zhou, X.C.; Zhu, H.; Shaw, R.; Brien, T.P.; Bozzuto, C.D.; Ooi, D.; Cantley, L.C. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 2001, 7, 1307–1319. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology 2019, 8, e1633235. [Google Scholar] [CrossRef]
- Cha, J.H.; Yang, W.H.; Xia, W.; Wei, Y.; Chan, L.C.; Lim, S.O.; Li, C.W.; Kim, T.; Chang, S.S.; Lee, H.H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e7. [Google Scholar] [CrossRef] [PubMed]
- Dreher, L.S.; Hoppe, T. ERADicate Tumor Progression with Metformin. Mol. Cell 2018, 71, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Gunton, J.E.; Delhanty, P.J.; Takahashi, S.; Baxter, R.C. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J. Clin. Endocrinol. Metab. 2003, 88, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Goode, J.; Paz, J.C.; Ouyang, K.; Screaton, R.; Fischer, W.H.; Chen, J.; Tabas, I.; Montminy, M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 2012, 485, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Hirshman, M.F.; Nygren, J.O.; Svanfeldt, M.; Båvenholm, P.N.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendić, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.W.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.E.; Wu, M.S.; Ventre, J.R.; Doebber, T.W.; Fujii, N.L.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kieffer, T.J. New aspects of an old drug: Metformin as a glucagon-like peptide 1 (GLP-1) enhancer and sensitiser. Diabetologia 2011, 54, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Maida, A.; Lamont, B.J.; Cao, X.; Drucker, D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia 2011, 54, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, J.M.; Croom, D.K.; Minnick, D.T. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem. Biophys. Res. Commun. 2004, 324, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Thazhath, S.S.; Bound, M.J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: Stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes Res. Clin. Pract. 2014, 106, e3–e6. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Irwin, N.; Duffy, N.A.; Gault, V.A.; O’harte, F.P.; Flatt, P.R. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur. J. Pharmacol. 2006, 547, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Duffy, N.; McKillop, A.; Ardill, J.; O’Harte, F.; Flatt, P.; Bell, P. Inhibition of dipeptidyl peptidase IV activity by oral metformin in Type 2 diabetes. Diabet. Med. 2005, 22, 654–657. [Google Scholar] [CrossRef]
- Fadini, G.P.; Albiero, M.; Menegazzo, L.; De Kreutzenberg, S.; Avogaro, A. The increased dipeptidyl peptidase-4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin-treated patients. Diabetes Obes. Metab. 2012, 14, 518–522. [Google Scholar] [CrossRef]
- Hinke, S.A.; Kühn-Wache, K.; Hoffmann, T.; Pederson, R.A.; McIntosh, C.H.; Demuth, H.-U. Metformin effects on dipeptidylpeptidase IV degradation of glucagon-like peptide-1. Biochem. Biophys. Res. Commun. 2002, 291, 1302–1308. [Google Scholar] [CrossRef]
- Migoya, E.; Bergeron, R.; Miller, J.; Snyder, R.; Tanen, M.; Hilliard, D.; Weiss, B.; Larson, P.; Gutierrez, M.; Jiang, G. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin. Pharmacol. Ther. 2010, 88, 801–808. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jee, J.-H.; Park, S.; Lee, M.-S.; Kim, K.-W.; Lee, M.-K. Metformin enhances glucagon-like peptide 1 via cooperation between insulin and Wnt signaling. J. Endocrinol. 2014, 220, 117–128. [Google Scholar] [CrossRef]
- Yi, F.; Sun, J.; Lim, G.E.; Fantus, I.G.; Brubaker, P.L.; Jin, T. Cross talk between the insulin and Wnt signaling pathways: Evidence from intestinal endocrine L cells. Endocrinology 2008, 149, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Miller, S.; Nicholls, A.W.; Baker, D.; Van Horn, S.; Thomas, E.; Rajpal, D.; Spivak, A.; Brown, J.R.; Nunez, D.J. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE 2014, 9, e100778. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Niraula, S.; Stambolic, V.; Goodwin, P.J. Metformin in cancer: Translational challenges. J. Mol. Endocrinol. 2012, 48, R31–R43. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Frasca, F. IGF and insulin receptor signaling in breast cancer. J. Mammary Gland Biol. Neoplasia 2008, 13, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, G.M.; Leclerc, G.J.; Kuznetsov, J.N.; DeSalvo, J.; Barredo, J.C. Metformin Induces Apoptosis through AMPK-Dependent Inhibition of UPR Signaling in ALL Lymphoblasts. PLoS ONE 2013, 8, e74420. [Google Scholar] [CrossRef] [PubMed]
- Conza, D.; Mirra, P.; Calì, G.; Insabato, L.; Fiory, F.; Beguinot, F.; Ulianich, L. Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism. Cells 2021, 10, 1067. [Google Scholar] [CrossRef]
- Saito, S.; Furuno, A.; Sakurai, J.; Sakamoto, A.; Park, H.-R.; Shin-ya, K.; Tsuruo, T.; Tomida, A. Chemical Genomics Identifies the Unfolded Protein Response as a Target for Selective Cancer Cell Killing during Glucose Deprivation. Cancer Res. 2009, 69, 4225–4234. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Kanigur Sultuybek, G.; Soydas, T.; Yenmis, G. NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin. Exp. Pharmacol. Physiol. 2019, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Et Biophys. Sin. 2017, 50, 133–143. [Google Scholar] [CrossRef]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr.-Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef] [PubMed]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Urbach, D.R.; Lipscombe, L.L.; Bell, C.M.; Kulkarni, G.; Austin, P.C.; Fleshner, N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3069–3075. [Google Scholar] [CrossRef]
- Tan, B.X.; Yao, W.X.; Ge, J.; Peng, X.C.; Du, X.B.; Zhang, R.; Yao, B.; Xie, K.; Li, L.H.; Dong, H. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 2011, 117, 5103–5111. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Molinolo, A.A.; Martin, D.; Younis, R.H.; Maruyama, T.; Patel, V.; Chen, W.; Schneider, A.; Gutkind, J.S. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev. Res. 2012, 5, 562–573. [Google Scholar] [CrossRef]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S.; et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet. Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.Z.; Mor, N.; Paek, H.; Blitzer, A.; Strome, M. Metformin Prevents the Progression of Dysplastic Mucosa of the Head and Neck to Carcinoma in Nondiabetic Patients. Ann. Otol. Rhinol. Laryngol. 2017, 126, 340–343. [Google Scholar] [CrossRef] [PubMed]
- DeCensi, A.; Puntoni, M.; Guerrieri-Gonzaga, A.; Cazzaniga, M.; Serrano, D.; Lazzeroni, M.; Vingiani, A.; Gentilini, O.; Petrera, M.; Viale, G.; et al. Effect of Metformin on Breast Ductal Carcinoma In Situ Proliferation in a Randomized Presurgical Trial. Cancer Prev. Res. 2015, 8, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Deschênes-Simard, X.; Pollak, M.N.; Ferbeyre, G. Metformin, aging and cancer. Aging (Albany NY) 2013, 5, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.M.; Stürmer, T.; Hong, J.-L.; Castillo, W.C.; Bae-Jump, V.; Funk, M.J. Metformin and the risk of endometrial cancer: A population-based cohort study. Gynecol. Oncol. 2015, 136, 341–347. [Google Scholar] [CrossRef]
- Greenhill, C. Metformin improves survival and recurrence rate in patients with diabetes and gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Karnewar, S.; Neeli, P.K.; Panuganti, D.; Kotagiri, S.; Mallappa, S.; Jain, N.; Jerald, M.K.; Kotamraju, S. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018, 1864, 1115–1128. [Google Scholar] [CrossRef]
- Galal, M.A.; Abdel Jabar, M.; Zhra, M.; Abdel Rahman, A.M.; Aljada, A. Absolute quantification of senescence mediators in cells using multiple reaction monitoring liquid chromatography-Tandem mass spectrometry. Anal. Chim. Acta 2021, 1184, 339009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ruan, X.-L.; Xue, Y.-X.; Yang, S.; Shi, M.; Wang, L.-N. Metformin reduces the senescence of renal tubular epithelial cells in diabetic nephropathy via the MBNL1/miR-130a-3p/STAT3 pathway. Oxidative Med. Cell. Longev. 2020, 2020, 8708236. [Google Scholar] [CrossRef] [PubMed]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, W.; Ni, X.; Little, P.J.; Xu, S.; Tang, L.; Weng, J. Metformin, macrophage dysfunction and atherosclerosis. Front. Immunol. 2021, 12, 682853. [Google Scholar] [CrossRef] [PubMed]
