Lentivirus-Mediated BCL-XL Overexpression Inhibits Stem Cell Apoptosis during Ex Vivo Expansion and Provides Competitive Advantage Following Xenotransplantation
Abstract
1. Introduction
2. Results
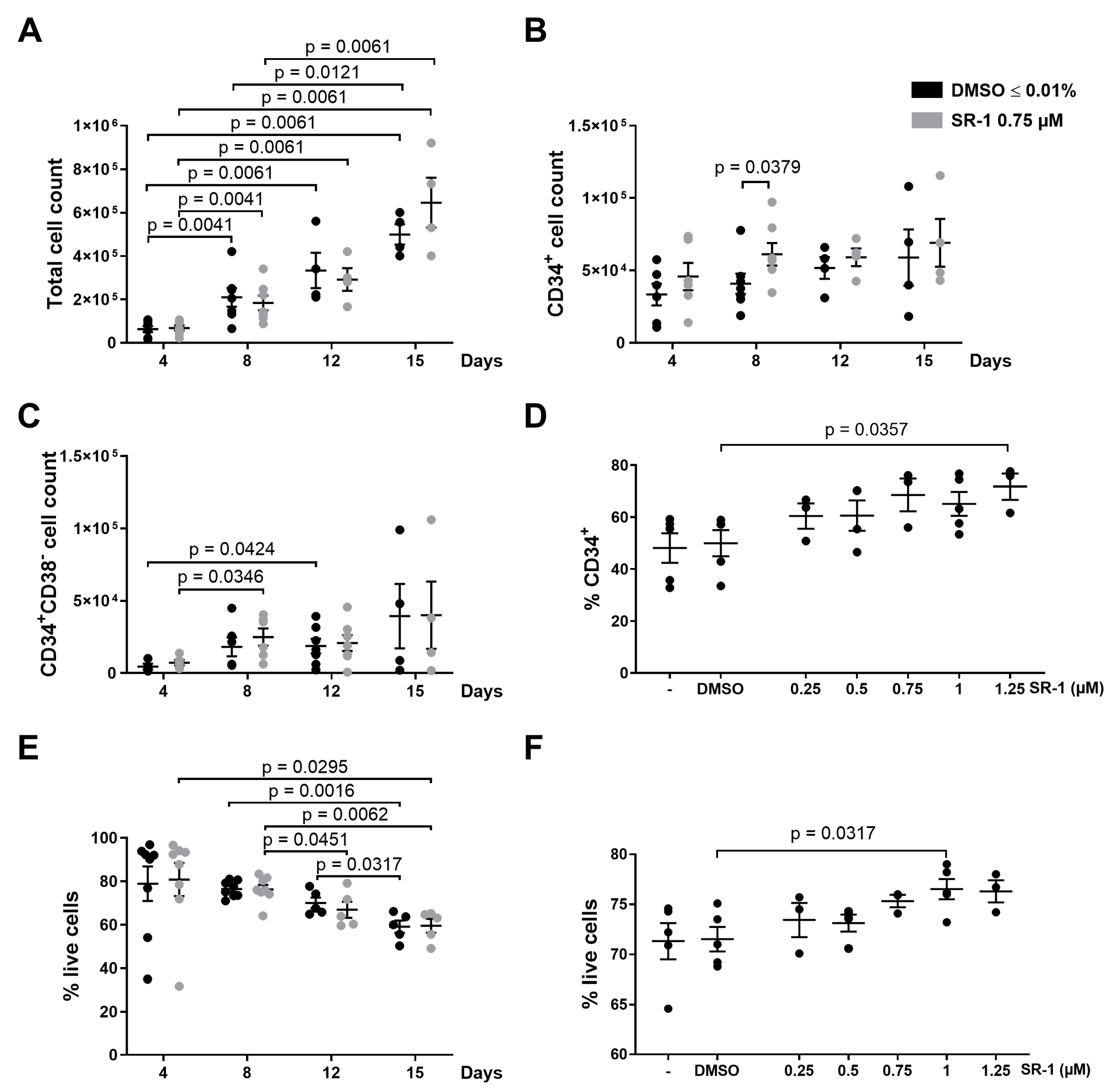
2.1. Apoptosis of CD34+ Cells Occurs during Ex Vivo Expansion
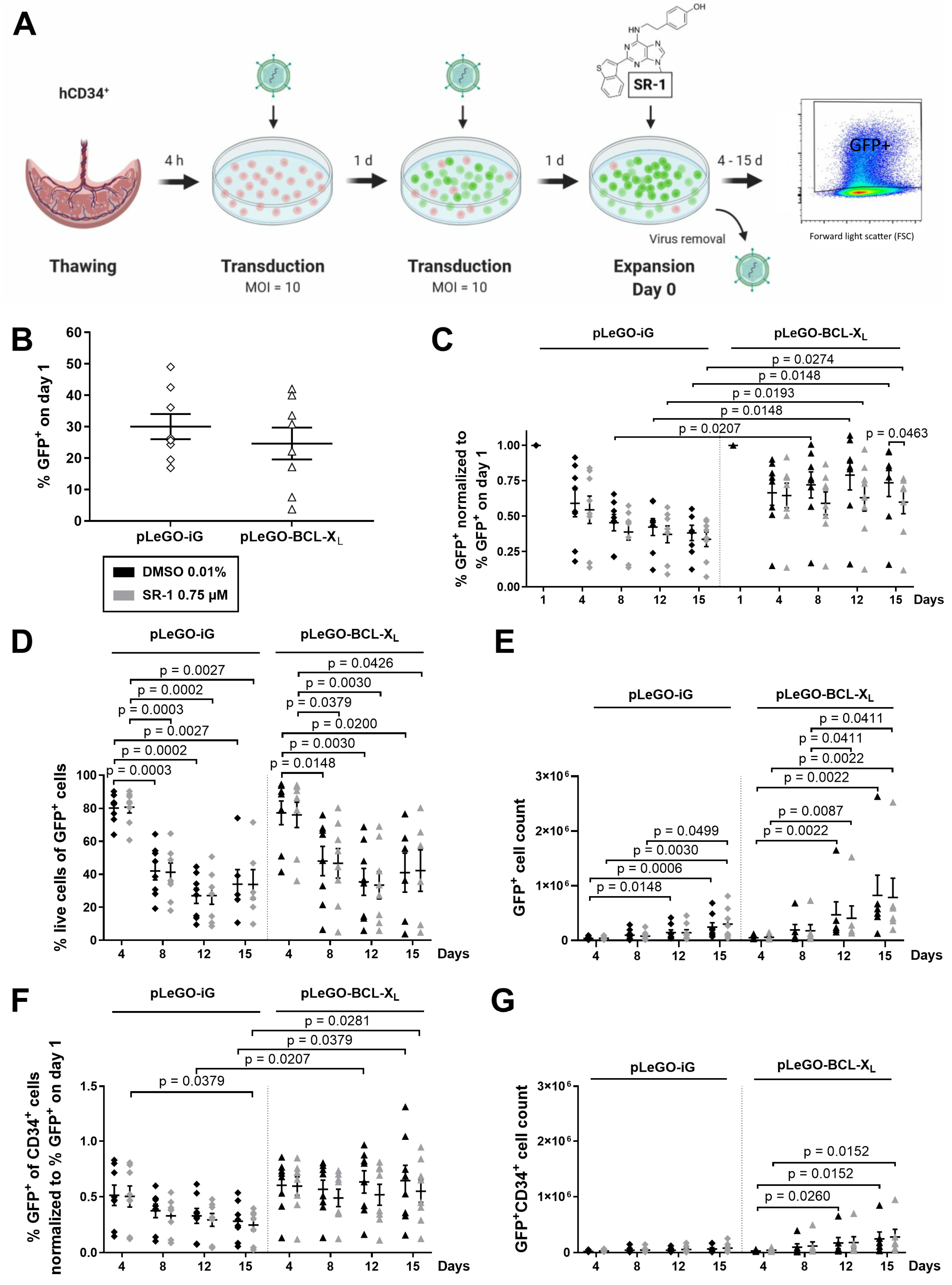
2.2. CD34+ Cells Overexpressing BCL-XL Accumulate during Ex Vivo Expansion
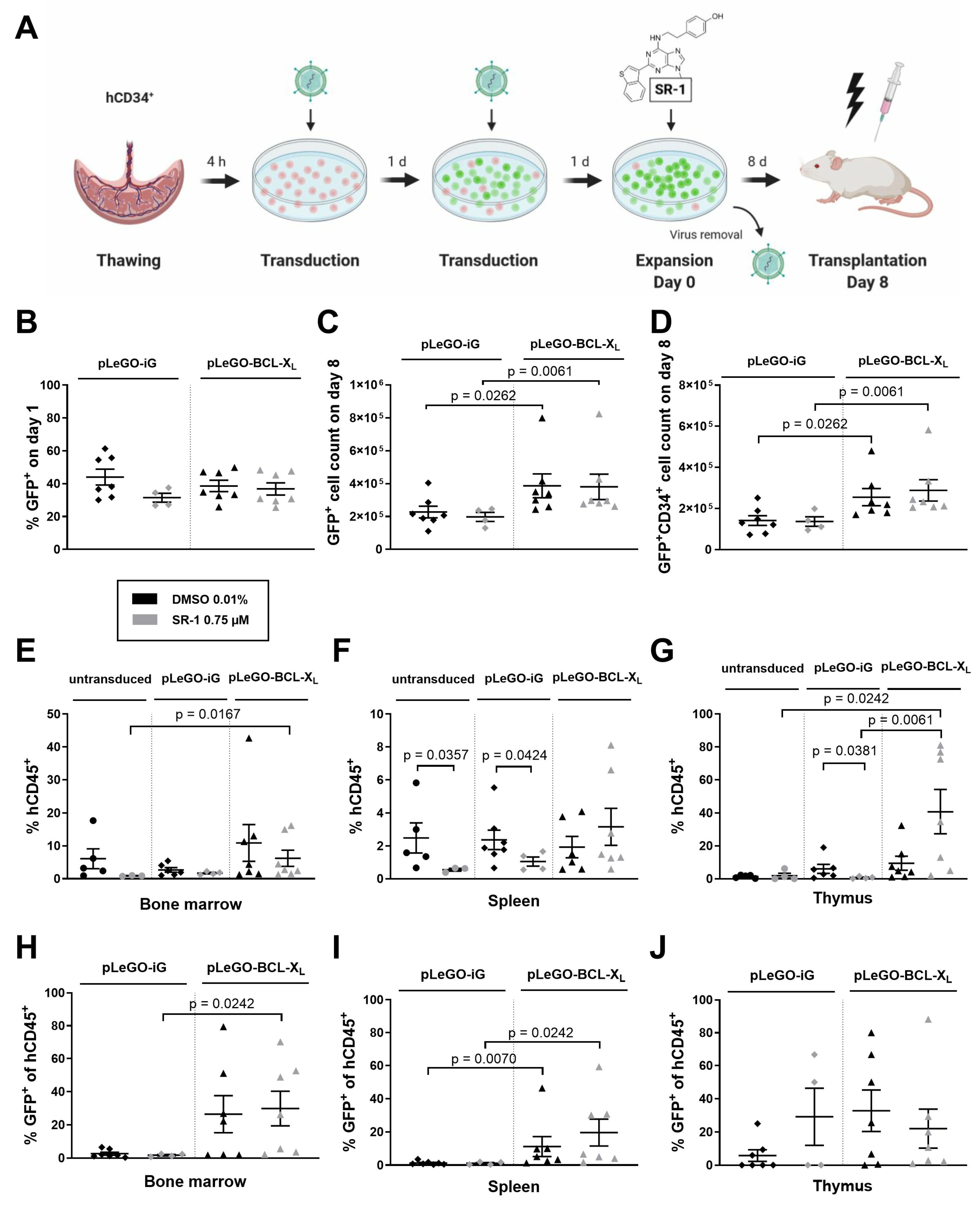
2.3. BCL-XL Overexpression Increases the Reconstitution Potential of In Vitro Expanded Stem and Progenitor Cells
2.4. Inhibitors Targeting Caspases and Necroptosis Show no Beneficial Effects during Ex Vivo Expansion
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Lentiviral Transduction
4.3. Xenotransplantation
4.4. Flow Cytometric Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, L.J.; Young, J.C.; Osborne, L.J.; Luens, K.M.; Scollay, R.; Hill, B.L. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp. Hematol. 1999, 27, 1019–1028. [Google Scholar] [CrossRef]