- Śmieszek, A.; Stręk, Z.; Kornicka, K.; Grzesiak, J.; Weiss, C.; Marycz, K. Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels-An Ex Vivo Study. Int. J. Mol. Sci. 2017, 18, 872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Chen, L.; Feng, X.; Liu, Z.; Hu, L.; Zeng, Z.; Jia, X.; Liang, M.; Shi, B. Negative regulation of Sirtuin 1 by AMP-activated protein kinase promotes metformin-induced senescence in hepatocellular carcinoma xenografts. Cancer Lett. 2017, 411, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, M.; Parris, A.B.; Feng, X.; Yang, X. p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2015, 464, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Damodaran, S.; Khemees, T.A.; Filon, M.J.; Schultz, A.; Gawdzik, J.; Etheridge, T.; Malin, D.; Richards, K.A.; Cryns, V.L. Synthetic lethal metabolic targeting of androgen-deprived prostate cancer cells with metformin. Mol. Cancer Ther. 2020, 19, 2278–2287. [Google Scholar] [CrossRef]
- Menendez, J.A.; Cufí, S.; Oliveras-Ferraros, C.; Martin-Castillo, B.; Joven, J.; Vellon, L.; Vazquez-Martin, A. Metformin and the ATM DNA damage response (DDR): Accelerating the onset of stress-induced senescence to boost protection against cancer. Aging 2011, 3, 1063. [Google Scholar] [CrossRef]
- Williams, C.C.; Singleton, B.A.; Llopis, S.D.; Skripnikova, E.V. Metformin induces a senescence-associated gene signature in breast cancer cells. J. Health Care Poor Underserved 2013, 24, 93. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Herrmann, A.L.; Däschle, A.; Kuhn, B.J.; Strobel, T.D.; Lohrey, C.; Bulkescher, J.; Krijgsveld, J.; Hoppe-Seyler, F. Effects of Metformin on the virus/host cell crosstalk in human papillomavirus-positive cancer cells. Int. J. Cancer 2021, 149, 1137–1149. [Google Scholar] [CrossRef]
- Onken, B.; Driscoll, M. Metformin induces a dietary restriction–like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS ONE 2010, 5, e8758. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, J.; Wu, X.; Zhang, G.; Li, T.; Wang, X.; Zhang, H.; Wang, C.C.; Liu, G.H. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018, 17, e12765. [Google Scholar] [CrossRef]
- Radisauskas, R.; Kuzmickiene, I.; Milinaviciene, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. J. Clin. Investig. 2019, 129, 3006–3017. [Google Scholar] [CrossRef]
- Cortes, J.E.; Jean Khoury, H.; Kantarjian, H.; Brümmendorf, T.H.; Mauro, M.J.; Matczak, E.; Pavlov, D.; Aguiar, J.M.; Fly, K.D.; Dimitrov, S. Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am. J. Hematol. 2016, 91, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Llaverias, G.; Danilo, C.; Mercier, I.; Daumer, K.; Capozza, F.; Williams, T.M.; Sotgia, F.; Lisanti, M.P.; Frank, P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011, 178, 402–412. [Google Scholar] [CrossRef]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S.; Group, J.S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef]
- Ghahremanfard, F.; Mirmohammadkhani, M.; Shahnazari, B.; Gholami, G.; Mehdizadeh, J. The valuable role of measuring serum lipid profile in cancer progression. Oman Med. J. 2015, 30, 353. [Google Scholar] [CrossRef]
- Huang, P.; Nedelcu, D.; Watanabe, M.; Jao, C.; Kim, Y.; Liu, J.; Salic, A. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell 2016, 166, 1176–1187.e14. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.E.; Scott, M.P. Communicating with hedgehogs. Nat. Rev. Mol. Cell Biol. 2005, 6, 306–317. [Google Scholar] [CrossRef]
- Sheng, R.; Chen, Y.; Yung Gee, H.; Stec, E.; Melowic, H.R.; Blatner, N.R.; Tun, M.P.; Kim, Y.; Kallberg, M.; Fujiwara, T.K.; et al. Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nat. Commun. 2012, 3, 1249. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar] [PubMed]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; Van Der Welle, R.E.; Mydock-McGrane, L.; Jiang, X.; Van Eijkeren, R.J.; Davis, O.B.; Louie, S.M. Lysosomal cholesterol activates mTORC1 via an SLC38A9–Niemann-Pick C1 signaling complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Tan, X.L.; Jian-Yu, E.; Lin, Y.; Rebbeck, T.R.; Lu, S.E.; Shang, M.; Kelly, W.K.; D’Amico, A.; Stein, M.N.; Zhang, L.; et al. Individual and joint effects of metformin and statins on mortality among patients with high-risk prostate cancer. Cancer Med. 2020, 9, 2379–2389. [Google Scholar] [CrossRef]
- Hu, D.; Guo, Y.; Wu, R.; Shao, T.; Long, J.; Yu, B.; Wang, H.; Luo, Y.; Lu, H.; Zhang, J.; et al. New Insight Into Metformin-Induced Cholesterol-Lowering Effect Crosstalk Between Glucose and Cholesterol Homeostasis via ChREBP (Carbohydrate-Responsive Element-Binding Protein)-Mediated PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Regulation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e208–e223. [Google Scholar] [CrossRef]
- Sharma, A.; Bandyopadhayaya, S.; Chowdhury, K.; Sharma, T.; Maheshwari, R.; Das, A.; Chakrabarti, G.; Kumar, V.; Mandal, C.C. Metformin exhibited anticancer activity by lowering cellular cholesterol content in breast cancer cells. PLoS ONE 2019, 14, e0209435. [Google Scholar] [CrossRef]
- Jiang, X.N.; Zhang, Y.; Wang, W.G.; Sheng, D.; Zhou, X.Y.; Li, X.Q. Alteration of Cholesterol Metabolism by Metformin Is Associated with Improved Outcome in Type II Diabetic Patients with Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 608238. [Google Scholar] [CrossRef]
- Nikolaos, T. Obesity as a Risk Factor for Cancer. EPRA Int. J. Res. Dev. (IJRD) 2023, 8, 101–104. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A. Association of obesity with survival outcomes in patients with cancer: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Z.; Pedersen, L.; Halberg, N. Cellular mechanisms linking cancers to obesity. Cell Stress 2021, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Annett, S.; Moore, G.; Robson, T. Obesity and cancer metastasis: Molecular and translational perspectives. Cancers 2020, 12, 3798. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, C.; Schehler, B.; Schneider, H. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2012, 121, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Morley, J.E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes. Res. 1998, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.P.; Alemzadeh, R.; Langley, G.; D’Angelo, L.; Smith, P.; Holshouser, S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metab. Clin. Exp. 2001, 50, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.H.; Jhun, J.Y.; Byun, J.K.; Jeong, J.H.; Lee, S.-Y.; Kim, J.K.; Choi, J.Y.; Cho, M.-L. Metformin prevents fatty liver and improves balance of white/brown adipose in an obesity mouse model by inducing FGF21. Mediat. Inflamm. 2016, 2016, 5813030. [Google Scholar] [CrossRef]
- Breining, P.; Jensen, J.B.; Sundelin, E.I.; Gormsen, L.C.; Jakobsen, S.; Busk, M.; Rolighed, L.; Bross, P.; Fernandez-Guerra, P.; Markussen, L.K. Metformin targets brown adipose tissue in vivo and reduces oxygen consumption in vitro. Diabetes Obes. Metab. 2018, 20, 2264–2273. [Google Scholar] [CrossRef]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef] [PubMed]
- Karise, I.; Bargut, T.C.; del Sol, M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed. Pharmacother. 2019, 111, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Mabhida, S.E.; Jack, B.U.; Nyambuya, T.M.; Nkambule, B.B.; Basson, A.K.; Tiano, L.; Dludla, P.V. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 2227. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Townsend, L.K.; McKie, G.L.; Medeiros, P.J.; Gurd, B.J.; Hazell, T.J. Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J. Appl. Physiol. 2017, 123, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.K.; Chari, M.; Wang, P.Y.; Lam, T.K. Central lactate metabolism regulates food intake. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E491–E496. [Google Scholar] [CrossRef] [PubMed]
- Chari, M.; Lam, C.K.; Wang, P.Y.; Lam, T.K. Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes 2008, 57, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Albrechtsen, N.J.W.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M. Bile acids are important direct and indirect regulators of the secretion of appetite-and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Mansuy, V.; Voirol, M.-J.; Pellerin, L.; Pralong, F.P. The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metab. Clin. Exp. 2011, 60, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Algire, C.; Zakikhani, M.; Blouin, M.J.; Shuai, J.H.; Pollak, M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr.-Relat. Cancer 2008, 15, 833–839. [Google Scholar] [CrossRef]
- Phoenix, K.N.; Vumbaca, F.; Fox, M.M.; Evans, R.; Claffey, K.P. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res. Treat. 2010, 123, 333–344. [Google Scholar] [CrossRef]
- Checkley, L.A.; Rho, O.; Angel, J.M.; Cho, J.; Blando, J.; Beltran, L.; Hursting, S.D.; DiGiovanni, J. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev. Res. 2014, 7, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yendamuri, S.; Barbi, J.; Pabla, S.; Petrucci, C.; Punnanitinont, A.; Nesline, M.; Glenn, S.T.; Depietro, P.; Papanicalou-Sengos, A.; Morrison, C. Body mass index influences the salutary effects of metformin on survival after lobectomy for stage I NSCLC. J. Thorac. Oncol. 2019, 14, 2181–2187. [Google Scholar] [CrossRef]
- Vedire, Y.R.; Mukherjee, S.; Dondapati, S.; Yendamuri, S. Association between visceral obesity, metformin use, and recurrence risk in early-stage colorectal cancer. Sci. Rep. 2023, 13, 8401. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur. J. Cancer 2014, 50, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Park, Y.-M.; Bookwalter, D.; O’Brien, K.; Jackson, C.; Weinberg, C.; Sandler, D. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann. Oncol. 2021, 32, 351–359. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Yuan, J.; Bi, Y.; Wang, C.; Liu, Y. The effect of metformin on biomarkers and survivals for breast cancer- a systematic review and meta-analysis of randomized clinical trials. Pharmacol. Res. 2019, 141, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Buxó, M.; Ferri Iglesias, M.J.; Verdura, S.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-Lopez, N. The C allele of ATM rs11212617 associates with higher pathological complete remission rate in breast cancer patients treated with neoadjuvant metformin. Front. Oncol. 2019, 9, 193. [Google Scholar] [CrossRef]
- Vazquez-Martin, A.; Oliveras-Ferraros, C.; Menendez, J.A. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle 2009, 8, 88–96. [Google Scholar] [CrossRef]
- Hu, T.; Chung, Y.M.; Guan, M.; Ma, M.; Ma, J.; Berek, J.S.; Hu, M.C. Reprogramming ovarian and breast cancer cells into non-cancerous cells by low-dose metformin or SN-38 through FOXO3 activation. Sci. Rep. 2014, 4, 5810. [Google Scholar] [CrossRef]
- Campagnoli, C.; Berrino, F.; Venturelli, E.; Abbà, C.; Biglia, N.; Brucato, T.; Cogliati, P.; Danese, S.; Donadio, M.; Zito, G. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin. Breast Cancer 2013, 13, 433–438. [Google Scholar] [CrossRef]
- Shi, B.; Hu, X.; He, H.; Fang, W. Metformin suppresses breast cancer growth via inhibition of cyclooxygenase-2. Oncol. Lett. 2021, 22, 615. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.R.; Borges, V.F.; Betts, C.B.; Guo, Q.; Kapoor, P.; Martinson, H.A.; Jindal, S.; Schedin, P. Cyclooxygenase-2–dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J. Clin. Investig. 2014, 124, 3901–3912. [Google Scholar] [CrossRef]
- Faria, J.; Negalha, G.; Azevedo, A.; Martel, F. Metformin and Breast Cancer: Molecular Targets. J. Mammary Gland Biol. Neoplasia 2019, 24, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Wang, Y.; Tang, S.; Sun, X.; Feng, X.; Li, Y.; Bao, G.; Li, P.; Mao, X. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015, 6, 44579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Chen, Y.; Fang, L.; Guan, C.; Bai, F.; Ma, M.; Lyu, J.; Meng, Q.H. Metformin Inhibits Tumorigenesis and Tumor Growth of Breast Cancer Cells by Upregulating miR-200c but Downregulating AKT2 Expression. J. Cancer 2017, 8, 1849–1864. [Google Scholar] [CrossRef]
- Pulito, C.; Mori, F.; Sacconi, A.; Goeman, F.; Ferraiuolo, M.; Pasanisi, P.; Campagnoli, C.; Berrino, F.; Fanciulli, M.; Ford, R.J. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017, 3, 17022. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, X.; Liu, J.; Sun, B.; Tang, H.; Zhang, H. miR-27a-mediated antiproliferative effects of metformin on the breast cancer cell line MCF-7. Oncol. Rep. 2016, 36, 3691–3699. [Google Scholar] [CrossRef]
- Cabello, P.; Pineda, B.; Tormo, E.; Lluch, A.; Eroles, P. The antitumor effect of metformin is mediated by miR-26a in breast cancer. Int. J. Mol. Sci. 2016, 17, 1298. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.S.; Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Edgerton, S.M.; Anderson, S.M.; Thor, A.D.; Richer, J.K. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm. Cancer 2014, 5, 374–389. [Google Scholar] [CrossRef]
- Zimmermann, M.; Arachchige-Don, A.P.; Donaldson, M.S.; Patriarchi, T.; Horne, M.C. Cyclin G2 promotes cell cycle arrest in breast cancer cells responding to fulvestrant and metformin and correlates with patient survival. Cell Cycle 2016, 15, 3278–3295. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.Z.; Dias, M.M.; Ropelle, E.R.; Osório-Costa, F.; Rossato, F.A.; Vercesi, A.E.; Saad, M.J.A.; Carvalheira, J.B.C. Metformin Amplifies Chemotherapy-Induced AMPK Activation and Antitumoral Growth. Clin. Cancer Res. 2011, 17, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Miskimins, W.K. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J. Mol. Signal. 2008, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Li, G.-Y.; Wang, B.; Han, S.-X.; Sun, X.; Jiang, Y.-N.; Shen, Y.-W.; Zhou, C.; Feng, J.; Lu, S.-Y.; et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J. Exp. Clin. Cancer Res. 2019, 38, 235. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.; Fan, Z.; Edgerton, S.M.; Liu, B.; Deng, X.-S.; Arnadottir, S.S.; Richer, J.K.; Anderson, S.M.; Thor, A.D. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle 2013, 12, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Samuel, S.M.; Varghese, E.; Kubatka, P.; Büsselberg, D. High glucose represses the anti-proliferative and pro-apoptotic effect of metformin in triple negative breast cancer cells. Biomolecules 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Wahdan-Alaswad, R.; Edgerton, S.; Salem, H.; Thor, A. Metformin targets glucose metabolism in triple negative breast cancer. J. Oncol. Transl. Res. 2018, 4, 129. [Google Scholar] [PubMed]
- Menendez, J.A.; Oliveras-Ferraros, C.; Cufí, S.; Corominas-Faja, B.; Joven, J.; Martin-Castillo, B.; Vazquez-Martin, A. Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle 2012, 11, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Costas-Chavarri, A.; Nandakumar, G.; Temin, S.; Lopes, G.; Cervantes, A.; Cruz Correa, M.; Engineer, R.; Hamashima, C.; Ho, G.F.; Huitzil, F.D.; et al. Treatment of Patients with Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Higurashi, T.; Nakajima, A. Metformin and Colorectal Cancer. Front. Endocrinol. 2018, 9, 622. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Uchiyama, T.; Suzuki, K.; Nozaki, Y.; Yoneda, K.; Fujita, K.; Yoneda, M.; et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol. Carcinog. 2010, 49, 662–671. [Google Scholar] [CrossRef]
- Xie, J.; Xia, L.; Xiang, W.; He, W.; Yin, H.; Wang, F.; Gao, T.; Qi, W.; Yang, Z.; Yang, X.; et al. Metformin selectively inhibits metastatic colorectal cancer with the KRAS mutation by intracellular accumulation through silencing MATE1. Proc. Natl. Acad. Sci. USA 2020, 117, 13012–13022. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. The Emerging Role of Metformin in the Prevention and Treatment of Colorectal Cancer: A Game Changer for the Management of Colorectal Cancer. Curr. Diabetes Rev. 2022, 18, e051121197762. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kitayama, J.; Horie, H.; Koinuma, K.; Ohzawa, H.; Yamaguchi, H.; Kawahira, H.; Mimura, T.; Lefor, A.K.; Sata, N. Metformin changes the immune microenvironment of colorectal cancer in patients with type 2 diabetes mellitus. Cancer Sci. 2020, 111, 4012–4020. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, D.H.; Kim, J.L.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Jeong, Y.A.; Lee, S.Y.; Lee, S.I.; Lee, Y.Y.; et al. Metformin enhances TRAIL-induced apoptosis by Mcl-1 degradation via Mule in colorectal cancer cells. Oncotarget 2016, 7, 59503–59518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiao, J.; Liu, J.; Liu, J.; Shu, G.; Yin, G. Metformin suppresses the growth of colorectal cancer by targeting INHBA to inhibit TGF-β/PI3K/AKT signaling transduction. Cell Death Dis. 2022, 13, 202. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Hu, L. Epithelial-Mesenchymal Transition Phenotype, Metformin, and Survival for Colorectal Cancer Patients with Diabetes Mellitus II. Gastroenterol. Res. Pract. 2017, 2017, 2520581. [Google Scholar] [CrossRef]