- Costa, M.H.G.; de Soure, A.M.; Cabral, J.M.S.; Ferreira, F.C.; da Silva, C.L. Hematopoietic Niche—Exploring Biomimetic Cues to Improve the Functionality of Hematopoietic Stem/Progenitor Cells. Biotechnol. J. 2018, 13, 1700088. [Google Scholar] [CrossRef] [PubMed]
- Boitano, A.E.; Wang, J.; Romeo, R.; Bouchez, L.C.; Parker, A.E.; Sutton, S.E.; Walker, J.R.; Flaveny, C.A.; Perdew, G.H.; Denison, M.S.; et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 2010, 329, 1345–1348. [Google Scholar] [CrossRef]
- Wagner, J.E.; Brunstein, C.G.; Boitano, A.E.; DeFor, T.E.; McKenna, D.; Sumstad, D.; Blazar, B.R.; Tolar, J.; Le, C.; Jones, J.; et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell 2016, 18, 144–155. [Google Scholar] [CrossRef]
- Afreen, S.; Weiss, J.M.; Strahm, B.; Erlacher, M. Concise Review: Cheating Death for a Better Transplant. Stem Cells 2018, 36, 1646–1654. [Google Scholar] [CrossRef]
- Labi, V.; Bertele, D.; Woess, C.; Tischner, D.; Bock, F.J.; Schwemmers, S.; Pahl, H.L.; Geley, S.; Kunze, M.; Niemeyer, C.M.; et al. Haematopoietic stem cell survival and transplantation efficacy is limited by the BH3-only proteins Bim and Bmf. EMBO Mol. Med. 2013, 5, 122–136. [Google Scholar] [CrossRef]