- Mogavero, A.; Maiorana, M.V.; Zanutto, S.; Varinelli, L.; Bozzi, F.; Belfiore, A.; Volpi, C.C.; Gloghini, A.; Pierotti, M.A.; Gariboldi, M. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci. Rep. 2017, 7, 15992. [Google Scholar] [CrossRef]
- Yip, K.L.; Tsai, T.N.; Yang, I.P.; Miao, Z.F.; Chen, Y.C.; Li, C.C.; Su, W.C.; Chang, T.K.; Huang, C.W.; Tsai, H.L.; et al. Metformin Enhancement of Therapeutic Effects of 5-Fluorouracil and Oxaliplatin in Colon Cancer Cells and Nude Mice. Biomedicines 2022, 10, 955. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.C.; Ku, J.L. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget 2017, 8, 56546–56557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, S.; Lu, X.; Shi, Z.; Liu, H.; Zhu, B. Metformin enhances the sensitivity of colorectal cancer cells to cisplatin through ROS-mediated PI3K/Akt signaling pathway. Gene 2020, 745, 144623. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Zhang, S.-J.; Xue, D.; Feng, Y.-Q.; Li, Y.; Huang, X.; Cui, Q.; Wang, B.; Feng, J.; Bao, T.; et al. Overcoming chemoresistance in non-angiogenic colorectal cancer by metformin via inhibiting endothelial apoptosis and vascular immaturity. J. Pharm. Anal. 2023, 13, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell-cell adhesion: Linking Wnt/beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y. Metformin attenuates cells stemness and epithelial-mesenchymal transition in colorectal cancer cells by inhibiting the Wnt3a/beta-catenin pathway. Mol. Med. Rep. 2019, 19, 1203–1209. [Google Scholar] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, A.; Zheng, S.; Chen, C.; Lyu, J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control J. Moffitt Cancer Cent. 2022, 29, 10732748221099227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, L.; Zhou, Q.; He, K.; Teng, L. Preventative and Therapeutic Effects of Metformin in Gastric Cancer: A New Contribution of an Old Friend. Cancer Manag. Res. 2020, 12, 8545–8554. [Google Scholar] [CrossRef]
- Li, P.; Zhang, C.; Gao, P.; Chen, X.; Ma, B.; Yu, D.; Song, Y.; Wang, Z. Metformin use and its effect on gastric cancer in patients with type 2 diabetes: A systematic review of observational studies. Oncol. Lett. 2018, 15, 1191–1199. [Google Scholar] [CrossRef]
- Dulskas, A.; Patasius, A.; Kaceniene, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Smailyte, G. A Cohort Study of Antihyperglycemic Medication Exposure and Gastric Cancer Risk. J. Clin. Med. 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Santoni, G.; Xie, S.-H.; Lagergren, J. Improved prognosis in gastric adenocarcinoma among metformin users in a population-based study. Br. J. Cancer 2021, 125, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bahardoust, M.; Mousavi, S.; Moezi, Z.D.; Yarali, M.; Tayebi, A.; Olamaeian, F.; Tizmaghz, A. Effect of Metformin Use on Survival and Recurrence Rate of Gastric Cancer After Gastrectomy in Diabetic Patients: A Systematic Review and Meta-analysis of Observational Studies. J. Gastrointest. Cancer 2023. [Google Scholar] [CrossRef]
- Fang, W.; Cui, H.; Yu, D.; Chen, Y.; Wang, J.; Yu, G. Increased expression of phospho-acetyl-CoA carboxylase protein is an independent prognostic factor for human gastric cancer without lymph node metastasis. Med. Oncol. 2014, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Nam, S.; Kook, M.C.; Kim, K.T.; Liu, X.; Yao, H.; Jung, H.R.; Lemos, R., Jr.; Seo, H.H.; Park, H.S.; et al. HNF4alpha is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut 2016, 65, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Gong, J.; Iwama, H.; Kitanaka, A.; Tani, J.; Miyoshi, H.; Nomura, K.; Mimura, S.; Kobayashi, M.; Aritomo, Y.; et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Juan, Y.N.; Yang, J.S.; Chiu, H.Y. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int. J. Oncol. 2019, 54, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Fang, W.; Xia, T.; Chen, Y.; Gao, Y.; Jiao, X.; Huang, S.; Wang, J.; Li, Z.; Xie, K. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget 2015, 6, 12748–12762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, J.; Zhou, R. Combination of metformin and oxaliplatin inhibits gastric cancer cell proliferation and induces apoptosis. Acta Biochim. Pol. 2022, 69, 321–326. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Metformin Use and Gastric Cancer Risk in Diabetic Patients After Helicobacter pylori Eradication. J. Natl. Cancer Inst. 2019, 111, 484–489. [Google Scholar] [CrossRef]
- Wan, J.; Zhou, J.; Zhao, H.; Wang, M.; Wei, Z.; Gao, H.; Wang, Y.; Cui, H. Sonic hedgehog pathway contributes to gastric cancer cell growth and proliferation. BioResearch Open Access 2014, 3, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Chen, Y.Z.; Chou, N.H.; Chen, Y.C.; Wu, C.C.; Liu, L.F.; Yang, Y.F.; Yeh, C.Y.; Kung, M.L.; Tu, Y.T.; et al. Metformin inhibits gastric cancer cell proliferation by regulation of a novel Loc100506691-CHAC1 axis. Mol. Ther. Oncolytics 2021, 22, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: A population-based study. Gastroenterology 2004, 127, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology 2004, 127 (Suppl. 1), S27–S34. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Richardson, P.A.; Everhart, J.E. The role of diabetes in hepatocellular carcinoma: A case-control study among United States Veterans. Am. J. Gastroenterol. 2001, 96, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Polesel, J.; Zucchetto, A.; Montella, M.; Dal Maso, L.; Crispo, A.; La Vecchia, C.; Serraino, D.; Franceschi, S.; Talamini, R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 2009, 20, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Shieh, J.J.; Chang, C.C.; Chen, T.T.; Lin, J.T.; Wu, M.S.; Lin, J.H.; Wu, C.Y. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: Population-based and in vitro studies. Gut 2013, 62, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Yu, J.; Cheng, A.S.; Wong, G.L.; Chan, H.Y.; Chu, E.S.; Ng, E.K.; Chan, F.K.; Sung, J.J.; Chan, H.L. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int. J. Cancer 2009, 124, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Groner, B.; Lucks, P.; Borghouts, C. The function of Stat3 in tumor cells and their microenvironment. Semin. Cell Dev. Biol. 2008, 19, 341–350. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, W.; Wu, F.; Wang, C.; Yu, L.; Tang, L.; Qiu, B.; Li, Y.; Guo, L.; Wu, M.; et al. Prognostic Significance of AMPK Activation and Therapeutic Effects of Metformin in Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, N.; Shimano, H.; Hasegawa, K.; Ohashi, K.; Matsuzaka, T.; Najima, Y.; Sekiya, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur. J. Cancer 2005, 41, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.; Hwang, B.J.; Dewi, R.E.; Twaddel, W.; Goloubeva, O.G.; Wong, K.K.; Saxena, N.K.; Biswal, S.; Girnun, G.D. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev. Res. 2012, 5, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xia, L.; Geng, W.; Xu, D.; Zhong, C.; Zhang, J.; Xia, Q. Metformin suppresses interleukin-22 induced hepatocellular carcinoma by upregulating Hippo signaling pathway. J. Gastroenterol. Hepatol. 2021, 36, 3469–3476. [Google Scholar] [CrossRef]
- Vacante, F.; Senesi, P.; Montesano, A.; Paini, S.; Luzi, L.; Terruzzi, I. Metformin counteracts HCC progression and metastasis enhancing KLF6/p21 expression and downregulating the IGF axis. Int. J. Endocrinol. 2019, 2019, 7570146. [Google Scholar] [CrossRef]
- Hu, A.; Hu, Z.; Ye, J.; Liu, Y.; Lai, Z.; Zhang, M.; Ji, W.; Huang, L.; Zou, H.; Chen, B. Metformin exerts anti-tumor effects via Sonic hedgehog signaling pathway by targeting AMPK in HepG2 cells. Biochem. Cell Biol. 2022, 100, 142–151. [Google Scholar] [CrossRef]
- Li, C.-Q.; Liu, Z.-Q.; Liu, S.-S.; Zhang, G.-T.; Jiang, L.; Chen, C.; Luo, D.-Q. Transcriptome analysis of liver cancer cell Huh-7 treated with metformin. Front. Pharmacol. 2022, 13, 822023. [Google Scholar] [CrossRef]
- Wang, M.-D.; Wang, N.-Y.; Zhang, H.-L.; Sun, L.-Y.; Xu, Q.-R.; Liang, L.; Li, C.; Huang, D.-S.; Zhu, H.; Yang, T. Fatty acid transport protein-5 (FATP5) deficiency enhances hepatocellular carcinoma progression and metastasis by reprogramming cellular energy metabolism and regulating the AMPK-mTOR signaling pathway. Oncogenesis 2021, 10, 74. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Saber, S.; Youssef, M.E.; Gaafar, A.G.A.; Eissa, H.; Abd-Eldayem, M.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Shahien, M.A. Empagliflozin adjunct with metformin for the inhibition of hepatocellular carcinoma progression: Emerging approach for new application. Biomed. Pharmacother. 2022, 145, 112455. [Google Scholar] [CrossRef]
- Gao, X.; Qiao, X.; Xing, X.; Huang, J.; Qian, J.; Wang, Y.; Zhang, Y.; Zhang, X.; Li, M.; Cui, J. Matrix stiffness-upregulated microRNA-17-5p attenuates the intervention effects of metformin on HCC invasion and metastasis by targeting the PTEN/PI3K/Akt pathway. Front. Oncol. 2020, 10, 1563. [Google Scholar] [CrossRef]
- Sun, R.; Zhai, R.; Ma, C.; Miao, W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 2020, 9, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Abdollah, M.R.; Elmazar, M.M.; El-Fawal, H.A.; Abdelnaser, A. Effects of metformin combined with antifolates on HepG2 cell metabolism and cellular proliferation. Front. Oncol. 2022, 12, 828988. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, M.; Park, M.; Kim, S.Y.; Shim, M.S.; Lee, C.Y.; Choi, D.H.; Cho, Y. Metformin and dichloroacetate suppress proliferation of liver cancer cells by inhibiting mTOR complex 1. Int. J. Mol. Sci. 2021, 22, 10027. [Google Scholar] [CrossRef]
- Saber, S.; Ghanim, A.M.; El-Ahwany, E.; El-Kader, E.M.A. Novel complementary antitumour effects of celastrol and metformin by targeting IκBκB, apoptosis and NLRP3 inflammasome activation in diethylnitrosamine-induced murine hepatocarcinogenesis. Cancer Chemother. Pharmacol. 2020, 85, 331–343. [Google Scholar] [CrossRef]
- Harati, R.; Vandamme, M.; Blanchet, B.; Bardin, C.; Praz, F.; Hamoudi, R.A.; Desbois-Mouthon, C. Drug-drug interaction between metformin and sorafenib alters antitumor effect in hepatocellular carcinoma cells. Mol. Pharmacol. 2021, 100, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, D.; Wu, T.; Hu, X.; Deng, J.; Yan, X.; Wu, J.; Xu, S.; Yang, X.; Li, G. Phenformin synergistically sensitizes liver cancer cells to sorafenib by downregulating CRAF/ERK and PI3K/AKT/mTOR pathways. Am. J. Transl. Res. 2021, 13, 7508. [Google Scholar]
- Tian, Y.; Tang, B.; Wang, C.; Sun, D.; Zhang, R.; Luo, N.; Han, Z.; Liang, R.; Gao, Z.; Wang, L. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget 2016, 7, 46230. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar]
- Dearden, S.; Stevens, J.; Wu, Y.-L.; Blowers, D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 2013, 24, 2371–2376. [Google Scholar] [CrossRef]
- Arrieta, O.; Barrón, F.; Padilla, M.-Á.S.; Avilés-Salas, A.; Ramírez-Tirado, L.A.; Jiménez, M.J.A.; Vergara, E.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Cardona, A.F. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor–mutated lung adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, e192553. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, S.-M.; Shin, D.W.; Cho, M.; Cho, J.H.; Kim, J. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J. Thorac. Oncol. 2021, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, Y.; Gong, Y.; He, J.; Chen, X. Metformin and lung cancer risk of patients with type 2 diabetes mellitus: A meta-analysis. Biomed. Rep. 2015, 3, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Wu, G.N.; Yuan, D.M. Association of the metformin with the risk of lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2013, 2, 259–263. [Google Scholar] [PubMed]
- Troncone, M.; Cargnelli, S.M.; Villani, L.A.; Isfahanian, N.; Broadfield, L.A.; Zychla, L.; Wright, J.; Pond, G.; Steinberg, G.R.; Tsakiridis, T. Targeting metabolism and AMP-activated kinase with metformin to sensitize non-small cell lung cancer (NSCLC) to cytotoxic therapy: Translational biology and rationale for current clinical trials. Oncotarget 2017, 8, 57733. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, Y.; Hopmans, S.N.; Sanli, T.; Barron, C.; Tsiani, E.; Cutz, J.C.; Pond, G.; Wright, J.; Singh, G.; Tsakiridis, T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br. J. Cancer 2013, 108, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dong, W.; Shen, H.; Xu, J.; Zhu, L.; Liu, Q.; Du, J. Combinational Therapy Enhances the Effects of Anti-IGF-1R mAb Figitumumab to Target Small Cell Lung Cancer. PLoS ONE 2015, 10, e0135844. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dong, W.; Qu, X.; Shen, H.; Xu, J.; Zhu, L.; Liu, Q.; Du, J. Metformin Enhances the Therapy Effects of Anti-IGF-1R mAb Figitumumab to NSCLC. Sci. Rep. 2016, 6, 31072. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Peng, H.; Yang, X.; Wu, W.; Zhao, Y.; Chen, D.; Chen, L.; Liu, J. Metformin mediated microRNA-7 upregulation inhibits growth, migration, and invasion of non-small cell lung cancer A549 cells. Anti-Cancer Drugs 2020, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, B.; Wu, J.; Zhang, H.; Wu, B. Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncol. Lett. 2014, 8, 1269–1274. [Google Scholar] [CrossRef]
- Teixeira, S.F.; Guimarães Idos, S.; Madeira, K.P.; Daltoé, R.D.; Silva, I.V.; Rangel, L.B. Metformin synergistically enhances antiproliferative effects of cisplatin and etoposide in NCI-H460 human lung cancer cells. J. Bras. De Pneumol. Publicacao Of. Da Soc. Bras. De Pneumol. E Tisilogia 2013, 39, 644–649. [Google Scholar] [CrossRef]
- Lin, C.C.; Yeh, H.H.; Huang, W.L.; Yan, J.J.; Lai, W.W.; Su, W.P.; Chen, H.H.; Su, W.C. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am. J. Respir. Cell Mol. Biol. 2013, 49, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Peng, T.; Zhang, K.; Lin, C.; Han, R.; Lu, C.; He, Y. Metformin restores crizotinib sensitivity in crizotinib-resistant human lung cancer cells through inhibition of IGF1-R signaling pathway. Oncotarget 2016, 7, 34442. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Choi, Y.J.; Park, J.W. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014, 74, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Bernal, P.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Orozco-Morales, M.; Olivera-Ramírez, A.; Ávila-Moreno, F.; Colín-González, A.L.; Cardona, A.F.; Rosell, R.; Arrieta, O. Will We Unlock the Benefit of Metformin for Patients with Lung Cancer? Lessons from Current Evidence and New Hypotheses. Pharmaceuticals 2022, 15, 786. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Kim, Y.; Kim, D.; Cho, E.Y.; Han, J.; Kim, H.K.; Shim, Y.M.; Kim, D.H. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J. Cell. Mol. Med. 2019, 23, 2872–2889. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.H.; Zhang, Y.G.; Wu, Z.; Liu, X.; Yang, J.W.; Ji, H.L. Effects of metformin on survival outcomes of lung cancer patients with type 2 diabetes mellitus: A meta-analysis. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2016, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Lim, M.C.; Baek, M.-H.; Park, Y.-H.; Kim, S. Impact of metformin on survival outcome in ovarian cancer: A nationwide population-based cohort study. J. Gynecol. Oncol. 2021, 32, e65. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.; Giri, S.; Hartmann, L.C.; Shridhar, V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell. Mol. Med. 2009, 15, 166–178. [Google Scholar] [CrossRef]