- Kollek, M.; Voigt, G.; Molnar, C.; Murad, F.; Bertele, D.; Krombholz, C.F.; Bohler, S.; Labi, V.; Schiller, S.; Kunze, M.; et al. Transient apoptosis inhibition in donor stem cells improves hematopoietic stem cell transplantation. J. Exp. Med. 2017, 214, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Harris, A.W.; Bath, M.L.; Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990, 348, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Whittingham, S.; Vaux, D.L.; Bath, M.L.; Adams, J.M.; Cory, S.; Harris, A.W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA 1991, 88, 8661–8665. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Broxmeyer, H.E. New approaches for improving engraftment after cord blood transplantation. Biol. Blood Marrow Transplant. 2010, 16, S126–S132. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Buckley, S.M.; Lewis, I.D.; Goldman, A.I.; Wagner, J.E.; van der Loo, J.C.M. Homing defect of cultured human hematopoietic cells in the NOD/SCID mouse is mediated by Fas/CD95. Exp. Hematol. 2003, 31, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Flach, J.; Milyavsky, M. Replication stress in hematopoietic stem cells in mouse and man. Mutat. Res. 2018, 808, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Traycoff, C.M.; Orazi, A.; Ladd, A.C.; Rice, S.; McMahel, J.; Srour, E.F. Proliferation-induced decline of primitive hematopoietic progenitor cell activity is coupled with an increase in apoptosis of ex vivo expanded CD34+ cells. Exp. Hematol. 1998, 26, 53–62. [Google Scholar] [PubMed]
- Jetmore, A.; Plett, P.A.; Tong, X.; Wolber, F.M.; Breese, R.; Abonour, R.; Orschell-Traycoff, C.M.; Srour, E.F. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood 2002, 99, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Jung, S.K.; Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 2020, 34, 3136–3148. [Google Scholar] [CrossRef]
- Murphy, W.J. Making a Better Hematopoietic Stem Cell—Timing Is Everything. N. Engl. J. Med. 2018, 378, 89–91. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. StemRegenin-1 Expanded vs Unexpanded UCB for High Risk Heme Malignancies—Full Text View—ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02765997?term=stemregenin+1&draw=2&rank=1 (accessed on 11 March 2022).