- Lengyel, E.; Litchfield, L.M.; Mitra, A.K.; Nieman, K.M.; Mukherjee, A.; Zhang, Y.; Johnson, A.; Bradaric, M.J.; Lee, W.S.; Romero, I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obstet. Gynecol. 2015, 212, 479.e1–479.e10. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chan, D.K.; Haugrud, A.B.; Miskimins, W. Mechanisms by Which Low Glucose Enhances the Cytotoxicity of Metformin to Cancer Cells Both In Vitro and In Vivo. PLoS ONE 2014, 9, e108444. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Wan, J.; Wang, W.; Wang, L.; Yuan, Y.; Yang, Z.; Liu, X.; Ming, L. Low glucose and metformin-induced apoptosis of human ovarian cancer cells is connected to ASK1 via mitochondrial and endoplasmic reticulum stress-associated pathways. J. Exp. Clin. Cancer Res. CR 2019, 38, 77. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Lee, H.-Y.; Lee, C. Metformin targets Axl and Tyro3 receptor tyrosine kinases to inhibit cell proliferation and overcome chemoresistance in ovarian cancer cells. Int. J. Oncol. 2015, 47, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-s.; Wang, S.; Deng, A.; Liu, B.; Edgerton, S.M.; Lind, S.E.; Wahdan-Alaswad, R.S.; Thor, A.D. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 2012, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Zong, C.S.; Xia, W.; Yamaguchi, H.; Ding, Q.; Xie, X.; Lang, J.; Lai, C.-C.; Chang, C.-J.; Huang, W.C.; et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008, 10, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Niu, N.; Wei, T.; Tozawa, H.; Chen, X.; Zhang, C.; Zhang, J.; Wada, Y.; Kapron, C.M.; Liu, J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, E.; Huang, S. STAT3 as a central regulator of tumor metastases. Curr. Mol. Med. 2009, 9, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Mavria, G.; Vercoulen, Y.; Yeo, M.; Paterson, H.F.; Karasarides, M.; Marais, R.M.; Bird, D.; Marshall, C.J. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Et Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, K.N.; Vumbaca, F.; Claffey, K.P. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERα negative MDA-MB-435 breast cancer model. Breast Cancer Res. Treat. 2008, 113, 101–111. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, J. Mechanisms of metformin inhibiting cancer invasion and migration. Am. J. Transl. Res. 2020, 12, 4885–4901. [Google Scholar]
- Wu, B.; Li, S.; Sheng, L.; Zhu, J.; Gu, L.; Shen, H.; La, D.-D.; Hambly, B.D.; Bao, S.; Di, W. Metformin inhibits the development and metastasis of ovarian cancer. Oncol. Rep. 2012, 28, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.; Graham, R.P.; Maguire, J.L.; Giri, S.; Shridhar, V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 2011, 13, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yeung, S.C.; Hassan, M.M.; Konopleva, M.; Abbruzzese, J.L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009, 137, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Eibl, G.; Rozengurt, E. Metformin: Review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Eibl, G.; Rozengurt, E. KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin. Cancer Biol. 2019, 54, 50–62. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Rozengurt, E. Mechanistic target of rapamycin (mTOR): A point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front. Physiol. 2014, 5, 357. [Google Scholar] [CrossRef] [PubMed]
- Rozengurt, E.; Soares, H.P.; Sinnet-Smith, J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: An unintended consequence leading to drug resistance. Mol. Cancer Ther. 2014, 13, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Á.; Vera, I.; Diaz, M.P.; Navarro, C.; Rojas, M.; Torres, W.; Parra, H.; Salazar, J.; De Sanctis, J.B.; Bermúdez, V. The YAP/TAZ signaling pathway in the tumor microenvironment and carcinogenesis: Current knowledge and therapeutic promises. Int. J. Mol. Sci. 2021, 23, 430. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Drzewoski, J.; Świderska, E.; Strycharz, J.; Gabryanczyk, A.; Kasznicki, J.; Bogdańska, M.; Śliwińska, A. Metformin Induces Apoptosis in Human Pancreatic Cancer (PC) Cells Accompanied by Changes in the Levels of Histone Acetyltransferases (Particularly, p300/CBP-Associated Factor (PCAF) Protein Levels). Pharmaceuticals 2023, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Berker, B.; Emral, R.; Demirel, C.; Corapcioglu, D.; Unlu, C.; Kose, K. Increased insulin-like growth factor-I levels in women with polycystic ovary syndrome, and beneficial effects of metformin therapy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2004, 19, 125–133. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Pritchard, K.I.; Ennis, M.; Clemons, M.; Graham, M.; Fantus, I.G. Insulin-lowering effects of metformin in women with early breast cancer. Clin. Breast Cancer 2008, 8, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Mutgan, A.C.; Besikcioglu, H.E.; Wang, S.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol. Cancer 2018, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, Z.; Jia, Z.; Xu, C. Inhibitory effect of metformin combined with gemcitabine on pancreatic cancer cells in vitro and in vivo. Mol. Med. Rep. 2016, 14, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-W.; Li, Z.-S.; Zou, D.-W.; Jin, Z.-D.; Gao, J.; Xu, G.-M. Metformin induces apoptosis of pancreatic cancer cells. World J. Gastroenterol. 2008, 14, 7192. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Z.; Ali, S.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Banerjee, S.; Kong, D.; Li, Y.; Thakur, S. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev. Res. 2012, 5, 355–364. [Google Scholar] [CrossRef]
- Nair, V.; Sreevalsan, S.; Basha, R.; Abdelrahim, M.; Abudayyeh, A.; Hoffman, A.R.; Safe, S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: Role of specificity protein (Sp) transcription factors. J. Biol. Chem. 2014, 289, 27692–27701. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Sinnett-Smith, J.; Wang, J.; Soares, H.P.; Young, S.H.; Eibl, G.; Rozengurt, E. Dose-dependent AMPK-dependent and independent mechanisms of berberine and metformin inhibition of mTORC1, ERK, DNA synthesis and proliferation in pancreatic cancer cells. PLoS ONE 2014, 9, e114573. [Google Scholar] [CrossRef] [PubMed]
- Sinnett-Smith, J.; Kisfalvi, K.; Kui, R.; Rozengurt, E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: Dependence on glucose concentration and role of AMPK. Biochem. Biophys. Res. Commun. 2013, 430, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Ro, J.Y. Prostatic intraepithelial neoplasia: A potential precursor lesion of prostatic adenocarcinoma. Yonsei Med. J. 1995, 36, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.S.; Giovannucci, E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2006, 15, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Filioussi, K.; Tsantes, A. Diabetes mellitus and risk of prostate cancer: A meta-analysis. Diabetologia 2004, 47, 1071–1078. [Google Scholar] [CrossRef]
- Tseng, C.H. Diabetes and risk of prostate cancer: A study using the National Health Insurance. Diabetes Care 2011, 34, 616–621. [Google Scholar] [CrossRef]
- Tseng, C.H. Prostate cancer mortality in Taiwanese men: Increasing age-standardized trend in general population and increased risk in diabetic men. Ann. Med. 2011, 43, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Lee, T.C.; Cheng, S.M.; Tu, S.T.; Yen, M.H.; Tseng, C.H. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp. Diabetes Res. 2012, 2012, 413782. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Chua, S., Jr.; Gao, Y.T.; Gentzschein, E.; Chang, L.; Deng, J.; Stanczyk, F.Z. Prostate cancer risk and serum levels of insulin and leptin: A population-based study. J. Natl. Cancer Inst. 2001, 93, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Gallus, S.; Bosetti, C.; Tzonou, A.; Lagiou, P.; Negri, E.; Trichopoulos, D.; La Vecchia, C. Diabetes and the risk of prostate cancer. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2002, 11, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.W.; Kuo, D.Y.; Liao, J.M.; Wang, P.S.; Yu, C.H. Growth Modulation of Diabetic Factors and Antidiabetic Drugs on Prostate Cancer Cell Lines. Chin. J. Physiol. 2016, 59, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Abel, U.; Grobholz, R.; Hermani, A.; Trojan, L.; Angel, P.; Mayer, D. Up-regulation of insulin-like growth factor axis components in human primary prostate cancer correlates with tumor grade. Hum. Pathol. 2005, 36, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.E.; Gleave, M.E.; Zakikhani, M.; Bell, R.H.; Piura, E.; Vickers, E.; Cunningham, M.; Larsson, O.; Fazli, L.; Pollak, M. Insulin receptor expression by human prostate cancers. Prostate 2009, 69, 33–40. [Google Scholar] [CrossRef]
- Kojima, S.; Inahara, M.; Suzuki, H.; Ichikawa, T.; Furuya, Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2009, 16, 161–167. [Google Scholar] [CrossRef]
- Hwang, I.C.; Park, S.M.; Shin, D.; Ahn, H.Y.; Rieken, M.; Shariat, S.F. Metformin association with lower prostate cancer recurrence in type 2 diabetes: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zingales, V.; Distefano, A.; Raffaele, M.; Zanghi, A.; Barbagallo, I.; Vanella, L. Metformin: A Bridge between Diabetes and Prostate Cancer. Front. Oncol. 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Zhang, X.L.; Luo, Z.G.; Yan, J.J.; Pan, S.H.; Ying, X.R.; Pan, J.G.; Zhang, G.F. Metformin therapy and prostate cancer risk: A meta-analysis of observational studies. Int. J. Clin. Exp. Med. 2015, 8, 13089–13098. [Google Scholar] [PubMed]
- Chen, C.B.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Metformin and the risk of prostate cancer across racial/ethnic groups: A population-based cohort study. Prostate Cancer Prostatic Dis. 2017, 20, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Laurent, K.; Loubat, A.; Giorgetti-Peraldi, S.; Colosetti, P.; Auberger, P.; Tanti, J.F.; Le Marchand-Brustel, Y.; Bost, F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008, 27, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A.J.; Venier, N.A.; Vandersluis, A.D.; Besla, R.; Sugar, L.M.; Kiss, A.; Fleshner, N.E.; Pollak, M.; Klotz, L.H.; Venkateswaran, V. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012, 15, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Nomiyama, T.; Kawanami, T.; Hamaguchi, Y.; Terawaki, Y.; Tanaka, T.; Murase, K.; Motonaga, R.; Tanabe, M.; Yanase, T. Combined Treatment with Exendin-4 and Metformin Attenuates Prostate Cancer Growth. PLoS ONE 2015, 10, e0139709. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Laurent, K.; Giuliano, S.; Larbret, F.; Ponzio, G.; Gounon, P.; Le Marchand-Brustel, Y.; Giorgetti-Peraldi, S.; Cormont, M.; Bertolotto, C.; et al. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010, 70, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Ahmad, N.; Hodges, K.; Kuang, S.; Ratliff, T.; Liu, X. Inhibition of polo-like kinase 1 (Plk1) enhances the antineoplastic activity of metformin in prostate cancer. J. Biol. Chem. 2015, 290, 2024–2033. [Google Scholar] [CrossRef]
- Pennanen, P.; Syvälä, H.; Bläuer, M.; Savinainen, K.; Ylikomi, T.; Tammela, T.L.J.; Murtola, T.J. The effects of metformin and simvastatin on the growth of LNCaP and RWPE-1 prostate epithelial cell lines. Eur. J. Pharmacol. 2016, 788, 160–167. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, Q.; Tang, Q.; Zheng, F.; Wu, J.; Yang, L.; Hann, S.S. Activation of AMPKα mediates additive effects of solamargine and metformin on suppressing MUC1 expression in castration-resistant prostate cancer cells. Sci. Rep. 2016, 6, 36721. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Gao, J.M.; Liang, J.Q.; Xi, J.M.; Fu, M.; Wu, Y.J. Vitamin D3 potentiates the growth inhibitory effects of metformin in DU145 human prostate cancer cells mediated by AMPK/mTOR signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 711–717. [Google Scholar] [CrossRef]
- Biernacka, K.M.; Persad, R.A.; Bahl, A.; Gillatt, D.; Holly, J.M.; Perks, C.M. Hyperglycaemia-induced resistance to Docetaxel is negated by metformin: A role for IGFBP-2. Endocr.-Relat. Cancer 2017, 24, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Akinyeke, T.; Matsumura, S.; Wang, X.; Wu, Y.; Schalfer, E.D.; Saxena, A.; Yan, W.; Logan, S.K.; Li, X. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis 2013, 34, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Demir, U.; Koehler, A.; Schneider, R.; Schweiger, S.; Klocker, H. Metformin anti-tumor effect via disruption of the MID1 translational regulator complex and AR downregulation in prostate cancer cells. BMC Cancer 2014, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Sacco, A.; Morcavallo, A.; Squatrito, S.; Migliaccio, A.; Morrione, A.; Maggiolini, M.; Belfiore, A. Metformin inhibits androgen-induced IGF-IR up-regulation in prostate cancer cells by disrupting membrane-initiated androgen signaling. Endocrinology 2014, 155, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Esquenet, M.; Goossens, K.; Heyns, W.; Verhoeven, G. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res. 1997, 57, 1086–1090. [Google Scholar] [PubMed]
- Loubière, C.; Goiran, T.; Laurent, K.; Djabari, Z.; Tanti, J.F.; Bost, F. Metformin-induced energy deficiency leads to the inhibition of lipogenesis in prostate cancer cells. Oncotarget 2015, 6, 15652–15661. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.E.; Leong, K.G.; Yue, P.; Li, L.; Jhunjhunwala, S.; Chen, D.; Seo, K.; Modrusan, Z.; Gao, W.Q.; et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: Implications for androgen-deprivation therapy. Cancer Res. 2012, 72, 527–536. [Google Scholar] [CrossRef]
- Rycaj, K.; Cho, E.J.; Liu, X.; Chao, H.P.; Liu, B.; Li, Q.; Devkota, A.K.; Zhang, D.; Chen, X.; Moore, J.; et al. Longitudinal tracking of subpopulation dynamics and molecular changes during LNCaP cell castration and identification of inhibitors that could target the PSA-/lo castration-resistant cells. Oncotarget 2016, 7, 14220–14240. [Google Scholar] [CrossRef]
- Tong, D.; Liu, Q.; Liu, G.; Xu, J.; Lan, W.; Jiang, Y.; Xiao, H.; Zhang, D.; Jiang, J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2017, 389, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cheng, J.C.; Chang, H.M.; Leung, P.C. COX2 and PGE2 mediate EGF-induced E-cadherin-independent human ovarian cancer cell invasion. Endocr.-Relat. Cancer 2014, 21, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Vo, B.T.; Morton, D., Jr.; Komaragiri, S.; Millena, A.C.; Leath, C.; Khan, S.A. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology 2013, 154, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Jorvig, J.E.; Chakraborty, A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anti-Cancer Drugs 2015, 26, 160–166. [Google Scholar] [CrossRef]
- Qin, G.; Xu, F.; Qin, T.; Zheng, Q.; Shi, D.; Xia, W.; Tian, Y.; Tang, Y.; Wang, J.; Xiao, X.; et al. Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget 2015, 6, 41794–41808. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, D.; Liu, G.; Xu, J.; Do, K.; Geary, K.; Zhang, D.; Zhang, J.; Zhang, Y.; Li, Y.; et al. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β1/STAT3 axis-regulated EMT. Cell Death Dis. 2017, 8, e3007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, B.; Wang, Y.; Zhang, M.; Fu, S.; Gao, H.; Peng, R.; Zhang, L.; Tang, J. Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int. J. Mol. Med. 2014, 33, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Banerjee, S.; Kong, D.; Li, Y.; Sarkar, F.H. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007, 67, 8293–8300. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Jia, Z.; Le, X.; Dai, B.; Wei, D.; Huang, S.; Tan, D.; Xie, K. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009, 69, 3501–3509. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, C.; Wang, L.; Ma, Q.; Xia, P.; Qi, M.; Yang, M.; Han, B. Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: Involvement of the tumor suppressor miR30a and its target gene SOX4. Biochem. Biophys. Res. Commun. 2014, 452, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Yamamura, S.; Majid, S.; Shahryari, V.; Hirata, H.; Tanaka, Y.; Dahiya, R. MicroRNA-708 induces apoptosis and suppresses tumorigenicity in renal cancer cells. Cancer Res. 2011, 71, 6208–6219. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; McDonnell, K.; Choi, H.; Gao, D.; Hahn, M.; Joshi, N.; Park, S.M.; Catena, R.; Do, Y.; Brazin, J.; et al. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell 2013, 23, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, J.; Wu, Y.; Wang, Z.; Guo, Y.; Lee, P.; Li, X. Metformin induces ER stress-dependent apoptosis through miR-708-5p/NNAT pathway in prostate cancer. Oncogenesis 2015, 4, e158. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Ali, R.; Bankhead, C.R.; Bethel, M.A.; Cairns, B.J.; Camisasca, R.P.; Crowe, F.L.; Farmer, A.J.; Harrison, S.; Hirst, J.A.; et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: Systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia 2012, 55, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3β-MCL-1 axis. Cancer Cell 2019, 35, 798–815.e5. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wondisford, F.E. Metformin action: Concentrations matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef]
- Stambolic, V.; Woodgett, J.R.; Fantus, I.G.; Pritchard, K.I.; Goodwin, P.J. Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Res. Treat. 2009, 114, 387–389. [Google Scholar] [CrossRef]
- Gui, D.Y.; Sullivan, L.B.; Luengo, A.; Hosios, A.M.; Bush, L.N.; Gitego, N.; Davidson, S.M.; Freinkman, E.; Thomas, C.J.; Vander Heiden, M.G. Environment Dictates Dependence on Mitochondrial Complex I for NAD+ and Aspartate Production and Determines Cancer Cell Sensitivity to Metformin. Cell Metab. 2016, 24, 716–727. [Google Scholar] [CrossRef]