- Angelos, M.G.; Ruh, P.N.; Webber, B.R.; Blum, R.H.; Ryan, C.D.; Bendzick, L.; Shim, S.; Yingst, A.M.; Tufa, D.M.; Verneris, M.R.; et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood 2017, 129, 3428–3439. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Fares, I.; Chagraoui, J.; Gareau, Y.; Gingras, S.; Ruel, R.; Mayotte, N.; Csaszar, E.; Knapp, D.J.H.F.; Miller, P.; Ngom, M.; et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 2014, 345, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Roy, J.; Lachance, S.; Delisle, J.-S.; Marinier, A.; Busque, L.; Roy, D.-C.; Barabé, F.; Ahmad, I.; Bambace, N.; et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: A single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020, 7, e134–e145. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins. Cold Spring Harb. Perspect. Biol. 2020, 12, a036319. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, M.A.; Zakharov, I.I.; Onishchenko, G.E. Apoptotic Features in Non-Apoptotic Processes. Biochemistry (Mosc) 2022, 87, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A long way to go: Caspase inhibitors in clinical use. Cell Death Dis. 2021, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Bartsch, U.; Stocking, C.; Fehse, B. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 2008, 16, 698–706. [Google Scholar] [CrossRef]
- Mazurier, F.; Fontanellas, A.; Salesse, S.; Taine, L.; Landriau, S.; Moreau-Gaudry, F.; Reiffers, J.; Peault, B.; Di Santo, J.P.; de Verneuil, H. A novel immunodeficient mouse model--RAG2 x common cytokine receptor gamma chain double mutants—Requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J. Interferon Cytokine Res. 1999, 19, 533–541. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehnle, P.M.A.; Wu, Y.; Koleci, N.; Bohler, S.; Erlacher, M. Lentivirus-Mediated BCL-XL Overexpression Inhibits Stem Cell Apoptosis during Ex Vivo Expansion and Provides Competitive Advantage Following Xenotransplantation. Int. J. Mol. Sci. 2024, 25, 4105. https://doi.org/10.3390/ijms25074105
Zehnle PMA, Wu Y, Koleci N, Bohler S, Erlacher M. Lentivirus-Mediated BCL-XL Overexpression Inhibits Stem Cell Apoptosis during Ex Vivo Expansion and Provides Competitive Advantage Following Xenotransplantation. International Journal of Molecular Sciences. 2024; 25(7):4105. https://doi.org/10.3390/ijms25074105
Chicago/Turabian StyleZehnle, Patricia M. A., Ying Wu, Naile Koleci, Sheila Bohler, and Miriam Erlacher. 2024. "Lentivirus-Mediated BCL-XL Overexpression Inhibits Stem Cell Apoptosis during Ex Vivo Expansion and Provides Competitive Advantage Following Xenotransplantation" International Journal of Molecular Sciences 25, no. 7: 4105. https://doi.org/10.3390/ijms25074105
APA StyleZehnle, P. M. A., Wu, Y., Koleci, N., Bohler, S., & Erlacher, M. (2024). Lentivirus-Mediated BCL-XL Overexpression Inhibits Stem Cell Apoptosis during Ex Vivo Expansion and Provides Competitive Advantage Following Xenotransplantation. International Journal of Molecular Sciences, 25(7), 4105. https://doi.org/10.3390/ijms25074105