- Wang, D.-S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, F.; Guan, J.; Zhou, L.; Chen, B.A. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Sorokin, D.V.; Tatarskiy, V.V., Jr.; Prokhorov, N.S.; Semina, S.E.; Berstein, L.M.; Krasil’nikov, M.A. The phenomenon of acquired resistance to metformin in breast cancer cells: The interaction of growth pathways and estrogen receptor signaling. IUBMB Life 2016, 68, 281–292. [Google Scholar] [CrossRef]
- Seo, D.S.; Joo, S.; Baek, S.; Kang, J.; Kwon, T.K.; Jang, Y. Metformin Resistance Is Associated with Expression of Inflammatory and Invasive Genes in A549 Lung Cancer Cells. Genes 2023, 14, 1014. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Kuppusamy, G. Metformin in breast cancer: Preclinical and clinical evidence. Curr. Probl. Cancer 2020, 44, 100488. [Google Scholar] [CrossRef]
- Del Barco, S.; Vazquez-Martin, A.; Cufí, S.; Oliveras-Ferraros, C.; Bosch-Barrera, J.; Joven, J.; Martin-Castillo, B.; Menendez, J.A. Metformin: Multi-faceted protection against cancer. Oncotarget 2011, 2, 896–917. [Google Scholar] [CrossRef]
- Wensink, M.J.; Lu, Y.; Tian, L.; Shaw, G.M.; Rizzi, S.; Jensen, T.K.; Mathiesen, E.R.; Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Eisenberg, M.L. Preconception antidiabetic drugs in men and birth defects in offspring: A nationwide cohort study. Ann. Intern. Med. 2022, 175, 665–673. [Google Scholar] [CrossRef]
- Cai, T.; Hu, Y.; Ding, B.; Yan, R.; Liu, B.; Cai, L.; Jing, T.; Jiang, L.; Xie, X.; Wang, Y. Effect of metformin on testosterone levels in male patients with type 2 diabetes mellitus treated with insulin. Front. Endocrinol. 2021, 12, 813067. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.-N.; Li, F.-F.; Sun, R.; Jing, T.; Lee, K.-O.; Ma, J.-H. Rapid changes in serum testosterone in men with newly diagnosed type 2 diabetes with intensive insulin and metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.W.; Dai, L.K.; Jean, W. Metformin-related vitamin B12 deficiency. Age Ageing 2006, 35, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Kos, E.; Liszek, M.J.; Emanuele, M.A.; Durazo-Arvizu, R.; Camacho, P. Effect of metformin therapy on vitamin D and vitamin B12 levels in patients with type 2 diabetes mellitus. Endocr. Pract. 2012, 18, 179–184. [Google Scholar] [CrossRef] [PubMed]
- De Jager, J.; Kooy, A.; Lehert, P.; Wulffelé, M.G.; Van der Kolk, J.; Bets, D.; Verburg, J.; Donker, A.J.; Stehouwer, C.D. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: Randomised placebo controlled trial. BMJ 2010, 340, c2181. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine 2019, 98, e17918. [Google Scholar] [CrossRef]
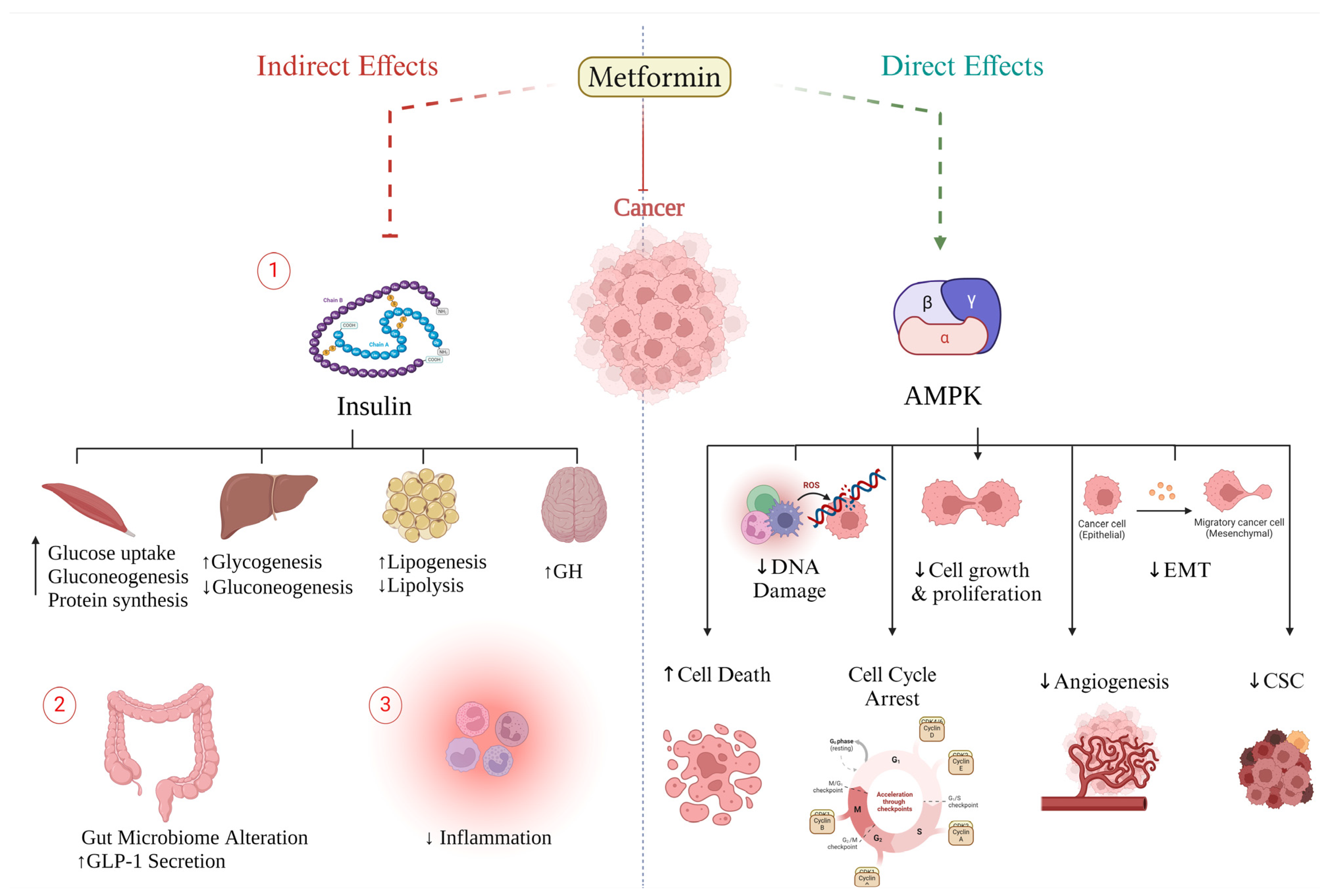

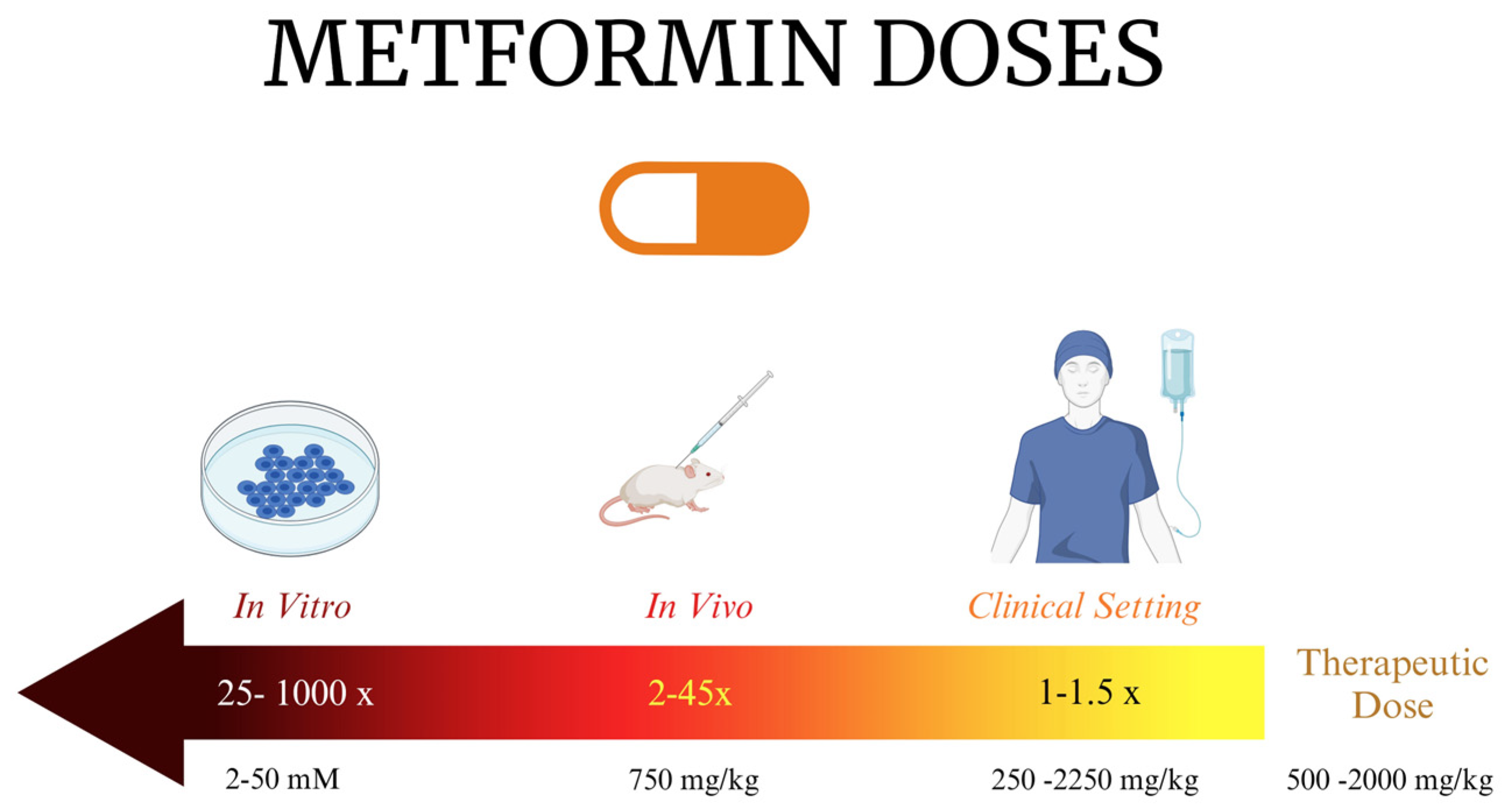
| Aging Hallmark | Significance | Metformin Impact | |
|---|---|---|---|
| At Molecular Level | Outcome | ||
| Dysregulated nutrient signaling | Distorted metabolic homeostasis |
| Improved signaling |
| Lost proteostasis | Cell dysfunction and reduced viability |
| Suppressed protein misfolding and improved autophagy |
| Mitochondrial dysfunction | Energy and homeostasis are affected |
| Mitochondrial biogenesis upregulation and delayed aging |
| Low-grade inflammation | Epigenetic changes, no protein stability, and stem cell dysfunction | ↑ Sirt-1 → ↓ NF-κB → ↓ cytokines (TNF-α & IL-6) | Inhibition of inflammation |
| Telomere attrition | Accelerated aging and diseases | AMPK regulates telomere transcription through telomere repeat RNA | Prevented telomere shortening |
| DNA damage | Genomic instability | ↑ ATM, ↑ Checkpoint Kinases-2 ↓ AKT | Antioxidant effect and DNA damage prevention |
| Stem cell exhaustion | Impaired tissue regenerative capacity and dysfunction | ↑ antioxidation, ↑ AMPK ↓ mTORC1 ↑ Nrf2 → ↑GPx7 | Delayed premature cellular senescence and extended lifespan of stem cells |
| Senescence and SASP | ↑ Sirt1 ↑ MBNL-1 → ↑miR-130a-3p → ↓ STAT3 ↓ NLRC4 ↓ NF-κB | Reduced premature senescence and SASP | |
| MiRNA | Regulatory Effect | Effector Gene | Reference |
|---|---|---|---|
| miR-200c | ↑ | ↓ AKT2 | [229] |
| miR-21-5p | ↓ | ↑ AMPK → ↓ mTOR | [230] |
| miR-27a | ↓ | ↑ AMPKα2 | [231] |
| miR-26a | ↑ | ↓ PTEN, ↓ EZH2, ↓ BCL-2 | [232] |
| miR-193b | ↑ | ↓ FAS | [233] |
| Interventional Studies | |||||
|---|---|---|---|---|---|
| NCT Number | Conditions | Interventions | Outcome Measures | Phases | Location |
| NCT05759312 | Ovarian clear-cell carcinoma | Zimberelimab | PFS, OS, DCR, duration of response, and recurrence pattern | Phase I and II | US |
| NCT04926155 | Metastatic prostate cancer | ADT and abiraterone | PFS, OS, and radiographic PFS | Phase II | China |
| NCT04925063 | Metastatic prostate cancer | ADT and abiraterone | Castration-resistant prostate cancer-free survival, OS, and radiographic PFS | Phase II | China |
| NCT05921942 | Colorectal cancer | FOLFOX protocol | DCR, PFS, OS, and IL-6 levels | Phase III | Egypt |
| NCT03379909 | Bladder cancer | ORR, time to recurrence, toxicity | Phase II | Netherlands | |
| NCT06030622 | Metastatic pancreatic cancer | Simvastatin and digoxin | Phase I | US | |
| NCT01529593 | Metastatic cancer refractory to standard therapy | Temsirolimus | Maximum Tolerated Dose (MTD) of temsirolimus and metformin and clinical tumor response | Phase I | US |
| NCT05929495 | Glioblastoma | Temozolamide | PFS at 6 months post-treatment | Phase II | Italy |
| NCT02336087 | Pancreatic adenocarcinoma | Gemcitabine hydrochloride/paclitaxel albumin-stabilized nanoparticles | Feasibility of, compliance with, and toxicity of combination, PFS, OS, | Phase I | US |
| NCT04758000 | Osteosarcoma | Placebo | DFS and toxicity | Phase II | Italy |
| NCT04945148 | Glioblastoma | Radiation IMRT, temozolomide | OS, ORR, PFS, safety, and tolerability | Phase II | Italy |
| NCT05445791 | Non-small-cell lung cancer | Placebo | OS, ORR, PFS | RCT: Phase III | Mexico |
| NCT01042379 | Breast cancer | Standard therapy | pCR, RFS, OS | RCT: Phase III | US |
| NCT05660083 | HER2-negative breast cancer | L-NMMA | Define recommended dose, ORR, PFS | Phase II | US |
| NCT05023967 | Breast cancer | Placebo | Frequency of DLT occurrence, ki67 changes, changes in the expressions of PP2A/GSK3β/MCL-1 axis and circulating biomarkers | RCT: Phase II | Italy |
| NCT05326984 | Acute lymphoblastic leukemia | Prednisone; vincristine; doxorubicin; L-asparaginase; etoposide; cytarabine; methotrexate; and 6-mercaptopurine | Decrease in ABCB1 gene expression, increase in AMPK gene expression, OS | RCT | Mexico |
| NCT02186847 | Lung cancer | Carboplatin and radiation therapy | PFS, OS, local–regional progression | Phase II | US |
| NCT01430351 | Glioblastoma | Mefloquine/memantine hydrochloride/temozolomide | PFS and toxicity | Phase I | US |
| NCT05680662 | Triple-negative breast cancer | Combination product: quercetin, EGCG, metformin, zinc | DFS at 3 and 10 yrs, toxicity | RCT: Early Phase I | |
| NCT05507398 | Breast cancer | Placebo and atorvastatin | RCT: Phase IV | ||
| NCT04248998 | Triple-negative breast cancer | Preoperative chemotherapy | pCR, RFS, OS, AA and lipid profile modifications, concentration of insulin and IGF-I | RCT: Phase II | Italy |
| Prospective Observational Studies | |||||
| NCT Number | Conditions | Interventions | Outcome Measures | Location | |
| NCT04947020 | Rectal cancer | OS, DFS | Poland | ||
| NCT05192850 | Recurrent endometrial carcinoma | Placebo | Endometrial cancer recurrence, PFS, OS | US | |
| NCT04245644 | Pancreatic cancer | Targeted drugs, such as aspirin, B-blockers, metformin, ACE inhibitors, statins | DFS, OS, pancreatic cancer progression | Italy | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. https://doi.org/10.3390/ijms25074083
Galal MA, Al-Rimawi M, Hajeer A, Dahman H, Alouch S, Aljada A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. International Journal of Molecular Sciences. 2024; 25(7):4083. https://doi.org/10.3390/ijms25074083
Chicago/Turabian StyleGalal, Mariam Ahmed, Mohammed Al-Rimawi, Abdurrahman Hajeer, Huda Dahman, Samhar Alouch, and Ahmad Aljada. 2024. "Metformin: A Dual-Role Player in Cancer Treatment and Prevention" International Journal of Molecular Sciences 25, no. 7: 4083. https://doi.org/10.3390/ijms25074083
APA StyleGalal, M. A., Al-Rimawi, M., Hajeer, A., Dahman, H., Alouch, S., & Aljada, A. (2024). Metformin: A Dual-Role Player in Cancer Treatment and Prevention. International Journal of Molecular Sciences, 25(7), 4083. https://doi.org/10.3390/ijms25074083




